Abstract
Purpose of Review
We describe the mechanisms and risk factors related to non-gastrointestinal waterborne hospital-acquired infections, including bloodstream infections. Included are some characteristics of the main bacteria described in the literature.
Recent Findings
In the last two decades, the number of water living bacteria that had been identified as causing healthcare-associated waterborne infections has expanded. Among these, Legionella, Enterobacteriaceae, Pseudomonas aeruginosa, other non-fermenting bacteria, and nontuberculous mycobacteria are included. We describe some of the main characteristics of the bacteria associated with the infections in the hospital setting, mostly bloodstream infections.
Summary
No single approach guarantees that hospital water will be safe for vulnerable patients, but a combination of engineering, chlorination surveillance, hygiene measures, and clinical care strategies can minimize the risk. Microbial contamination of the water supply in healthcare facilities is better prevented than remediated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last four decades, modern medicine has increased the life expectancy of patients with chronic life-threatening diseases, previously considered terminal, at the cost of increasing vulnerability of human beings, and frequently affecting both humoral and cellular immunity [1]. To this, we have to add the use of invasive devices, mainly vascular catheters that constitute a direct bridge between the external environment, including bath water, and the bloodstream; these intravascular devices put patients at risk of infection particularly bloodstream infection (BSI). Waterborne healthcare-associated infections (HAIs) have been increasingly recognized in the last decades as a source of pneumonia (Legionella) [2•]. More recently, water for human use has been related as a potential cause of HAI, in particular bacteremia in patients with invasive devices. Multiple microorganisms can grow in nonsterile water, and if preventive measures are not established to strictly avoid the contact between water and these devices, it can become a source of bacteremia. On the other hand, microorganisms that grow in hospital water incur significant morbidity and mortality [3••].
Environment
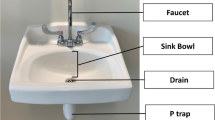
Hospital water and water-related devices, as well as moist environments and aqueous solutions, can serve as a reservoir of waterborne pathogens in healthcare settings where water temperatures can become suitable for bacterial growth. In addition, the complex structure of hospital water systems can lead to stagnation and corrosion and favor microorganisms’ biofilm formation [4••]. A variety of water reservoirs have been linked to nosocomial outbreaks including the following: drinking water, tap water, ice machines, hospital water systems, sinks, showers, bathing and tub immersion, electronic faucets, flower vases, decorative water wall fountains, heater-cooler units, eyewash stations, and dental-unit water stations [4••].
Maintenance managers of healthcare facilities are responsible for the integrity and conservation of water systems for the distribution of drinking and non-potable water for various uses. Municipal drinking water and even hospital tap water are not expected to be free of pathogens. Municipal water undergoes routine microbiological surveillance to assure safe levels of important community pathogens such as coliform bacteria. Further processing may occur at the building for purposes such as supplementary disinfection, water softening, and water heating for hot water [2•].
Transmission of pathogens from a water reservoir may occur by direct or indirect contact, ingestion and aspiration of contaminated water, or inhalation of aerosols [4••].
Hosts
Most waterborne pathogens that cause infections in hospital do so in a small proportion of exposed patients. Certain pathogens are related with host characteristics that predispose to nosocomial infections (ex. transplantation, chronic comorbid conditions, immunosuppressive therapies, history of tobacco use, central venous catheters, other implanted devices or mechanical ventilation) [3••].
This article reviews the epidemiology of waterborne HAIs, describing the most frequent pathogens involved.
Bacteria related with waterborne BSI
Several bacteria are associated with waterborne non-gastrointestinal infections, mainly bacteremia; several of these have been described in different epidemic outbreaks (Table 1). The most important are described below.
Acinetobacter spp.
Acinetobacter spp. has been identified in natural surface water samples in high proportions; it has been found in 1 to 5.5% of the heterotrophic plate count flora in drinking water, and in 5 to 92% of different water samples [5]. It has been also identified in the skin and respiratory tract in some healthy individuals. In a survey of untreated groundwater supplies in the USA, this microorganism was isolated in nearly 40% of the groundwater supplies [6]. The same study revealed that slime production of this microorganism, a recognized virulence factor for A. calcoaceticus, was not different between water isolates and clinical strains, suggesting the pathogenic potential for strains isolated from groundwater when it gets to a vulnerable subject [5, 6].
The identification of potential sources for HAI of these microorganisms is crucial to apply preventive interventions. Some published case reports found the microorganism in blood cultures and water supplies in patients with exposure of intravenous catheter insertion sites while bathing or showering, highlighting the importance of using an efficient physical barrier between the catheter insertion site and the environment with a plastic protection above semipermeable dressing that covers the insertion site of the catheter [7]. An outbreak reported in a newborn nursery was related to a breach in the aseptic technique during intravenous medication administration, along with environment factors through air conditioner condensate that predisposed to airborne dissemination via contaminated aerosols [8].
Aeromonas spp.
Aeromonas spp. has been cultured from more than 90% of natural water samples throughout the USA and Puerto Rico. Some studies have shown a higher burden in warmer environments with peaks during the summer, with 42 to 67% of clinical-associated infections in warmer months [9]. A. hydrophila is the most commonly identified species in humans, particularly in older population, followed by A. caviae, and A. veronii [10]. They have been found in chlorinated drinking water supplies in some countries, in low quantities (< 10 CFU/mL) in drinking water distribution systems, and it has been associated with biofilm production [10]. Extra-intestinal infections come from environmental origins, soil, or water contact [11].
The presentation of Aeromonas-related bacteremia includes fever (74 to 89%), hypotension (61%), jaundice (57%), and chills (46%), and it is more common in immunocompromised hosts. One-third of cases can be polymicrobial [10]. Bone infections in immunocompromised host are a result of blood bone spread, while in healthy individuals, it is most commonly a result of trauma of the tissue next to the bone, often after contamination with freshwater [12].
Burkholderia cepacia
Burkholderia cepacia bloodstream outbreaks have been described in ICUs and hemodialysis services due to contamination of fluids and disinfectant agents [13]. The severity is given by the virulence of the microorganism. It is more likely found in immunocompromised patients and epidemic outbreaks associated with the administration of contaminated parenteral solutions or antiseptic agents have been reported [14]. Outbreaks have been reported in hemodialysis units where B. cepacia has been concomitantly isolated from blood cultures obtained from catheter lumens and chlorhexidine bottles. Pulsed field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) were used to study blood cultures [13].
Another outbreak in central venous catheters in Lebanon reported that contaminated tap water was used to dilute alcohol for skin antisepsis; when used for decontamination of heparin vial caps, the catheter and bloodstream were also contaminated with B. cepacia. The same clone was identified from blood cultures, tap water, and alcohol [15].
Elizabethkingia spp.
Elizabethkingia spp. are an uncommon but threatening infection among people with underlying medical conditions [16]. It is considered an emerging pathogen in the healthcare environment [16]. The first report of a primarily community-associated Elizabethkingia outbreak was in January 2016, when the Wisconsin Division of Public Health notified the Centers for Disease Control (CDC) of six E. anophelis BSI among residents of four counties during the previous 13 days. A thorough investigation revealed 63 cases in residents of 12 Wisconsin counties, two patients in Illinois, and one patient in Michigan. Twenty-five of the 48 affected patients (43%) were admitted to the intensive care unit (ICU); 10/61 (14%) required hemodialysis and 18/63 (29%) died. Only one sample of water containing material used by a patient while ill, grew the same microorganism, but the ultimate source of the outbreak was not identified [17, 18].
A study of an outbreak of E. meningoseptica in an ICU revealed that sinks used for hand hygiene and also used to dispose patient secretions were four times more contaminated than sinks that were only used for hand hygiene [19].
Enterobacter spp.
According to the National Nosocomial Infections Surveillance System (NNIS), Enterobacter spp. accounted for 5 to 7% of all nosocomial bacteremia in the USA from 1976 to 1989. However, in recent publications, these nosocomial pathogens were almost exclusively isolated from ICU patients; they represent in some reviews the fifth most common pathogens recovered from blood (5.3%) [20].
The isolation of Enterobacter spp. from blood is two- to threefold higher in specialized units, such as cancer centers, and two- to threefold lower in community hospitals. E. cloacae predominates in most series (46 to 91% of isolates) followed by E. aerogenes (9 to 43%), E. agglomerans, E. sakazakii, and others. Around 14 to 53% of bacteremia that involve Enterobacter spp. are polymicrobial [21].
A Malaysian study of 11 babies, 9 of them premature, who presented a nosocomial outbreak of bacteremia caused by E. gergoviae in the neonatal ICU of a general hospital, found the same microorganism isolated from the dextrose/saline used for the dilution of parenteral antibiotics and from the hands of a healthcare worker in the nursery [22, 23].
Halomonas spp.
Halomonas phocaeensis was identified in a nosocomial outbreak of BSI in neonatal ICU related to fresh frozen plasma warming. This first description of H. phocaeensis bacteremia illustrates the infection risks associated with poorly controlled water hygiene in healthcare settings [24].
Halomonas spp. was recognized as human pathogen causing BSI in dialyzed patients attributed to contamination of machines in a dialysis center with bicarbonate fluid products used to prepare dialysis solutions. It has been documented that they are biofilm producers [25, 26].
Legionella pneumophila
Legionella pneumophila grows within free-living amoebae, which can tolerate a wide range of temperatures (between 20 and 50 °C), and other environmental conditions [3••]. It was first described as the cause of pneumonia among 182 delegates to the American Legion Convention in Philadelphia in 1976 [27].
Legionella spp. are ubiquitous in aquatic habitats, including rivers, ponds, hot springs, and lakes; all are recognized as potential human pathogens [28]. Although most Legionnaire’s disease is community acquired, approximately 10% of cases are thought to result from hospital water exposure [3••]. The disease is typically transmitted to patients through aerosols that arise from showers, ice machines, decorative fountains, humidifiers, and other water sources in hospitals [29]. The most vulnerable and most affected patients in the hospital are those with advanced age and chronic lung disease. The mortality rate of nosocomial Legionnaire’s disease is approximately 32%, fourfold higher than the community-acquired infection, likely because of the underlying comorbidities of hospitalized patients [3••].
Prevention of healthcare-associated Legionella infection requires a multidisciplinary approach involving healthcare epidemiologists and hospital officers, hospital designers, architects, and maintenance engineers, as well as the clinicians and the microbiology laboratory [3••]. But when the water system of a hospital is already contaminated with Legionella, different options exist including super-heating and flushing the system with or without shock chlorination. These methods have been effective in terminating existing outbreaks [30]. Hospitals providing care for immunocompromised patients must conduct close clinical surveillance for Legionnaire’s disease among hospitalized patients. The Centers for Disease Control (CDC) recommends that facilities with an already nosocomial outbreak of Legionnaire’s disease conduct ongoing microbiologic surveillance to detect recontamination of their water supply. The use of environmental surveillance for primary prevention (when there has been no outbreak) remains controversial [31].
Nontuberculous mycobacteria
Nontuberculous mycobacteria (NTM) are ubiquitous microorganisms in the environment. Most are found in wet soil, aural water, and even tap water; survive in hot water systems; and resist chlorination. Plumbing features, such as stagnation, and warm water, also foster proliferation of NTM [32].
NTM, including both rapid-growing and slow-growing species, have caused waterborne healthcare-associated outbreaks and infections as follows: M. abscessus with tap water; M. avium with potable water; M. canariasense in the hospital water supply; M. chelonae with ice and ice machines; M. chimaera with heater-cooler units; M. fortuitum with hospital water system and showers; M. genavense with tap water; M. mucogenicum with bathing and tub immersion, electronic faucets, sinks, showers, and hospital water systems; M. neoaurum with hospital water systems; M. phocaicum with showers; M. porcinum with ice and ice machines and tap water; and M. simiae with tap water [4••, 33].
The invasive forms of disease are most common in immunocompromised hosts as in AIDS, cancer, and hematological and solid organ transplant patients and in those receiving immunosuppressive drugs including biologic therapies with anti-tumor necrosis factor molecules [34].
Many true outbreaks and several pseudo-outbreaks with NTM have been reported. HAI outbreaks and sporadic cases have included central line BSI, sternal wound infections, cosmetic surgery–associated soft tissue infections, and BSI related with dialysis. Most reported outbreaks involved exposure of invasive devices or non-intact skin to tap water [33].
Ochrobactrum spp.
Ochrobactrum infections are especially identified in immunocompromised patients. The outbreaks that have been reported affect patients receiving chemotherapy or organ transplant recipients. Poor hand hygiene was the primary source of the infection, responsible for catheter flushing solution contamination [35, 36].
Pseudomonas aeruginosa
Pseudomonas spp. is commonly associated with biofilms in hospital water sources. Therapy pools, tubs, and daily bathing in bed can be sources of transmission [30]. Being recognized as one of the most threatening microorganisms in hospitalized immunosuppressed patients, together with the increasing incidence of antimicrobial resistance in the last decades, has made P. aeruginosa an emerging infection control problem [37]. The source of many of P. aeruginosa infections remains unclear, but most are related with environmental contamination, including tap water [38].
It is well recognized that immunocompromised patients are an important risk group for these infections; a study of an hematology ward where water samples were cultured and compared to clinical isolates from hematologic patients, using PFGE, found that P. aeruginosa isolated from blood cultures from three patients were indistinguishable from water strains [39]. The transmission was related to the infusion therapy procedure tray used to transport intravenous drugs to patients contaminated by water colonized by P. aeruginosa [39].
Ralstonia pickettii
Ralstonia spp. is a group of environmental low-virulent Gram-negative non-fermenting bacilli, slow-growing bacteria that can take almost 3 days to visualize colonies [1]. It can cause infection when contaminated solutions or intravenous medication is administered, causing nosocomial outbreaks of BSI, most commonly in immunocompromised hosts (organ transplant recipients, leukemia, or HIV patients) [40].
R. pickettii is the most clinically important pathogen from the Ralstonia genus. It causes bacteremia due to contaminated solutions, water for injection, saline solutions, hemodialysis water supplies, and sterile drug solutions [14, 41]. It has been described in a case of peritoneal dialysis-associated peritonitis, where different factors influenced bacterial proliferation and biofilms, being detected in high numbers in dialysis water treatment facilities equipped with chlorinated polyvinyl chloride piping [42, 43].
These organisms are prevalent in many different types of water supplies (including hospital water supplies), being well adapted to survive in low-nutrient conditions. It has also been reported in chlorinated polyvinyl tubbing [44, 45].
Serratia marcescens
Serratia marcescens is the primary species of the Serratia genus associated with disease. It can be isolated from different clinical specimens including blood, tracheal aspirates, and urine [46]. S. marcescens thrives in moist environments, including intravenous solutions, indwelling intravenous catheters, soap, and disinfectants, all of which have been described as the source of outbreaks [47].
Different outbreaks have been reported in situations which predispose to deficits in hygienic behavior, and a lack of standard infection control, for example the use of nonsterile used for intravenous administration [46, 48].
Sphingomonas
Sphingomonas paucimobilis bacteremia has been reported in patients who received hydromorphone analgesia syringes [49]. In another study, it was isolated from the blood of a patient with leukemia. The genotype of this microorganism was the same from tap water [50].
Stenotrophomonas maltophilia
Stenotrophomonas maltophilia has recently emerged as a significant pathogen of nosocomial infections in immunosuppressed or critically ill patients, with up to 53% mortality reported [51]. The intrinsic resistance to disinfectants and to commonly used antibiotics poses a threat to prevention and makes treatment difficult. The most frequent clinical manifestation is pneumonia in cancer and ICU patients. It has been associated with the use of broad-spectrum antimicrobials, mechanical ventilation, advanced age, and high APACHE II score [51]. A study conducted in a teaching hospital in China found that the incidence of S maltophilia bacteremia was from 3.4 to 15.4 episodes per 1000 admissions over a 9-year period. Malignancy was the most common comorbidity, and hemodialysis and septic shock were associated with death [52]. Another study from Beijing analyzed 76 BSI episodes secondary to S. maltophilia between 2010 and 2018 in a tertiary care hospital, and found that risk factors for infection were solid organ transplantation, hematological malignancy, and neutropenia. Altogether, risk factors associated with death were as follows: mechanical ventilation, hemodialysis, and septic shock [53, 54].
The second most frequent clinical manifestation was catheter-related BSI (CRBSI). A study reported that removal of central venous catheter was associated with a favorable outcome in patients with S. maltophilia bacteremia [51]. A 10-year case-control study from 2006 through 2016 performed at a tertiary-care hospital in patients with S. maltophilia bacteremia, and hematologic malignancies showed a higher overall 30-day mortality rate for patients with bacteremia compared to controls (61.0 and 32.2%; P < 0.001) [52].
Vibrio cholerae Non-O1, Non-O139
Vibrio species are known to be ubiquitously found in marine environments, while their survival is mandated by the water’s temperature and salinity; the incidence of the infections increases during summer [55]. The invasive form of non-O1, non-O139 V. cholerae infections is mainly seen in immunocompromised hosts. The main risk factors that have been reported are as follows: hepatic cirrhosis, liver diseases, alcoholism, diabetes mellitus, and hematologic malignancies [55].
In a review of 48 patients with non-O1, non-O139 V. cholerae bacteremia with skin and soft tissue infections (most commonly are cellulitis and necrotizing fasciitis), these were associated with aquatic environments exposure, and at least one comorbidity such as liver disease and/or alcohol abuse [56].
Yersinia enterocolitica
The principal route of Y. enterocolitica infection is through contaminated food or water [57]. Although bacteremia and sepsis are rare, there are some cases associated with blood cell transfusion [58, 59].
Prevention
Hand hygiene of healthcare personnel is the most important preventive measure to avoid HAI [60]. It is ironical that transmission in the setting of appropriate hand hygiene may occur when hands become contaminated during handwashing in a sink with a contaminated aerator, faucet, or drain. This method of transmission has been reported in several outbreaks [60].
No single approach guarantees that hospital water will be safe for vulnerable patients, but a combination of engineering and hygiene measures along with clinical strategies can minimize the risk [1]. Engineering measures in the built environment include avoidance of features in plumbing systems that predispose to stagnation, and maintenance of separate cold and hot water systems until near the point of use [61]. Decorative fountains should not be placed inside healthcare facilities [30]. Clinical strategies include aggressive surveillance for nosocomial waterborne infection [1]. Infection control specialists should be quick to respond to increases in rates of known and emerging waterborne pathogens [60].
Direct aerosol transmission from water to patients (from a shower, room humidifier, cooling tower); indirect transmission from fomites that had contact with contaminated water; inappropriate use of nonsterile water for tasks that require higher measures of caution, such as care of ventilated patients and rinsing of respiratory therapy or endoscopic equipment in tap water or exposure of implanted devices to water; or transmission on the hands of healthcare personnel [3••]. All of the above represent opportunities for prevention through hospital policies, education and monitoring of healthcare personnel practices, and proper cleaning and maintenance of equipment [3••].
In patients with catheters, the optimal care for cleaning and taking care of it is essential for preventing waterborne CRBSI. Some strategies are shown in Table 2.
The delivery of drinking water to ever-increasing populations for which there are finite sources of freshwater is a common problem in middle- and low-income countries [62].
When water chlorination is not guaranteed because of diverse reasons (lack of supplies, shortage of electricity), chlorine surveillance of hospital water at different areas should be mandatory as it is an important preventive policy of waterborne HAI [62, 63]. A number of alternatives to chlorination have been developed that are in active use in many parts of the world, but the risks associated with their by-products are even less well established than for chlorination [63]. A useful guideline for chlorine testing is water chlorination locally to maintain it at a concentration ≥ 0.5 mg/L in every point of use [62].
Conclusions
In the last two decades, the number of water-living bacteria that have been identified as causing healthcare-associated waterborne infections has expanded. No single approach guarantees that hospital water will be safe for vulnerable patients, but a combination of engineering, chlorination surveillance, hygiene measures, and clinical care strategies can minimize the risk. Microbial contamination of healthcare facility water supply is better prevented than remediated.
Reference and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sharma S, Sachdeva P, Virdi JS. Emerging water-borne pathogens. Appl Microbiol Biotechnol. 2003;61:424–8. https://doi.org/10.1007/s00253-003-1302-y.
• Gamage SD, Ambrose M, Kralovic SM, Roselle GA. Water safety and Legionella in health care: priorities, policy, and practice. Infect Dis Clin N Am. 2016;30:689–712. https://doi.org/10.1016/j.idc.2016.04.004This review emphasizes the importance of maintaining an adequate water distribution system in hospitals, in order to have sufficient quality water that reduces the risk of infections associated with it.
•• Decker BK, Palmore TN. Hospital water and opportunities for infection prevention. Curr Infect Dis Rep. 2014;16(10):432. https://doi.org/10.1007/s11908-014-0432-yThis article is an excellent review of the main pathogens causing water-associated infections and related prevention measures.
•• Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis. 2016;62:1423–35. https://doi.org/10.1093/cid/ciw122This manuscript reflects some of the most important pathogens associated with water transmission and their relationship to outbreaks.
Bifulco JM, Shirey JJ, Bissonnette GK. Detection of Acinetobacter spp. in rural drinking water supplies. Appl Environ Microbiol. 1989;55:2214–9.
Bartram J, Cotruvo J, Exner M, Fricker C, Glasmacher A. Heterotrophic plate counts and drinking-water safety: the significance of HPCs for water quality and human health. WHO Emerging Issues in Water and Infectious Disease Series. London: IWA Publishing; 2003.
Volkow P, Sánchez-Girón F, Rojo-Gutiérrez L, Cornejo-Juárez P. Hospital-acquired waterborne bloodstream infection by Acinetobacter baumannii from tap water: a case report. Infect Dis Clin Pract. 2013;21:405–6.
McDonald LC, Walker M, Carson L, Arduino M, Aguero SM, Gomez P, et al. Outbreak of Acinetobacter spp. bloodstream infections in a nursery associated with contaminated aerosols and air conditioners. Pediatr Infect Dis J. 1998;17:716–22. https://doi.org/10.1097/00006454-199808000-00011.
Katz MJ, Parrish NM, Belani A, Shah M. Recurrent aeromonas bacteremia due to contaminated well water. Open Forum Infect Dis. 2015;2:ofv142. Published 2015 Oct 20. https://doi.org/10.1093/ofid/ofv142.
Ko WC, Chuang YC. Aeromonas bacteremia: review of 59 episodes. Clin Infect Dis. 1995;20:1298–304. https://doi.org/10.1093/clinids/20.5.1298.
Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. ScientificWorldJournal. 2012;2012:625023. https://doi.org/10.1100/2012/625023.
Qu F, Cui EB, Xia GM, He JY, Hong W, Li B, et al. The clinical features and prognosis of Aeromonas septicaemia in hepatic cirrhosis: a report of 50 cases. Zhonghua Nei Ke Za Zhi. 2003;42:840–2.
Montaño-Remacha C, Márquez-Cruz MD, Hidalgo-Guzmán P, Sánchez-Porto A, Téllez-Pérez FP. Brote de bacteriemia por Burkholderia cepacia en una unidad de hemodiálisis de Cádiz, 2014 [An outbreak of Burkholderia cepacia bacteremia in a hemodialysis unit, Cadiz, 2014]. Enferm Infecc Microbiol Clin. 2015;33:646–50. https://doi.org/10.1016/j.eimc.2015.02.013.
Moreira BM, Leobons MBGO, Pellegrino FLPC, Santos M, Teixeira LM, de Andrade Marques E, et al. Ralstonia pickettii and Burkholderia cepacia complex bloodstream infections related to infusion of contaminated water for injection. J Hosp Infect. 2005;60:51–5. https://doi.org/10.1016/j.jhin.2004.09.036.
Nasser RM, Rahi AC, Haddad MF, Daoud Z, Irani-Hakime N, Almawi WY. Outbreak of Burkholderia cepacia bacteremia traced to contaminated hospital water used for dilution of an alcohol skin antiseptic. Infect Control Hosp Epidemiol. 2004;25:231–9. https://doi.org/10.1086/502384.
Chew KL, Cheng B, Lin RTP, Teo JWP. Elizabethkingia anophelis is the dominant Elizabethkingia species found in blood cultures in Singapore. J Clin Microbiol. 2018;56:e01445–17. Published 2018 Feb 22. https://doi.org/10.1128/JCM.01445-17.
Elbadawi LI, Borlaug G, Gundlach K, Monson T, Noble-Wang J, Moulton-Meissner H, et al. A large and primarily community associated outbreak of Elizabethkingia anophelis infections, Wisconsin, 2015–2016. Open Forum Infects Dis. 2016;3(Suppl 1). https://doi.org/10.1093/ofid/ofw195.09.
Perrin A, Larsonneur E, Nicholson AC, Edwards DJ, Gundlach KM, Whitney AM, et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat Commun. 2017;8:15483. Published 2017 May 24. https://doi.org/10.1038/ncomms15483.
Balm MN, Salmon S, Jureen R, Teo C, Mahdi R, Seetoh T, et al. Bad design, bad practices, bad bugs: frustrations in controlling an outbreak of Elizabethkingia meningoseptica in intensive care units. J Hosp Infect. 2013;85:134–40. https://doi.org/10.1016/j.jhin.2013.05.012.
Jarvis WR, Martone WJ. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29 Suppl A:19–24. https://doi.org/10.1093/jac/29.suppl_a.19.
Sanders WE Jr, Sanders CC. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997;10:220–41.
Ganeswire R, Thong KL, Puthucheary SD. Nosocomial outbreak of Enterobacter gergoviae bacteraemia in a neonatal intensive care unit. J Hosp Infect. 2003;53:292–6. https://doi.org/10.1053/jhin.2002.1371.
Macias AE, Huertas M, de Leon SP, Munoz JM, Chavez AR, Sifuentes-Osornio J, et al. Contamination of intravenous fluids: a continuing cause of hospital bacteremia. Am J Infect Control. 2010;38:217–21. https://doi.org/10.1016/j.ajic.2009.08.015.
Berger P, Barguellil F, Raoult D, Drancourt M. An outbreak of Halomonas phocaeensis sp. nov. bacteraemia in a neonatal intensive care unit. J Hosp Infect. 2007;67:79–85. https://doi.org/10.1016/j.jhin.2007.06.018.
Stevens DA, Johnson N. Mystery solved? Halomonas and dialysis infections. Diagn Microbiol Infect Dis. 2017;88:1–2. https://doi.org/10.1016/j.diagmicrobio.2017.01.021.
Stevens DA, Hamilton JR, Johnson N, Kim KK, Lee JS. Halomonas, a newly recognized human pathogen causing infections and contamination in a dialysis center: three new species. Medicine (Baltimore). 2009;88:244–9. https://doi.org/10.1097/MD.0b013e3181aede29.
Moriguchi S, Abe M, Kimura M, Yoshino C, Baba M, Okada C, et al. The diagnosis of Legionella pneumophila Serogroup 5 bacteremic pneumonia during severe neutropenia using loop-mediated isothermal amplification. Intern Med. 2018;57:1045–8. https://doi.org/10.2169/internalmedicine.9810-17.
Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28:95–133. https://doi.org/10.1128/CMR.00029-14.
Fields BS, Benson RF, Besser RE. Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev. 2002;15:506–26. https://doi.org/10.1128/cmr.15.3.506-526.2002.
Decker BK, Palmore TN. Waterborne pathogen detection: more than just "location, location, location…". Infect Control Hosp Epidemiol. 2014;35:130–1. https://doi.org/10.1086/675067.
Stout JE, Muder RR, Mietzner S, et al. Role of environmental surveillance in determining the risk of hospital-acquired legionellosis: a national surveillance study with clinical correlations. Infect Control Hosp Epidemiol. 2007;28:818–24. https://doi.org/10.1086/518754.
Bian SN, Zhang LF, Zhang YQ, Yang QW, Wang P, Xu YC, et al. Clinical and laboratory characteristics of patients with nontuberculous mycobacterium bloodstream infection in a tertiary referral hospital in Beijing, China. Chin Med J. 2016;129:2220–5. https://doi.org/10.4103/0366-6999.189920.
Edun B, Shah A, Durkin M, Whitmire M, Patterson-Williams S, Albrecht H, et al. Non-tuberculous mycobacterial bloodstream infections in patients with indwelling vascular catheters - the role of sickle cell anaemia. Infect Dis (Lond). 2017;49:341–6. https://doi.org/10.1080/23744235.2016.1262058.
Tagashira Y, Kozai Y, Yamasa H, Sakurada M, Kashiyama T, Honda H. A cluster of central line-associated bloodstream infections due to rapidly growing nontuberculous mycobacteria in patients with hematologic disorders at a Japanese tertiary care center: an outbreak investigation and review of the literature. Infect Control Hosp Epidemiol. 2015;36:76–80. https://doi.org/10.1017/ice.2014.14.
Caroleo B, Malandrino P, Liberto A, Condorelli D, Patanè F, Maiese A, et al. Catheter-related bloodstream infections: a root cause analysis in a series of simultaneous Ochrobactrum anthropi infections. Curr Pharm Biotechnol. 2019;20:609–14. https://doi.org/10.2174/1389201020666190405182025.
Ezzedine H, Mourad M, Van Ossel C, Logghe C, Squifflet JP, Renault F, et al. An outbreak of Ochrobactrum anthropi bacteraemia in five organ transplant patients. J Hosp Infect. 1994;27:35–42. https://doi.org/10.1016/0195-6701(94)90066-3.
Breathnach AS, Cubbon MD, Karunaharan RN, Pope CF, Planche TD. Multidrug-resistant Pseudomonas aeruginosa outbreaks in two hospitals: association with contaminated hospital waste-water systems. J Hosp Infect. 2012;82:19–24. https://doi.org/10.1016/j.jhin.2012.06.007.
Bicking Kinsey C, Koirala S, Solomon B, Rosenberg J, Robinson BF, Neri A, et al. Pseudomonas aeruginosa outbreak in a neonatal intensive care unit attributed to hospital tap water. Infect Control Hosp Epidemiol. 2017;38:801–8. https://doi.org/10.1017/ice.2017.87.
Garvey MI, Bradley CW, Holden E. Waterborne Pseudomonas aeruginosa transmission in a hematology unit? Am J Infect Control. 2018;46:383–6. https://doi.org/10.1016/j.ajic.2017.10.013.
Nasir N, Sayeed MA, Jamil B. Ralstonia pickettii bacteremia: an emerging infection in a tertiary care hospital setting. Cureus. 2019;11:e5084. Published 2019 Jul 5. https://doi.org/10.7759/cureus.5084.
Chen YY, Huang WT, Chen CP, Sun SM, Kuo FM, Chan YJ, et al. An Outbreak of Ralstonia pickettii bloodstream infection associated with an intrinsically contaminated normal Saline solution. Infect Control Hosp Epidemiol. 2017;38:444–8. https://doi.org/10.1017/ice.2016.327.
Dombrowsky M, Kirschner A, Sommer R. PVC-piping promotes growth of Ralstonia pickettii in dialysis water treatment facilities. Water Sci Technol. 2013;68:929–33. https://doi.org/10.2166/wst.2013.332.
Zapardiel J, Blum G, Caramelo C, Fernandez-Roblas R, Rodriguez-Tudela JL, Soriano F. Peritonitis with CDC group IVc-2 bacteria in a patient on continuous ambulatory peritoneal dialysis. Eur J Clin Microbiol Infect Dis. 1991;10:509–11. https://doi.org/10.1007/bf01963939.
Ryan MP, Pembroke JT, Adley CC. Ralstonia pickettii: a persistent Gram-negative nosocomial infectious organism. J Hosp Infect. 2006;62:278–84. https://doi.org/10.1016/j.jhin.2005.08.015.
Ryan MP, Adley CC. Ralstonia spp.: emerging global opportunistic pathogens. Eur J Clin Microbiol Infect Dis. 2014;33:291–304. https://doi.org/10.1007/s10096-013-1975-9.
Åttman E, Korhonen P, Tammela O, Vuento R, Aittoniemi J, Syrjänen J, et al. A Serratia marcescens outbreak in a neonatal intensive care unit was successfully managed by rapid hospital hygiene interventions and screening. Acta Paediatr. 2018;107:425–9. https://doi.org/10.1111/apa.14132.
Moulton-Meissner H, Noble-Wang J, Gupta N, Hocevar S, Kallen A, Arduino M. Laboratory replication of filtration procedures associated with Serratia marcescens bloodstream infections in patients receiving compounded amino acid solutions. Am J Health Syst Pharm. 2015;72:1285–91. https://doi.org/10.2146/ajhp150141.
Volkow-Fernández P, Ponce de León-Rosales S, Sifuentes-Osornio J, Calva-Mercado JJ, Ruiz-Palacios GM, Cerbón MA. Epidemia de bacteremias primarias por una cepa endémica de Serratia marcescens en una unidad de terapia intensiva [An epidemic of primary bacteremia due to an endemic strain of Serratia marcescens in an intensive care unit]. Salud Publica Mex. 1993;35:440–7.
Wasiura J, Segal BH, Mullin KM. Cluster of Sphingomonas paucimobilis bacteremias linked to diversion of intravenous hydromorphone. N Engl J Med. 2019;381:584–5. https://doi.org/10.1056/NEJMc1902973.
Perola O, Nousiainen T, Suomalainen S, Aukee S, Kärkkäinen U-M, Kauppinen J, et al. Recurrent Sphingomonas paucimobilis -bacteraemia associated with a multi-bacterial water-borne epidemic among neutropenic patients. J Hosp Infect. 2002;50:m196–201. https://doi.org/10.1053/jhin.2001.1163.
Velázquez-Acosta C, Zarco-Márquez S, Jiménez-Andrade MC, Volkow-Fernández P, Cornejo-Juárez P. Stenotrophomonas maltophilia bacteremia and pneumonia at a tertiary-care oncology center: a review of 16 years. Support Care Cancer. 2018;26:1953–60. https://doi.org/10.1007/s00520-017-4032-x.
Kim SH, Cho SY, Kang CI, Seok H, Huh K, Ha Y, et al. Clinical predictors of Stenotrophomonas maltophilia bacteremia in adult patients with hematologic malignancy. Ann Hematol. 2018;97:343–50. https://doi.org/10.1007/s00277-017-3178-4.
Chen Y, Suo J, Du M, Chen L, Liu Y, Wang L, et al. Clinical features, outcomes, and risk factors of bloodstream infections due to Stenotrophomonas maltophilia in a tertiary-care hospital of China: a retrospective analysis. Biomed Res Int. 2019;2019:4931501. Published 2019 Dec 9. https://doi.org/10.1155/2019/4931501.
Eb Ebara H, Hagiya H, Haruki Y, Kondo E, Otsuka F. Clinical characteristics of Stenotrophomonas maltophilia bacteremia: a regional report and a review of a Japanese case series. Intern Med. 2017;56:137–42. https://doi.org/10.2169/internalmedicine.56.6141.
Zmeter C, Tabaja H, Sharara AI, Kanj SS. Non-O1, non-O139 Vibrio cholerae septicemia at a tertiary care center in Beirut, Lebanon; a case report and review. J Infect Public Health. 2018;11:601–4. https://doi.org/10.1016/j.jiph.2018.01.001.
Maraki S, Christidou A, Anastasaki M, Scoulica E. Non-O1, non-O139 Vibrio cholerae bacteremic skin and soft tissue infections. Infect Dis (Lond). 2016;48:171–6. https://doi.org/10.3109/23744235.2015.1104720.
Fàbrega A, Vila J. Yersinia enterocolitica: pathogenesis, virulence and antimicrobial resistance. Enferm Infecc Microbiol Clin. 2012;30:24–32. https://doi.org/10.1016/j.eimc.2011.07.017.
From the Centers for Disease Control. Yersinia enterocolitica bacteremia and endotoxin shock associated with red blood cell transfusions--United States, 1991. JAMA. 1991;265:2174–5.
Centers for Disease Control and Prevention (CDC). Red blood cell transfusions contaminated with Yersinia enterocolitica--United States, 1991–1996, and initiation of a national study to detect bacteria-associated transfusion reactions. MMWR Morb Mortal Wkly Rep. 1997;46:553–5.
Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee. Society for Healthcare Epidemiology of America. Association for Professionals in Infection Control. Infectious Diseases Society of America. Hand Hygiene Task Force. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 Suppl):S3–S40. https://doi.org/10.1086/503164.
Sehulster LM, Chinn RYW, Arduino MJ, Carpenter J, Donlan R, Ashford D, et al. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Chicago: American Society for Healthcare Engineering/American Hospital Association; 2004. https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf
Macías AE, Monroy R, Muñoz JM, Medina H, Ponce de León S. Cloración y contaminación bacteriana. Aguas turbulentas en los hospitales. [Chlorination and bacterial contamination. Hospitals with troubled waters]. Rev Investig Clin. 2006;58:470–4.
Bull RJ, Birnbaum LS, Cantor KP, Rose JB, Butterworth BE, Pegram R, et al. Water chlorination: essential process or cancer hazard? Fundam Appl Toxicol. 1995;28:155–66. https://doi.org/10.1006/faat.1995.1156.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gómez-Gómez B declares that she has no conflict of interest. Volkow-Fernández P declares that she has no conflict of interest. Cornejo-Juárez P declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Treatment and Prevention of Hospital Infections
Rights and permissions
About this article
Cite this article
Gómez-Gómez, B., Volkow-Fernández, P. & Cornejo-Juárez, P. Bloodstream Infections Caused by Waterborne Bacteria. Curr Treat Options Infect Dis 12, 332–348 (2020). https://doi.org/10.1007/s40506-020-00234-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-020-00234-5