Opinion Statement
Cystic echinococcosis (CE) is a chronic, complex, and neglected disease. Its treatment depends on a number of factors, such as location, size, and stage of the cysts, and availability of therapeutic options. Despite the wealth of scientific literature on treatment for echinococcosis, the current management of the disease is based on poor to moderate quality of evidence and recommendation strength. In addition, therapeutic strategies have been developed over time without systematic and adequate evaluation of their efficacy, effectiveness, and safety. This is due to the lack of large, longitudinal, controlled studies, which in turn is partly due to the chronicity of the disease which requires a follow-up of many years. The lack of adequate funding makes these costly trials impossible to implement. Although the recommended multidisciplinary and stage-specific approach may be available in referral centers, this is often not the case in many endemic countries, where the most affected populations have limited access to diagnosis and therapy, and where the risks associated with invasive procedures may be particularly high. The level of evidence on which clinicians have to rely is low. For the time being, patients should preferably be treated in referral centers only. Proper comparative clinical trials are urgently needed. Because of space constraints, this update will focus on the most frequent location of CE, the liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis is a chronic, complex, and neglected zoonotic disease, caused by the larval form of the tapeworm Echinococcus granulosus, which develops in organs and tissues of the intermediate hosts.
Epidemiology
E. granulosus occurs in all continents, with the highest prevalence in parts of Eurasia, Africa, Australia, and South America. Within the endemic zones, the prevalence of the parasites varies from sporadic to high.
The true incidence is difficult to determine because of the slow rate of cyst growth and variable clinical presentation. Reports based on hospital- and surgery-based surveys greatly underestimate the actual rates of infection, especially in low socio-economic groups with limited access to diagnosis and treatment.
Mass community-based surveys using portable ultrasound (US) scanners, conducted in many remote, rural areas of the world, including Tunisia, Libya, Tanzania, Kenya, Sudan, Ethiopia, Argentina, Uruguay, and China, showed the real burden of disease, uncovering infection rates of up to 6.6 % [1].
Pathogenesis
The adult intestinal form of E. granulosus is a small tapeworm 3–6-mm long, attached to the small intestine of the definitive canine host (dogs, coyotes, wolves). Cysts occur in herbivore intermediate hosts (sheep, cattle, goats).
In the typical dog-sheep cycle, Echinococcus eggs are passed in the feces of an infected dog and may be ingested by grazing sheep. They hatch into embryos in the intestine, penetrate the intestinal mucosa, and are carried by blood to major filtering organs, including the liver and lungs. After the developing embryos localize in a specific organ or site, they transform into cysts. The cyst contains small tapeworm heads or protoscolices.
These protoscolices are infective to dogs that may ingest viscera containing echinococcal cysts, when dogs are fed viscera of home-slaughtered sheep or other livestock. Protoscolices attach to the dog’s intestinal mucosa where they develop into mature adult tapeworms and then restart the biologic cycle.
Humans may become infected by ingesting tapeworm eggs passed from an infected carnivore. This occurs most frequently when individuals handle or contact infected dogs or other infected carnivores or inadvertently ingest food or drink contaminated with fecal material containing tapeworm eggs.
Increasingly, cases of CE in non-endemic countries are seen in immigrants from endemic countries.
Cyst Structure
In each anatomic site, cysts are surrounded by the periparasitic host tissue (pericyst), which encompasses the endocyst of larval origin. The endocyst has an outer, acellular laminated layer and an inner, or germinative, layer that gives rise to brood capsules and protoscolices (Fig. 1). The cyst is filled with clear fluid, numerous brood capsules, and protoscolices. Some cysts may also harbor daughter cysts of variable size. The growth rate of the cysts is variable. The average increase of cyst diameter is thought to be approximately 1 cm/year; however, data on natural history are scarce.
Diagnosis
The diagnosis of CE is based mainly on imaging methods and on serology, which has only a minor, confirmatory role. E. granulosus eggs are shed with the feces by the definitive hosts (canids) but not by intermediate hosts; therefore, direct diagnosis in human intermediate hosts is only possible through demonstration of viable protoscolices in the cyst, which can be obtained at surgery or by percutaneous aspiration.
Natural History
Although the exact sequence of structural and metabolic changes occurring to echinococcal cysts during their natural history is still unclear, longitudinal studies using ultrasound in untreated people have shown that cysts may evolve spontaneously from unilocular fluid-filled cysts to forms with semi-solid content and a calcified wall, passing through other morphologically different stages, including a detached endocyst and daughter vesicles. This morphological heterogeneity mirrors different states of the cysts’ biological activity and, most importantly, correlates with different response rates to nonsurgical treatments [2].
Clinical Presentation and Treatment Options
The clinical presentation of CE is protean and depends on many variables, including location, number, size and stage of the cysts, and presence of complications. There are four management options for CE, surgery, percutaneous treatment, medical treatment with benzimidazoles, and watch and wait or expectant management.
All these options, however, have developed over decades without adequate comparative evaluation of efficacy, effectiveness, rate of adverse events, relapse rates, and cost.
One key element in clinical decision-making for liver CE is the standardized WHO classification of echinococcal cysts proposed by the WHO IWGE in 2003.
The classification has made possible a stage-specific approach to hepatic CE. This approach is currently adopted in several referral centers and pending randomized clinical trials comparing systematically treatment options, remains the most rational way to manage patients with hepatic CE (Fig. 2).
Surgery
Surgery for hepatic CE maintains a central role in complicated cysts (rupture, biliary fistulas, compression of vital structures, bacterial superinfection, hemorrhage), cysts at high risk of rupture, or large cysts with many daughter vesicles, that are not suitable for percutaneous treatment approaches.
Surgery can be performed as an open procedure, with radical or conservative techniques, or through a laparoscopic approach, but criteria to be used to allocate patients to the various options are still the subject of debate.
In what is possibly the largest review of the literature on laparoscopic surgery for CE, which included including 914 patients with 1116 echinococcal cysts, the most common procedure was cystectomy (60.39 %), followed by partial pericystectomy (14.77 %) and pericystectomy (8.21 %); the rest were segmentectomies. Conversion to open laparotomy occurred in 4.92 % of cases, due to anatomical limitations or inaccessible locations. The overall mortality was 0.22 %, and morbidity was 15.07 %, with no intraoperative deaths reported. The most common complication was bile leakage (57/914) [3].
Radical surgery aims to remove the entire cyst, including the pericyst, with or without hepatic resection. In conservative procedures, only the parasitic material is removed, whereas part of the pericyst is left in place. The residual cavity is usually managed with omentoplasty.
A relatively new technique is subadventitial cystectomy. A fibrous membrane exists between the cyst and the pericyst, a structure covered by the compressed Glisson and hepatic vein systems, with a small gap between them, and the fibrous capsule. Along this space, the cysts can be completely separated from the liver to minimize hepatic injury and without spillage of their contents. This technique, called subadventitial cystectomy, is believed to reduce the number of postoperative biliary fistulas [4].
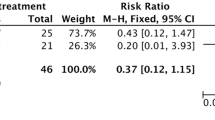
In a retrospective study comparing 52 patients who underwent subadventitial cystectomy and 27 patients who underwent resection of the protruding dome, the latter had 4 cases of long-term cavity-related complications, and the authors concluded that this renders subadventitial cystectomy preferable [5].
Radical procedures are generally found to bear greater intraoperative risks and less postoperative complications (biliary fistula, superinfection) and relapses compared with conservative surgical procedures but in these retrospective studies a strong bias could be from selection of candidate patients, surgical team experience, historical period considered and, with regard to relapse rates, the length of follow-up. A recent retrospective study on 170 patients, half of whom underwent radical surgery and the other half conservative surgery, could not demonstrate that radical surgery reduces recurrence [6].
A peculiar problem is represented by cysts adjacent to the inferior vena cava (IVC).
In a study of 32 patients with such cysts, radical surgery was performed in 20 of them, with a 28 % morbidity rate and a 3 % mortality rate. The authors conclude it should not be performed routinely in cysts located in segments VIII, I, and IV and in upper segments of the IVC in contact with the hepatic vein and/or right atrium. They also propose a cyst classification based on location of the cyst and length of contact and degrees of involvement of the IVC [7].
The main postoperative complications of surgery for CE are secondary bacterial infection of cysts and cystobiliary communications leading to biliary obstruction or obstructive cholangitis.
For cysts with bacterial superinfection, open surgery is recommended by surgeons although percutaneous management of this complication is an option.
Cysto biliary communications are best diagnosed preoperatively by MR cholangiography (MRC) [8]. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-MRC yields information that complements T2-weighted MRC findings and improves identification of cistobiliary communications [9].
The management of bilio-cystic fistulas has never been evaluated in randomized clinical trials. Two recent systematic reviews addressed the efficacy and safety of ERCP treatment with cysto-biliary communications [10, 11]. These analyses graded as level IV and grade C the evidence of ERCP use in the following cases: (1) preoperatively, to define cysto-biliary relations and to treat biliary obstruction, thus making surgery possible; (2) postoperatively, to decrease the incidence of external fistulization and biliary complications by avoiding surgical revision of the bile duct, or as the only intervention when complete evacuation of the biliary tract and cyst content is possible, or again, to facilitate the diagnosis and treatment of obstruction, cholangitis and external biliary fistulae.
Other non-surgical techniques that successfully treated biliary complications, especially in patients where ERCP failed, have also been published in case reports [12].
Benazzouz and colleagues treated 47 hydatid cysts with biliary rupture with an 80.8 % success rate without the need for surgery with percutaneous drainage alone with a large bore catheter, associated with endoscopic biliary clearance in case of failure. In the case of frank fistula, the first-line treatment was endoscopy alone, associated with percutaneous drainage in case of failure [12].
It should not be forgotten that even in the most experienced hands, serious complications can result from ERCP, including pancreatitis, bleeding, infection, or perforation [13].
Postoperative Complications
A study conducted in Chile on 126 patients with uncomplicated echinococcal cysts found an incidence of post operative morbidity was 10.3 %, with 76.9 % Clavien grade I or II. Medical complications were 6.5 %, and surgical complications were 4.1 %. There was no mortality and a recurrence rate of 0.8 % [14] with a median follow-up of 83 months.
Local recurrence and secondary echinococcosis is associated with spillage during removal of the cyst, incomplete removal of the endocyst and possibly the presence of unnoticed exophytic cyst development.
Perioperative ABZ prophylaxis continues to be recommended as a measure to minimize the risk of secondary cystic echinococcosis resulting from spillage of fluid [15, 16].
Percutaneous Treatments
Percutaneous treatments for abdominal cystic echinococcosis were introduced in the mid-1980s and over the years developed into an attractive alternative to surgery and benzimidazole derivatives for certain cyst stages.
Percutaneous treatment modalities aim either to destroy the germinal layer with scolecidal agents or to evacuate the entire endocyst.
The most popular method in the first group is PAIR (short for puncture, aspiration, injection of a scolecidal agent and re-aspiration), whereas several modified catheterization techniques belong to the second group, and are generally reserved for cysts that are difficult to drain, or tend to relapse after PAIR (multivesiculated cysts or cysts with predominantly solid content and daughter cysts).
Catheterization techniques are based on the aspiration of the “solid” contents of the cyst, the germinal, and the laminated layer, through a large-bore catheter or device.
The puncture of echinococcal cysts has long been discouraged because of risks of anaphylactic shock and spillage of the fluid; however, a systematic analysis on percutaneous aspiration of echinococcal cysts reported only 2 cases of lethal anaphylaxis (0.04 %) and 99 reversible anaphylactic reactions (1.8 %) were reported out of 5943 percutaneous aspiration procedures on 5517 hepatic and non-hepatic echinococcal cysts [17].
Peritoneal seeding has never been reported, but it is difficult to assess its actual rate because many series have a short follow-up time. Prophylactic administration of ABZ for at least 30 days after puncture is a cautionary measure that should always accompany PAIR [15].
PAIR is performed with several variants of the standard protocol and is generally successful (i.e., conducive to permanent solidification) in CE1 and CE3a cysts. A few studies with long-term follow-up indicate that multivesiculated cysts (i.e., CE2 and CE3b) tend to relapse repeatedly after PAIR [15, 18].
One of the advantages of PAIR conducted on cysts that are responsive to this type of procedure (small and mid-sized CE1 and CE3a) is that it can be performed as an outpatient procedure, as shown on a small retrospective study in which 35 cysts were treated with PAIR on an outpatient basis [19].
Catheterization techniques have mostly been applied to the treatment of partially solid or multivesiculated cysts, allowing the aspiration of the cyst content and evacuation of the parasite membranes. They present a useful alternative to surgery for those cyst stages poorly responsive to medical treatment and PAIR but no study has assessed their performance in randomized clinical trials [12]. Although these techniques have been applied safely and with a good short-term success rate, only a limited number of cases have been treated with each technique, and the overall length of follow-up may have been too short to assess the real relapse rate.
Medical Treatment
The use of benzimidazole (BZD) carbamates in the treatment of CE was introduced in the 1970s. While both albendazole (ABZ) and mebendazole (MBZ) have been proven effective against the larval stage of E. granulosus, ABZ is the current treatment of choice due to better absorption. ABZ is administered orally at a dose of 10–15 mg/kg per day generally for 3–6 mo; administration should be continuous without treatment interruptions, in contrast to the early recommendations issued in the 1980s.
However, the optimal dose and duration of treatment with ABZ have not been formally assessed. Studies are small and few have adequate controls.
Factors associated with treatment outcome include cyst stage, size, and location. Unilocular (CE1 and CE3a) cysts and small cysts (<6 cm) respond better and faster to ABZ treatment compared with multivesciculated (CE2 and CE3b) and larger cysts, with a lower relapse rate as shown in the systematic review by Stojkovic et al. [20].
Adverse effects of BMZ include headache (10 % of cases), gastrointestinal symptoms (56 %), hepatotoxicity, severe leukopenia, neutropenia or thrombocytopenia (<1 %), and alopecia (2 %).
Increases in aminotransferases (15 % cases) may be due to drug-related efficacy or to real drug-related toxicity. Risks observed in laboratory animals include embryotoxicity and teratogenicity. While teratogenicity is theoretical, it is nonetheless good practice to avoid use during pregnancy whenever possible.
If ABZ is not available or not tolerated, MBZ, the first BMZ tested against Echinococcus, may be used at a dosage of 40–50 mg/kg body weight, in three divided doses during fat-rich meals.
Praziquantel (PZQ) 40 mg/kg once a week in combination with ABZ seems more effective in killing protoscoleces than ABZ alone. Other clinical studies evaluating this combination are available but they do not clarify whether PZQ has a pharmacological effect in its own right or acts only by enhancing ABZ absorption. The usefulness of PZQ to avoid secondary echinococcosis needs confirmation [15].
The pharmacological activity mediating BZ action is based on binding to parasite β-tubulin, which produces a subsequent disruption of the tubulin-microtubule dynamic equilibrium preventing proper assembly of the cytoskeleton.
However, due to the fact that parasite and host beta-tubulin are highly similar [21], benzimidazole administration is associated with adverse side effects, is parasitostatic only and, often has to be continued for months or, in some locations, years.
Clearly, novel chemotherapeutic options are urgently needed. New perspectives in this area have been opened up by the recent characterization of the whole nuclear genomes of E. multilocularis and E. granulosus [22, 23].
These studies revealed extensive adaptations to parasitism in the tapeworm genomes, such as the loss of several pathways important for the de novo synthesis of amino acids nucleotides, fatty acids, and cholesterol, which have to be taken up from the host.
Cestodes appear to have expanded or evolved genes for the modulation of the host immune system. These studies identified promising drug targets such as G-protein-coupled receptors (GPCRs), ion channels, proteases, and kinases that are expressed in the clinically relevant metacestode stage [22, 23].
Substances are available that can be tested against these targets for antiparasitic activities in established in vitro and in vivo models for Echinococcus infections [21].
Another crucial finding was that cestodes seem to employ a highly modified stem cell system. The implications of these modifications on stem cell maintenance and dynamics could be related to the unlimited proliferation capacity typically observed in cestode larvae (e.g., Echinococcus).
Since the germinative cells are the only proliferating cell type in Echinococcus, scientists believe they must be the crucial drivers of frequently observed recurrences (e.g., unresponsiveness of CE2 and CE3b cysts to albendazole and PAIR).
Analysis has shown that germinative cells are also insensitive to the currently available chemotherapy, because they exclusively express a beta tubulin with limited affinity to benzimidazoles [24]. Efforts should therefore concentrate on targets expressed in germinative cells since this is the crucial cell type driving parasite proliferation, differentiation, and regeneration within the host.
Non-Surgical-Non-Medical Approaches
Curative or palliative non-surgical, non-chemical interventions in CE and AE were recently reviewed. In CE, some of these techniques, like radiofrequency thermal ablation (RFA), were shelved after initial attempts, while others, such as high-intensity focused ultrasound, appear promising but are still in a preclinical phase [12].
Watch and Wait
Experts in a few referral centers have long managed expectantly inactive cysts of the liver that remain free of complications for years. A recent paper has shown in detail for the first time that this option is viable in referral centers when strict follow-up can be implemented and patients are compliant [25••]. If the safety of this approach is confirmed on larger cohorts, this simple, inexpensive option can save an enormous amount of useless drug prescriptions and surgical inteventions, as a remarkable percentage of patients with hepatic CE harbor inactive, non-complicated cysts [26].
Although CE2 and CE3b cysts are probably best treated with surgery (there are no studies comparing these two treatments) in selected (i.e., uncomplicated) echinococcal cysts of the liver watch and wait can also be applied as a bridge to surgery [27•]).
Stage-Specific Approach
Despite the dearth of randomized clinical trials evaluating treatment options, with the ensuing lack of evidence, the clinical management of hepatic CE is facilitated by the standardization of US classification, which enables clinicians to identify the most rational option on the basis of cyst stage (Fig. 1).
Experience with this approach has shown that small (<5 cm) univesicular CE1 and C3a cysts tend to respond well to ABZ treatment, while larger cysts are treated preferentially with PAIR [15] plus ABZ. Giant cysts (>10 cm) should be treated with a catheter left in place until the drainage is minimum, usually about 3 weeks.
Unfortunately, as a recent review of the literature [28•] and a survey of clinicians managing patents with CE [29••] have shown, the standardized classification and stage-specific approach have so far been adopted by a minority of clinicians. This constitutes yet another obstacle to the improvement of quality of care offered to these patients; therefore, every effort should be made by clinicians in endemic and non-endemic countries alike, to choose a stage-specific approach, whenever possible.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schantz PM, Wang H, Qiu J, Liu FJ, Saito E, Emshoff A, et al. Echinococcosis on the Tibetan Plateau: prevalence and risk factors for cystic and alveolar echinococcosis in Tibetan populations in Qinghai Province, China. Parasitology. 2003;127(Suppl):S109–20.
Hosch W, Junghanss T, Stojkovic M, Brunetti E, Heye T, Kauffmann GW, et al. Metabolic viability assessment of cystic echinococcosis using high-field (1)H MRS of cyst contents. NMR Biomed. 2008;21:734–54.
Tuxun T, Zhang JH, Zhao JM, Tai QW, Abudurexti M, Ma HZ, et al. World review of laparoscopic treatment of liver cystic echinococcosis—914 patients. Int J Infect Dis. 2014;24:43–50.
Lv H, Jiang Y, Peng X, Zhang S, Wu X, Yang H, et al. Subadventitial cystectomy in the management of biliary fistula with liver hydatid disease. Acta Trop. 2015;141:223–8.
Mohkam K, Belkhir L, Wallon M, Darnis B, Peyron F, Ducerf C, et al. Surgical management of liver hydatid disease: subadventitial cystectomy versus resection of the protruding dome. World J Surg. 2014;38:2113–21.
El Malki HO, Souadka A, Benkabbou A, Mohsine R, Ifrine L, Abouqal R, et al. Radical versus conservative surgical treatment of liver hydatid cysts. Br J Surg. 2014;101:669–75.
Ramia JM, Serrablo A, De la Plaza R, Esarte J, Gijon L, Sarria L, et al. Is radical surgery feasible in liver hydatid cysts in contact with the inferior vena cava? World J Surg. 2014;38:2940–5.
Hosch W, Stojkovic M, Janisch T, Heye T, Werner J, Friess H, et al. MR imaging for diagnosing cysto-biliary fistulas in cystic echinococcosis. Eur J Radiol. 2008;66:262–7.
Kantarci M, Pirimoglu B, Ogul H, Bayraktutan U, Eren S, Aydinli B, et al. Can biliary-cyst communication be predicted by Gd-EOB-DTPA-enhanced MR cholangiography before treatment for hepatic hydatid disease? Clin Radiol. 2014;69:52–8.
Dziri C, Haouet K, Fingerhut A, Zaouche A. Management of cystic echinococcosis complications and dissemination: where is the evidence? World J Surg. 2009;33:1266–73.
Ramia JM, Figueras J, De la Plaza R, Garcia-Parreno J. Cysto-biliary communication in liver hydatidosis. Langenbecks Arch Surg. 2012;397:881–7.
Tamarozzi F, Vuitton L, Brunetti E, Vuitton DA, Koch S. Non-surgical and non-chemical attempts to treat echinococcosis: do they work? Parasite. 2014;21:75.
Dolay K, Akbulut S. Role of endoscopic retrograde cholangiopancreatography in the management of hepatic hydatid disease. World J Gastroenterol. 2014;20:15253–61.
Manterola C, Otzen T, Urrutia S. Risk factors of postoperative morbidity in patients with uncomplicated liver hydatid cyst. Int J Surg. 2014;12:695–9.
Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16.
Lissandrin R, Agliata S, Brunetti E. Secondary peritoneal echinococcosis causing massive bilateral hydronephrosis and renal failure. Int J Infect Dis. 2013;17:e141–2.
Neumayr A, Troia G, de Bernardis C, Tamarozzi F, Goblirsch S, Piccoli L, et al. Justified concern or exaggerated fear: the risk of anaphylaxis in percutaneous treatment of cystic echinococcosis-a systematic literature review. PLoS Negl Trop Dis. 2011;5:e1154.
Golemanov B, Grigorov N, Mitova R, Genov J, Vuchev D, Tamarozzi F, et al. Efficacy and safety of PAIR for cystic echinococcosis: experience on a large series of patients from Bulgaria. Am J Trop Med Hyg. 2011;84:48–51.
Koroglu M, Erol B, Gurses C, Turkbey B, Bas CY, Alparslan AS, et al. Hepatic cystic echinococcosis: percutaneous treatment as an outpatient procedure. Asian Pac J Trop Med. 2014;7:212–5.
Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, et al. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis. 2009;3:e524.
Brehm K, Koziol U. On the importance of targeting parasite stem cells in anti-echinococcosis drug development. Parasite. 2014;21:72.
Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sanchez-Flores A, Brooks KL, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63.
Zheng H, Zhang W, Zhang L, Zhang Z, Li J, Lu G, et al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat Genet. 2013;45:1168–75.
Schubert A, Koziol U, Cailliau K, Vanderstraete M, Dissous C, Brehm K. Targeting Echinococcus multilocularis stem cells by inhibition of the Polo-like kinase EmPlk1. PLoS Negl Trop Dis. 2014;8:e2870.
Piccoli L, Tamarozzi F, Cattaneo F, Mariconti M, Filice C, Bruno A, et al. Long-term sonographic and serological follow-up of inactive echinococcal cysts of the liver: hints for a “Watch-and-Wait” approach. PLoS Negl Trop Dis. 2014;8:e3057. This is the first clinical study showing that “watch and wait” is a viable option in selected patients with inactive cysts of the liver. This option can spare patients unnecessary surgery and save healthcare resources for more urgent needs especially in low resource areas with high CE prevalence.
Tamarozzi F, Rossi P, Galati F, Mariconti M, Nicoletti GJ, Rinaldi F et al. The Italian registry of cystic echinococcosis (RIEC): the firstprospective registry with a European future. Euro Surveill. 2015;20(18). doi:10.2807/1560-7917.ES2015.20.18.2115.
Rinaldi F, De Silvestri A, Tamarozzi F, Cattaneo F, Lissandrin R, Brunetti E. Medical treatment versus “Watch and Wait” in the clinical management of CE3b echinococcal cysts of the liver. BMC Infect Dis. 2014;14:492. This retrospective study shows that CE2 and CE3b cysts respond poorly to albendzole and can be managed expectantly, if uncomplicated, as a bridge to surgery.
Tamarozzi F, Nicoletti GJ, Neumayr A, Brunetti E. Acceptance of standardized ultrasound classification, use of albendazole, and long-term follow-up in clinical management of cystic echinococcosis: a systematic review. Curr Opin Infect Dis. 2014;27:425–31. Survey of the literature on CE from 2004-2013 (the decade following the issue of WHO IWGE ultrasound classification) shows that 71.2% of publications did not indicate any classification, whereas only 14% used the WHO IWGE classification.
Nabarro LE, Amin Z, Chiodini PL. Current Management of Cystic Echinococcosis; a survey of specialist practice. Clin Infect Dis 2014. Survey of 41 clinicians who manage CE patients, in 23 countries on 5 continents, showed that most clinicians do not follow WHO-IWGE guidance, for reasons that remain unclear.
Acknowledgments
We are grateful to Sam Goblirsch, MD, for reviewing the manuscript. This work was funded in part by the EU FP7 Programme under grant agreement n° 602051 (to E.Brunetti).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Enrico Brunetti declares that he has no conflict of interest.
Liliana Praticò declares that she has no conflict of interest.
Andreas Neumayr declares that he has no conflict of interest.
Marcello Maestri declares that he has no conflict of interest.
Francesca Tamarozzi declare that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Travel Medicine
Rights and permissions
About this article
Cite this article
Brunetti, E., Praticò, L., Neumayr, A. et al. Update on Treatment for Cystic Echinococcosis of the Liver. Curr Treat Options Infect Dis 8, 153–164 (2016). https://doi.org/10.1007/s40506-016-0079-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-016-0079-3