Abstract
Basal release of GUT peptides has been found to be altered in restrained eaters. Stress-induced secretion, however, has not yet been described, but could be a biological basis of overeating that exposes restrained eaters to a higher risk of becoming obese. The aim of the present study was to compare restrained and unrestrained eaters with respect to stress-induced release of the GUT peptides ghrelin and PYY. 46 young women were studied. Blood sampling for peptides was done before and after the Trier Social Stress Test. Ghrelin secretion after stress was significantly elevated in the restrained eaters, whereas no significant differences were detected for PYY. Stress-induced release of GUT peptides can be interpreted as a cause as well as a consequence of restrained eating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The eating disorders bulimia nervosa and binge eating disorder show prevalence rates up to 13.1 % in community samples [24].
Patients with bulimia nervosa usually are in the normal weight range. The core symptoms of the disorder are bulimic attacks: that means the uncontrolled intake of a large amount of food (up to 10,000 calories) in a short period of time. This behaviour may take place several times a day. Most of the patients practise vomiting after their bulimic attacks, but also the abuse of laxatives is very common to compensate the excessive intake. Psychologically, they are characterised by extreme concerns about weight and shape [7].
Binge eating disorder (BED) is an eating disorder characterised by the recurrent uncontrolled intake of large amounts of food. In contrast to bulimia nervosa, BED subjects do not practise compensatory behaviours such as vomiting or the use of laxatives after a binge episode. Most of these individuals with BED therefore are obese.
Binge eating disorder (BED) is now an actual eating disorder diagnosis in the DSM-5, which was released by the American Psychiatric Association in May 2013.
Due to their abnormal eating behaviour, both disorders show numerous biological aberrations in the endocrine and neurotransmitter systems.
For both disorders, current aetiological models (overview in [1]) are multifactorial, but postulate as a main vulnerability factor for the onset and maintenance repeated periods of dieting or the permanent practice of restrained eating. In particular, dieting has been linked to binge eating. This has been investigated in numerous laboratory studies, which have shown that overeating after stress-induced disinhibition is a stable characteristic of restrained eaters (e.g., [18]). Whether stress provokes short-term changes in peptides that regulate food intake is still unclear.
According to the reviews of [5, 17], it can be concluded that peripheral release of ghrelin and PYY is linked to the regulation of food intake in humans. Ghrelin promotes food intake, whereas PYY has an inhibitory effect.
Fasting plasma levels of ghrelin in patients with bulimia nervosa were significantly higher than those in controls [25]. Restrained eating was also significantly associated with higher ghrelin levels [22].
Acute psychological stress leads to a significant increase in ghrelin levels. This has been shown in an animal model [12] as well as in humans [20].
Patients with bulimia nervosa show blunted PYY response to test meals [11, 16]. Basal or stimulated concentrations of PYY have not been investigated in restrained eaters. There is some evidence that subjects under short-term caloric restriction show significantly decreased PYY levels [2].
Stress-related changes in PYY levels are only described in [4, 13].
The present investigation therefore addressed the question whether there is an altered release of stress-induced ghrelin and PYY, specifically in restrained eaters.
Method
All subjects were recruited by advertisement on the university campus. Weight had to be in the normal range (BMI 19–24). Exclusion criteria were clinical eating disorders in the past or at present and acute medical illness. With respect to these criteria, all subjects were checked by a medical doctor.
The study was approved by the ethical committee of medicine in Rheinland-Pfalz, Germany (ID: 837.159.096664). All subjects were paid for participation.
Classification into restrained and unrestrained eaters was done according to norm tables of the German version of the Three Factor Eating Questionnaire (cognitive restraint scale [19]). Subjects with a score ≤7 were classified as unrestrained eaters and those with a score ≥12 as restrained eaters.
The characteristics of the sample are depicted in Table 1.
With respect to these characteristics, no significant differences between the comparison groups were present.
There were no hints of an increased prevalence of obesity in the families of participants. The mean maximum BMI during the lifetime of the participants themselves was 23.5 kg/m2.
The stressor was the Trier Social Stress Test (TSST) [10]. The procedure combines a social stressor (public speech) with a mental stressor (arithmetic under time pressure) and is a validated tool to provoke psychobiological stress responses.
Blood sampling
All subjects were studied in the afternoon and asked to eat a meal 2 h before the start of the experiment and to finish eating at least 1.5 h before arriving at the laboratory. Blood samples were drawn through an indwelling butterfly catheter in a forearm vein. Blood samples for ghrelin and PYY were collected at time 0 (before stress) and 30 and 60 min after stress induction. PYY was measured in total form (PYY3-36 and PYY1-36). The blood was sampled in EDTA-treated tubes and immediately centrifuged to yield plasma for peptide determinations. For stabilisation of ghrelin and PYY Pefabloc as an irreversible serine protease inhibitor [4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride, AEBSF; from Sigma-Aldrich, Steinheim, GER] was added into EDTA-treated blood collection tubes to a final concentration of 1 mg/mL. The samples were processed immediately after blood withdrawal, kept on ice and centrifuged at 4 °C/1000g for 10 min. Ghrelin plasma aliquots were acidified with HCl to a final concentration of 0.05 N. Samples were stored at −20 °C until analysis with enzyme-linked immunoassays.
Ghrelin (total) and PYY (both 1–36 and 3–36) plasma levels were analysed with commercial ELISA-based assays in kits from Millipore-Corporation, St. Charles, MI, USA. Duplicate sample aliquots were treated according to the manufacturer’s instructions. Results were excluded from further analyses if the difference between duplicate results of a sample was >15 %CV. The intra- and interassay coefficients of variation for ghrelin total were <1.91/<7.74 % and <5.78/<16.50 % for PYY, respectively.
Manipulation check
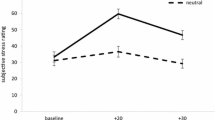
The success of stress induction was tested by using subjective ratings provided by visual analogue scales ranging from 0 (no stress) to 10 (maximum stress). The ratings were obtained before and 15 min after stress induction and analysed by MANOVA for repeated measurement. There was a significant main effect of stress: F (1,44) = 62.3, p < .001, partial ETA squared = 0.59, but no significant interaction effect with group (restrained vs. unrestrained). The ratings are presented in Table 2.
Stress hormone levels in the form of salivary cortisol are also available before and after stress induction. Basal cortisol level for the participants was 3.8 ± 2.4 nmol/L. Stress induction led to a significant increase in cortisol after 30 min to 5.7 ± 4.0 nmol/L (p < .003).
Statistical analysis for peptide release
Mean ghrelin values before, 30 and 60 min after stress induction were compared by MANOVA for repeated measurement testing linear trend over time (see O’Brien and Kaiser, 1985 for a detailed description of this procedure).
Post hoc contrasts between groups at different time points were tested in a hierarchical procedure. First, a global test of differences in linear trend was performed. Then, subsequent F tests of mean differences at 30 and 60 min after stress induction were conducted.
A measure of effect size was partial ETA squared. The same procedure, but only for the time points before and 60 min after stress induction, was performed for PYY.
Results
The data for ghrelin are shown in Table 3.
The MANOVA showed a main effect of stress on ghrelin release. F (2,44) = 7.5, p < .003. Partial ETA squared = 0.25. During stress ghrelin increased significantly.
Comparisons between groups at 30 and 60 min after stress revealed that restrained eaters had higher mean values at both measurement points.
-
At 30 min: F (1,46) = 8.96, p < .005, partial ETA squared = 0.16.
-
At 60 min: F (1,46) = 7.8, p < .008, partial ETA squared = 0.15.
The basal concentration of ghrelin was also significantly different with higher levels for the restrained eaters: F (1,45) = 7.5, p < .01, partial ETA squared = 0.14.
Table 4 shows the data for PYY.
The stress induction was not significantly linked to release of PYY in the restrained eaters, whose release remained at pre-stress level and did not influence lack of satiation during stress.
Discussion
Our data show that stress-induced peripheral ghrelin release is significantly higher in restrained eaters. Probably, this signals the brain to initiate the intake of food.
Restrained eaters already had significantly higher basal levels of ghrelin. The stress-induced difference remained significant with a comparable effect size.
The stress-induced elevation of ghrelin levels, however, is small and therefore has to be interpreted with some care until replication.
Stress was successfully induced for both comparison groups. This is proved by a significant increase in ratings of subjective feelings of stress measured by visual analogue scales. Also, cortisol significantly increased after stress induction.
Our results on baseline differences in ghrelin are comparable to the findings of [22]. The differences might be psychobiologically determined. High ghrelin levels would stimulate food intake and therefore pose a biological pressure to eat on a person. Restrained eaters are known to have an increased fear of becoming obese and counteract the biological pressure to eat by a more intense cognitive control of their food intake. Therefore, high ghrelin levels could be biological correlates of restrained eating behaviour.
Similar differences in basal ghrelin levels have also been found by [25], who investigated young women with bulimia nervosa. This disorder is strongly associated with restrained eating between the binge attacks [14].
PYY did not show stress-related alterations, but remained at pre-stress levels. This finding is also reported by [15] measuring postprandial PYY plasma levels.
Two explanations of our results may be considered. The increased stress-induced ghrelin release may partially be a cause of the restrained eating behaviour. High ghrelin secretion in stressful situations leads to increased food intake, which in turn would promote weight gain with the danger of becoming overweight in the long term. To prevent this, chronic or repeated dieting behaviour (that means restrained eating) has to be practised.
In obese women, a stress-related elevation of ghrelin has been found [6, 21]. The consequence may be a biologically determined higher motivation for food intake in stress situations which promotes further weight gain and maintenance of obesity. Therefore, obese women often practise repeated dieting or permanent restrained eating which is reflected in high scores on scales measuring cognitive restraint [9].
The other interpretation is that the increased ghrelin release is a consequence of the frequent dieting behaviour, seen in people classified as restrained eaters. Some support comes from a study of [22] who reported a significant correlation between the degree of restraint and circulating ghrelin in normal weight subjects. The data of [3] are also in agreement with this hypothesis. Obese women had significantly elevated ghrelin levels after a dieting period. In our study, data on the difference between lowest and highest weight during the last 12 months are available. If this difference is large, it can be taken as an indicator of multiple dieting. The correlation of this difference and basal ghrelin levels was 0.34 (p < .03) and could possibly contribute to explain differences in ghrelin levels, because dieting has been identified to increase ghrelin [8].
On the other hand no significant relationship between weight changes and ghrelin levels was reported by [23] in 200 post-menopausal women, which would argue against this interpretation.
References
Agras WS (ed) (2010) The Oxford handbook of eating disorders. Oxford University Press, New York
Chan JL, Stoyneva V, Kelesidis T, Raciti P, Mantzoros CS (2006) Peptide YY levels are decreased by fasting and elevated following caloric intake but are not regulated by leptin. Diabetologia 49:169–173. doi:10.1007/s00125-005-0041-2
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630. doi:10.1056/NEJMoa012908
Forbes SC, Cox HM (2014) Peptide YY, neuropeptide Y and corticotrophin-releasing factor modulate gastrointestinal motility and food intake during acute stress. Neurogastroenterol Motil 26(11):1605–1614. doi:10.1111/nmo.12428
Gibson CD, Carnell S, Ochner CN, Geliebter A (2010) Neuroimaging, gut peptides and obesity: novel studies of the neurobiology of appetite. J Neuroendocrinol 22:833–845. doi:10.1111/j.1365-2826.2010.02025.x
Gluck ME, Yahav E, Hashim SA, Geliebter A (2014) Ghrelin levels after a cold pressure stress test in obese women with binge eating disorder. Psychosom Med 76:74–79. doi:10.1097/PSY.0000000000000018
Grilo CM, Masheb RM, White MA (2010) Significance of overvaluation of shape/weight in binge-eating disorder: comparative study with overweight and bulimia nervosa. Obesity (Silver Spring) 18(3):499–504. doi:10.1038/oby.2009.280
Hill BR, Rolls BJ, Roe LS, De Souza MJ, Williams NI (2013) Ghrelin and peptide YY increase with weight loss during a 12-month intervention to reduce dietary energy density in obese women. Peptides 49:138–144. doi:10.1016/j.peptides.2013.09.009
Keskitalo K, Tuorila H, Spector TD, Cherkas LF, Knaapila A, Kaprio J, Silventoinen K, Perola M (2008) The Three-Factor Eating Questionnaire, body mass index, and responses to sweet and salty fatty foods: a twin study of genetic and environmental associations. Am J Clin Nutr 88(2):263–271
Kirschbaum C, Pirke KM, Hellhammer DH (1993) The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81. doi:10.1159/000119004
Kojima S, Nakahara T, Nagai N, Muranaga T, Tanaka M, Yasuhara D, Naruo T (2005) Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clin Endocrinol 62:74–78. doi:10.1111/j.1365-2265.2004.02176.x
Kristensson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C, Lindström E (2006) Acute psychological stress raises plasma ghrelin in the rat. Regul Pept 134:114–117. doi:10.1016/j.regpep.2006.02.003
Liang C, Luo H, Liu Y, Cao J, Xia H (2012) Plasma hormones facilitated the hypermotility of the colon in a chronic stress rat model. PloS One 7(2):e31774. doi:10.1371/journal.pone.0031774
Lowe MR, Thomas JG, Safer DL, Butryn ML (2007) The relationship of weight suppression and dietary restraint to binge eating in bulimia nervosa. Int J Eat Disord 40(7):640–644. doi:10.1002/eat.20405
Martins C, Robertson MD, Morgan LM (2010) Impact of restraint and disinhibition on PYY plasma levels and subjective feelings of appetite. Appetite 55:208–213. doi:10.1016/j.appet.2010.05.091
Monteleone P, Martiadis V, Rigamonti AE, Fabrazzo M, Giordani C, Muller EE, Maj M (2005) Investigation of peptide YY and ghrelin responses to a test meal in bulimia nervosa. Biol Psychiatry 57:926–931. doi:10.1016/j.biopsych.2005.01.004
Perry B, Wang Y (2012) Appetite regulation and weight control: the role of gut hormones. Nutr Diabetes 2(1):e26. doi:10.1038/nutd.2011.21
Polivy J, Coleman J, Herman CP (2005) The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int J Eat Disord 38(4):301–309. doi:10.1002/eat.20195
Pudel V, Westenhöfer J (1989) Fragebogen zum Eßverhalten (FEV)-Handanweisung. Hogrefe, Göttingen
Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y (2007) The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 32:693–702. doi:10.1016/j.psyneuen.2007.04.010
Sarker MR, Franks S, Caffrey J (2013) Direction of post-prandial ghrelin response associated with cortisol response, perceived stress and anxiety, and self-reported coping and hunger in obese women. Behav Brain Res 257:197–200. doi:10.1016/j.bbr.2013.09.046
Schur EA, Cummings DE, Callahan HS, Foster-Schubert K (2008) Association of cognitive restraint with ghrelin, leptin, and insulin levels in subjects who are not weight-reduced. Physiol Behav 93:706–712. doi:10.1016/j.physbeh.2007.11.025
Soni AC, Conroy MB, Mackey RH, Kuller LH (2011) Ghrelin, leptin, adiponectin, and insulin levels and concurrent and future weight change in overweight postmenopausal women. Menopause (New York, NY) 18(3):296–301. doi:10.1097/gme.0b013e3181f2e611
Stice E, Marti CN, Rohde P (2013) Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. J Abnorm Psychol 122(2):445–457. doi:10.1037/a0030679
Tanaka M, Naruo T, Muranaga T, Yasuhara D, Shiiya T, Nakazato M, Nozoe SI (2002) Increased fasting plasma ghrelin levels in patients with bulimia nervosa. Eur J Endocrinol 146:R1–R3. doi:10.1530/eje.0.146R001
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The study was approved by the ethical committee of medicine in Rheinland-Pfalz, Germany (ID: 837.159.096664).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hilterscheid, E., Laessle, R. Stress-induced release of GUT peptides in young women classified as restrained or unrestrained eaters. Eat Weight Disord 20, 435–439 (2015). https://doi.org/10.1007/s40519-015-0185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-015-0185-0