Abstract
Effect of chromium (both CrIII and CrVI) were studied on the oxidative damages and the induction of antioxidant defence in turnip. For this purpose turnip (Brassica rapa L.) plants were grown in refined sand under three different sources of chromium, i.e., chromium sulphate (CrIII), potassium chromate (CrVI) and potassium dichromate (CrVI) supplied at 250 µM concentrations for 4, 24 and 168 h (7 days) after 50 days of growth in normal nutrition. The extent of oxidative damage was most in dichromate as it caused a maximum increase in lipid peroxidation and H2O2 concentration in leaves of turnip plants. The growth and biomass of plants decreased, while the levels of chloroplastic pigment and iron increased in plants supplied CrVI (both potassium chromate and potassium dichromate). Exposure of plants to Cr led to increase in Cr concentration, more in the roots than in shoots. Excess supply of Cr (chromium sulphate, potassium chromate and potassium dichromate), though inhibited peroxidase activity (at 4 and 168 h exposure), stimulated catalase, ascorbate peroxidase and superoxide dismutase activities. Localization of different isoforms of APX and SOD on native gels revealed activation of certain isoform in the Cr-treated plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world is facing serious problem of heavy metal pollution. Uptake of heavy metals in high concentration is known to be toxic to plants as well as animals. Heavy metal induces disturbances in normal metabolic functions, inhibition of growth and cause premature death of plants (Marschner 1995). Chromium is a non-essential pollutant element for plants. Apart from the parent rock sources, chromium has various routes of entry into biotic components of the ecosystem. The major industrial wastes of electroplating, chrome plating, tanning and paint industry and municipal sludge contribute to Cr build-up along with other heavy metals to their phytotoxic levels through their discharges (Welch et al. 1991).
Chromium is the seventh most abundant metal in the earth’s crust and has been classified under the category of borderline elements (Nieboer and Richardson, 1980). In nature, Cr exists in two different stable oxidation states: trivalent (CrIII) and hexavalent (CrVI) chromium. Both CrIII and CrVI differ in terms of chemical properties, mobility, bioavailability and toxicity (Katz and Salem 1994). There are various other valency states of Cr, but they are unstable and short-lived in biological systems. CrVI is considered the most toxic form of Cr, which occur as a chromate [(CrO4)2−] or dichromate [(Cr2O7)2−] oxyanions. CrVI is reported to be taken up actively by the sulfate carrier and immediately converted to CrIII in roots, possibly by the ferric reductases (Zayed et al. 1998 ). In contrast, CrIII is taken up passively and retained by the cation-exchange sites of the cell walls (Marschner 1995). Chromium (CrVI) is more readily taken up by the plants in comparison to CrIII but there is a distinct restriction of translocation of both species to shoot irrespective of the concentration in the nutrient solution. The reason for higher CrVI uptake could be that it is taken up with the essential elements with a similar molecular mechanism like Fe and S. Poor translocation of CrIII to the shoots could be due to vacuolar sequesterization of most of the Cr in the root cells to render it non-toxic. In plants, excess Cr has been shown to interact with essential nutrient such as Fe, Mn, Cu, Zn and P (Dube et al. 2003). Chromium does not play any role in plant metabolism (Dixit et al. 2002). Chromium phytotoxicity can result in inhibition of seed germination, disturbed pigment concentration, nutrient imbalance, and inhibition of water uptake (Panda and Choudhury 2005), changes in ultrastructure, particularly vacuolation of root cell (Panda 2007), and induces oxidative stress, as it enhances the generation of reactive oxygen species (ROS) such as superoxide anion (O •−2 ), hydrogen peroxide (H2O2) and hydroxyl radicals (OH˙) leading to damage of DNA, proteins and membrane lipids by peroxidation reaction (Panda et al. 2003; Panda and Khan 2003; Choudhury and Panda 2005). Both CrIII and CrVI can reduce chlorophyll content and inhibit plant growth (Panda and Choudhury 2005; Oliveira 2012). Increase in chlorophyll and carotenoid concentrations caused by exposure to Cr have also been reported in bean and water hyacinth plants (Zeid 2001; Paiva et al. 2009).
The present study was undertaken to investigate the effect of equal concentrations (250 µM) of chromium sulphate (Cr2(SO4)3, a CrIII source, and potassium chromate (K2CrO4) and potassium dichromate (K2Cr2O7), both sources of CrVI, in nutrient media on the oxidative stress and antioxidant defence responses of turnip, a highly nutritious vegetable plant.
Materials and methods
Turnip (Brassica rapa L) var. Purple top white globe (P.T.W.G.) was grown in sand culture in a glasshouse at ambient temperature (18–25 °C). The experiment was conducted in polythene containers provided with a central drainage hole covered with an inverted watch glass lined with acid washed glass wool at the rim to control free drainage of extra nutrient solution. The glasshouse conditions during the experiment period were: photosynthetic photon flux density (12:00 noon) 900–1450 µmol m−2 s−1, daily maximum and minimum temperatures during 24 h ranged between 20–28 and 6.5–10 °C, respectively, and the relative humidity ranged from 38 to 48 %.
Initially the seedlings were supplied with a complete nutrient solution for 50 days. The nutrient solution contained 4 mM KNO3, 4 mM Ca(NO)2, 2 mM MgSO4, 1.5 mM NaH2PO4, 0.1 mM Fe-EDTA, 10 µM MnSO4, 33 µM H3BO3, 1 µM ZnSO4, 1 µM CuSO4, 0.2 µM Na2MoO4, 0.1 µM CoSO4, 0.1 µM NiSO4 and 0.1 mM NaCl (Hewitt 1966) using analytical grade reagents. Nutrient solution was supplied daily, except on Sundays when the pots were flushed with de-ionised water to prevent accumulation of soluble salts on the root surfaces. On day 51, when plants attained a reasonable growth and 1st and 2nd whorl of leaves were broad enough, the pots were divided in 4 lots having 4 pots in each lot. The 1st lot was supplied with a complete nutrient solution and served as control throughout the period of the experiment. In remaining lots, 3 sources of chromium, i.e., chromium sulphate [Cr2(SO4)3], a trivalent source, and potassium chromate (K2CrO4) and potassium dichromate (K2Cr2O7) both hexavalent sources, were supplied at 250 µM concentration for 7 days and finally plants were harvested at 57th day. After 4, 24 h, and 7 days exposure observations were recorded for root and shoot biomass, Cr and Fe concentration, chloroplastic pigments, H2O2 concentration, lipid peroxidation and activities of superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and peroxidase (POX).
Water status was evaluated in the leaf tissue of plants by measuring water potential and relative water content (RWC) as described previously by Tewari et al. (2010). For the estimation of plant biomass and tissue concentrations of Cr and Fe, turnip plants were sampled and separated into roots and tops, washed thoroughly with mild acid and tap water and then with de-ionised water, and weighed for biomass after drying in an oven at 80 °C for 48 h. The samples were digested in HNO3 and HClO4 (10:1 v/v) and the tissue Cr and Fe content of the clear digest were estimated on an atomic absorption spectrophotometer, and the results were expressed as µg per g dry weight.
For the estimation of chlorophyll and total carotenoid content leaf tissue were extracted in 80 % acetone, and absorbance of acetone extract was recorded spectrophotometrically (Lichtenthaler 1987). The H2O2 content was determined as H2O2-titanium complex formed by the reaction of tissue H2O2 and titanium tetrachloride by the method of Brennan and Frankel (1977). Lipid peroxidation was determined according to Heath and Packer (1968) using the thiobarbituric acid (TBA) to estimate malondialdehyde (MDA) content. The amount of MDA was calculated from the difference in absorbance at 532 and 600 nm using an extinction coefficient of 155 mM−1 cm−1.
Assay of the activities of SOD, CAT and POX were made in fourth fully expanded young leaves. 5 g fresh leaf tissue was homogenized in 10 ml chilled 50 mM potassium phosphate buffer (pH 7.0) containing 0.5 % (w/v) insoluble polyvinyl polypyrrolidone and 1.0 mM phenylmethylsulfonylfluoride in a chilled pestle and mortar kept in an ice bath. After filtering through 2 fold muslin cloth homogenate was centrifuged at 20,000×g for 10 min at 2 °C. For assay of APX, 5.0 mM sodium ascorbate was added to the extraction medium. Soluble proteins were measured in the enzyme extracts according to the method of Lowry et al. (1951).
Catalase (CAT, EC: 1.11.1.6) and peroxidase (POX, EC: 1.11.1.7) activities were measured according to the methods described by Pandey and Sharma (2003)and expressed as µmol H2O2 decomposed and change in absorbance, respectively per min per mg protein. Superoxide dismutase (SOD, EC: 1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm. The enzyme activity was expressed as units min−1 mg−1 protein. A unit of SOD activity is defined as the amount enzyme required to inhibit the photoreduction of NBT by 50 % (Beauchamp and Fridovich 1971). Ascorbate peroxidase (APX, EC: 1.11.1.11) activity was assayed by estimating change in absorbance at 290 nm (Nakano and Asada 1981). The activity of APX was calculated in terms µmol ascorbate oxidized min−1 mg protein−1.
Separation of different isoforms of APX and SOD was done using native-PAGE. Ten percent native gels were prepared and run at constant voltage (250 V) and current (30 mA) at 4 °C using a non-dissociating discontinuous buffer system. SOD isoforms were studied on 10 % (v/v) acrylamide gels using a photochemical method of Beauchamp and Fridovich (1971). The gels were soaked in 2.45 mM NBT for 20 min and then submerged in a solution containing 36 mM potassium phosphate buffer, 0.028 mM riboflavin and 28.0 mM TEMED. The gels were then washed over a clean glass plate and exposed to a light source (15 W fluorescent lamp). The SOD isoenzymes were seen as achromatic bands against the purple-blue background. Different SOD isoforms were identified by incubating gels either in 2 mM KCN for 5 min for inhibition of Cu/Zn-SOD or 5 mM H2O2 for 20 min for inhibition of Fe-SOD.
APX activity was localized on 10 % (v/v) native gels equilibrated in a solution containing 50 mM potassium phosphate buffer (pH 7.0), 4 mM ascorbic acid and H2O2 for 20 min (Mittler and Zilinskas 1993). The gels were washed in a buffer with gentle shaking for 1 min and kept in a solution containing 50 mM phosphate buffer (pH 7.8), 28 mM TEMED and 2.45 mM NBT with gentle agitation for 10 min in dark till all bands appear. After a brief wash with glass distilled water the achromatic bands of isoforms of APX were observed on a purple-blue background.
All measurements were made on samples drawn in triplicate and data presented are mean of three biological and two technical replicates. The data were analysed by analysis of variance (ANOVA) and the means were tested for significant differences by the Fisher LSD using Sigma-Stat 2.1 software.
Results and discussion
After 24 h of metal supply, plants treated with potassium dichromate (K2Cr2O7) showed loss of turgor, midday wilting, however, these plants recovered from wilting in the evening, though loss of turgor was quite evident. Plants supplied with potassium chromate (K2CrO4) also exhibited the same effect after 3 days of exposure. However, plants supplied with Cr2(SO4)3 did not exhibit midday wilting throughout the period of investigation. These visual observations of water loss in Cr-stressed plants were supported by decreases in water potential and relative water content (Table 1). Within 48 h of K2Cr2O7 supply, retardation in growth became evident. Growth depression and visible symptoms of Cr toxicity in turnip plants were similar to those reported earlier in radish (Dube et al. 2004). After 5 days of treatment, K2CrO4-supplied plants also exhibited similar symptoms. The K2CrO4-supplied plants later, exhibited complete cessation of growth and accelerated senescence. The visible effects of CrVI species (K2Cr2O7 and K2CrO4) observed as loss of turgor, severe retardation in growth and accelerated senescence were similar to those reported for other plant species (Sharma and Sharma 1996). The young emerging leaves failed to open properly, remained small in size, deformed and very curly. Affected leaves showed necrosis, which started from the margins. Deformity developed even in the roots of K2Cr2O7-supplied plants as longitudinal splits were developed in roots, which became brownish in colour. The severity of the symptom was in following order: K2Cr2O7 > K2CrO4 > Cr2(SO4)3.
Irrespective of the source, 250 µM Cr supply decreased biomass production. Effect of cationic form of Cr [Cr2(SO4)3] was statistically non-significant (Table 2). However, effect of CrIII [Cr2(SO4)3] was more pronounced on roots as compared to the top parts of plants, as indicated by significantly higher top/root ratio of dry matter accumulation after 7 days of Cr supply (Kotas and Stasicka 2000). K2CrO4- or K2Cr2O7-supplied plants exhibited lower top/root ratio compared to control plants (Table 2). The decrease in biomass might also be due to disturbed carbohydrate and nitrogen metabolism (Chatterjee and Chatterjee 2000) and reduction in protein synthesis or low photosynthetic rates due to CrVI toxicity and loss of physiological water (Pandey and Sharma 2003).
Tissue Cr and Fe concentration
Plants supplied with excess Cr by three different sources brought about an increase in Cr concentration in shoot and root (Table 2). After 4 h of exposure, the accumulation of Cr was higher in the shoot than in roots, indicating an efficient translocation of Cr from root to shoot tissue. However, prolonged metal supply (up to 168 h) retained most of the Cr in the root tissue, indicating restricted root to shoot translocation. These observations are similar to studies of Zayed et al. (1998) and Chatterjee and Chatterjee (2000), who reported low mobility of Cr. Higher accumulation of Cr in the roots of plants supplied with CrVI could be explained by its active uptake and transport nature, which is a metabolically driven process (Marschner 1995). Contrastingly, CrIII was taken up passively by the plants and retained at cation exchange sites of the cell wall (Marschner 1995). Exposure of plants to dichromate increased Fe concentration in shoots. When the exposure was only of 4 h, Cr2(SO4)3 (CrIII) and K2CrO4 supply reduced Fe concentration in turnip shoots, but the exposure of K2CrO4 for longer durations (24 and 168 h) increased the shoot iron concentration. Concentration of Fe was always higher in roots than the shoots (Table 2).
Chloroplastic pigments
The observed increase in chlorophyll concentration in Cr-treated turnip plants (Table 3) does not conform to the earlier reports on cauliflower (Chatterjee and Chatterjee 2000), cabbage (Pandey and Sharma 2003) and wheat (Liu et al. 2014). The increased chlorophyll concentration may be attributed to a possible improvement in the chloroplast ultrastructure as a direct consequence of increased Fe concentration. The positive effect of Cr concentrations on chlorophyll synthesis might also be attributed to the increased transport of Mg as a result of Cr treatment (Zeid 2001). The increase in chlorophyll a concentration in K2CrO4 and K2Cr2O7 (CrVI)-supplied plants resulted in increase in chl a/b ratio significantly. An increased carotenoid concentration might be an adaptive response to the increased buildup of ROS in CrVI—stressed plants. However, carotenoids to chl ratio decreased at this stage (Table 3).
Hydrogen peroxide and lipid peroxidation
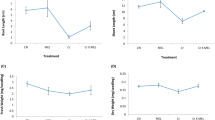
Hydrogen peroxide concentration (Fig. 1a) and lipid peroxidation (Fig. 1b) increased significantly after 24 and 168 h (7 days) of Cr supply. Higher production of H2O2 and O •−2 radical have been reported in many plant species exposed to Cr, and it has been implicated in the induction of oxidative stress (Dixit et al. 2002; Panda and Choudhury 2005, Pandey et al. 2009, Liu et al. 2014). The observed increase in lipid peroxidation, an indicator of increased oxidative damage severely affected the functioning of plasma membrane and finally lead to the death of the cells (Kappus 1985; Panda and Choudhury 2005). The greater lipid peroxidation recorded in K2Cr2O7-supplied plant, was two-fold higher than K2CrO4 and three-fold higher than Cr2(SO4)3 (Fig. 1b).
Antioxidant enzymes
The activities of antioxidant enzymes were altered by Cr exposure. Turnip plants exposed to Cr enhanced CAT and SOD activity, except at 4 h. Increased activities of CAT and SOD in Cr-exposed turnip plants (Table 4) are in accordance with earlier studies with other plant species (Shanker and Pathmanabhan 2004). It appears that the initial antioxidant defence against Cr-induced oxidative stress was provided by increased activity of SOD, an enzyme responsible for dismutation of O •−2 into H2O2. The accumulation of H2O2 in Cr–stressed plants is indicative of oxidative load and can be interpreted as a consequence of enhanced SOD activity (Panda and Upadhyay 2008). While Mn-SOD was not detected, Fe-SOD was found to be the predominant form of Cr-induced SOD in stressed plants. Treating turnip plants with Cr also enhanced Cu/Zn-SOD and the induction was in the order of: Cr3+ < CrO4 2− < Cr2O7 2− (Fig. 2). The expression of Fe-SOD was activated and an additional diffused-band of Fe-SOD, very close to the main band of Fe-SOD also appeared, especially by K2Cr2O7 (Fig. 2). These observations suggest that different Cr salts stimulated plants differently, as indicated by bands of various SOD isoforms. While the activities of CAT and SOD were suppressed initially (4 h of Cr exposure) but the activities increased with an advancement of period of Cr exposure (Table 4), while the activity of APX increased throughout the period of Cr exposure (Table 4). These observations suggest that APX might have provided sufficient antioxidant defence against H2O2 generation. Similar results have also been reported by Shanker and Pathmanabhan (2004) and Panda and Choudhury (2005) in other plant species. Though, CrIII [Cr2(SO4)3] did not appear to affect the intensity of APX isoforms at 24 h after the treatment, it increased the intensity of the bands as well as induced a few new isoforms of APX at 168 h (7 days) after the treatment. CrVI, both K2CrO4 and K2Cr2O7, particularly the latter, increased both numbers of bands of APX isoforms and their intensity, particularly 7 days after the treatment (Fig. 3). APX isoforms (Fig. 3), localized by activity staining on native gels, showed greater intensity with more number of bands under chromium stress as compared to control and corroborates increased level of H2O2 in Cr—stressed turnip plants. Decline in activity of peroxidase in plants (Table 4) exposed to different forms of Cr is in accordance with the earlier findings of Pandey and Sharma (2003) in cabbage.
Conclusion
Exposure of Cr induced oxidative stress in turnip, as indicated by an increased accumulation of MDA and H2O2, and the significant increases in the antioxidative enzymes SOD, APX and CAT. Enhanced chloroplastic pigments along with increases in Fe levels in CrVI—stressed leaves appears to be characteristic features of CrVI stressed turnip plants. While the turnip plants showed responses to the exposure of both CrIII and CrVI, CrVI was found more toxic than CrIII. Among the two sources of CrVI, K2Cr2O7 was more damaging than K2CrO4, possibly due to its lesser stability and because of the presence of two atoms of Cr per K2Cr2O7 molecule.
References
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and assays applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Brennan, T., & Frankel, C. (1977). Involvement of hydrogen peroxide in regulation of senescence in pear. Plant Physiology, 59, 411–416.
Chatterjee, J., & Chatterjee, C. (2000). Phytotoxicity of cobalt, chromium and copper in cauliflower. Environmental Pollution, 109, 69–74.
Choudhury, S., & Panda, S. K. (2005). Toxic effects, oxidative stress and ultrastructural changes in moss Toxithelium nepalense (Schwaegr) Broth. Under chromium and lead phytotoxicity. Water, Air, and Soil pollution, 167, 73–90.
Dixit, V., Pandey, V., & Shyam, R. (2002). Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L cv. Azad) root mitochondria. Plant, Cell and Environment, 25, 687–693.
Dube, B. K., Tewari, K., Chatterjee, J., & Chatterjee, C. (2003). Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere, 164, 793–801.
Dube, B. K., Sinha, P., Gopal, R., & Chatterjee, C. (2004). Chromium phytotoxicity alters metabolism in radish. Journal of Vegetable Crop Production, 10, 61–71.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts, I. kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 180–198.
Hewitt, E. J. (1966). Sand and water culture methods used in the study of plant nutrition (2nd ed.). London: Technical communication No. 22, Common Wealth Agricultural Bureau.
Kappus, H. (1985). Lipid peroxidation: Mechanisms, analysis, enzymology and biological relevance. In H. Sies (Ed.), Oxidative stress (pp. 273–310). London: Academic Press.
Katz, S. A., & Salem, H. (1994). The biological and environmental chemistry of chromium. New York: VCH Publishers.
Kotas, J., & Stasicka, Z. (2000). Chromium occurrence in the environment and methods of its speciation. Environmental Pollution, 107, 263–283.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
Liu, C., Du, W., Cai, H., Jia, Z., & Dong, H. (2014). Trivalent chromium pretreatment alleviates the toxicity of oxidative damage in wheat plants exposed to hexavalent chromium. Acta Physiologiae Plantarum, 36, 787–794.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Marschner, H. (1995). Mineral nutrition of higher plants. London: Academic Press.
Mittler, R., & Zilinskas, B. A. (1993). Detection of ascorbate peroxidase activity in native gels by inhibition of ascorbate-dependent reduction of nitroblue tetrazolium. Analytical Biochemistry, 212, 540–546.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880.
Nieboer, E., & Richardson, D. H. S. (1980). The replacement of nondescript term “heavy metals” by a biologically and chemically significant classification of metal ions. Environmental Pollution, B1, 3–26.
Oliveira, H. (2012). Chromium as an environmental pollutant: Insights on induced plant toxicity. Journal of Botany, 2012, 8.
Paiva, L. B., de Oliveira, J. G., Azevedo, R. A., Ribeiro, D. R., daSilva, M. G., & Vitoria, A. P. (2009). Ecophysiological responses of water hyacinth exposed to CrIII and CrVI. Environmental and Experimental Botany, 65, 403–409.
Panda, S. K. (2007). Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. Journal of Plant Physiology, 164, 1419–1428.
Panda, S. K., & Choudhury, S. (2005). Chromium stress in plants. Brazilian Journal of Plant Physiology, 17, 95–102.
Panda, S. K., & Khan, M. H. (2003). Antioxidant efficiency in rice (Oryza sativa L.) leaves under heavy metal toxicity. Journal of Plant Biology, 30, 23–29.
Panda, S. K., & Upadhyay, R. K. (2008). Biochemical changes and oxidative damage in Azolla pinnata L. under chromium phytotoxicity stress. Indian Journal of Plant Physiology, 13, 243–250.
Panda, S. K., Choudhury, I., & Khan, M. H. (2003). Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biologia Plantarum, 46, 289–294.
Pandey, N., & Sharma, C. P. (2003). Chromium interference in iron nutrition and water relations of cabbage. Environmetal and Experimental Botany, 49, 195–200.
Pandey, V., Dixit, V., & Shyam, R. (2009). Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts. Protoplasma, 236, 85–95.
Shanker, A. K., & Pathmanabhan, G. (2004). Speciation dependant antioxidative response in roots and leaves of Sorghum (Sorghum bicolor (L) Moench cv CO 27) under Cr(III) and Cr(VI) stress. Plant and Soil, 265, 141–151.
Sharma, D. C., & Sharma, C. P. (1996). Chromium uptake and toxicity effects on metabolic activities in wheat, Triticum aestivum L. cv. UP 2003. Indian Journal of Experimental Biology, 34, 689–691.
Tewari, R. K., Kumar, P., & Sharma, P. N. (2010). Morphology and oxidative physiology of boron-deficient mulberry plants. Tree Physiology, 30, 68–77.
Welch, R. M., Allaway, W. H., House, W. A., & Kubota, J. (1991). Geographic distribution of trace element problems. In J. J. Mortvedt, F. R. Cox, L. M. Schuman, & R. M. Welch (Eds.), Micronutrient in Agriculture (pp. 31–37). Wisconsin: Soil Science Society of America.
Zayed, A., Lytle, C. M., Qian, J. H., & Terry, N. (1998). Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta, 206, 293–299.
Zeid, I. M. (2001). Responces of Phaseolus vulgaris to chromium and cobalt treatments. Biologia Plantarum, 44, 111–115.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, J., Kumar, P., Sharma, P.N. et al. Chromium toxicity induces oxidative stress in turnip. Ind J Plant Physiol. 20, 220–226 (2015). https://doi.org/10.1007/s40502-015-0163-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-015-0163-6