Opinion statement
Purpose of review The management of treatment-resistant posttraumatic stress disorder (TRPTSD) is a complex clinical challenge, and many patients may continue to endure a heavy symptom burden, even despite the best available treatments. We review the recent literature to provide an update on the evidence base and offer guidance to clinicians on available approaches, including a number of novel and emerging options.
Recent findings If adequate trials of treatment with first-line antidepressants (SSRIs or venlafaxine) or trauma-focused psychotherapy have failed, dosage increase, switching to an alternative first-line option, or combining medication and psychotherapy are reasonable initial approaches. If these remain insufficient, augmentation strategies should be offered, including addition of second-generation antipsychotics (such as risperidone) or the adrenergic antagonist prazosin (especially if sleep disturbance or nightmares are problematic). Further options include the use of other antidepressants (most notably mirtazapine, duloxetine, and trazodone), and the anticonvulsants topiramate or lamotrigine, though the evidence for these is relatively weak. Having tried all these possibilities, the clinician may wish to suggest complementary approaches such as yoga, mindfulness meditation, or acupuncture for symptom reduction and overall well-being. Emerging alternatives, if available, could also be considered, such as augmentation of exposure therapy with d-cycloserine or MDMA, or the use of device-based brain stimulation (such as transcranial magnetic stimulation), though the evidence for these is still preliminary.
Summary Comprehensive assessment of TRPTSD, including a thorough evaluation of associated comorbidity, should inform individualized care, incorporating a process of shared decision-making. Despite the complex clinical challenge of TRPTSD, clinicians should remain hopeful however, that translational neuroscience and clinical trials of emerging approaches will allow progressively better treatment alternatives to be established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posttraumatic stress disorder (PTSD) presents after a severely traumatic experience with symptoms from four clusters: intrusion experiences (such as flashbacks and nightmares), avoidance (of trauma-related stimuli), negative alterations in mood and cognition (such as memory disturbance or inability to experience positive emotions), and alterations in arousal and reactivity (including hypervigilance and sleep disturbance) [1]. These symptoms cause distress and result in functional impairment [2••, 3]. Comorbid conditions are frequently present, most notably depressive, anxiety, and substance use disorders [4, 5], adding considerably to the disease burden. The lifetime prevalence of PTSD is approximately 3.9% globally [6], though it is higher in certain populations such as combat-exposed veterans, where it may be as high as 24% [7]. Despite a range of efficacious treatments [8••, 9], PTSD is frequently chronic and treatment resistant [10•].
While there is no standard definition for treatment-resistant PTSD (TRPTSD), a pragmatic view is that it is the persistence of PTSD despite adequate first-line treatment (including antidepressants and/or cognitive-behavioral therapy) [10•]. Only approximately 20–30% of patients achieve full remission following the use of first-line therapies [11], and while an additional 30–40% experience partial improvement, this still means that 70% are resistant to first-line treatment.
Though the demographic and clinical characteristic of TRPTSD still needs to be clarified, several known risk factors may be clinically relevant [2••, 10•, 12]: combat-related PTSD, and PTSD in refugees, may be more treatment resistant; a long duration since trauma exposure and chronically untreated symptoms predicts non-response as does severity of PTSD; and comorbid depression may increase treatment resistance, especially if suicidality is prominent. In terms of specific symptoms, psychosis is a sign of severity and predicts resistance [10•, 13], while sleep disturbance (including as a result of nightmares) is a hallmark PTSD symptom that is particularly refractory [2••, 14•]. On the other hand, an internal locus of control and problem-focused as opposed to emotion-focused coping in the wake of trauma, as well as strong social support, may be protective against TRPTSD [2••].
To inform best practice, and assist in developing new treatments, neurobiological research continues to be undertaken, and the Research Domain Criteria (RDoC) framework emphasizes the potential value of translational approaches to PTSD [15, 16•]. Alongside the role of monoamine neurotransmitters (serotonin, noradrenaline, and dopamine) in PTSD pathophysiology [16•, 17, 18], the glutamatergic system is recognized as of central importance [19], particularly given its role in learning and memory processes essential for fear acquisition and extinction [2••, 20]. In addition, on the macro-systems level, neuroimaging suggests an imbalance between amygdala hyperactivity and ventromedial prefrontal cortex (vmPFC) hypoactivity in PTSD that contributes to fear acquisition, behavioral dysregulation, and hyperarousal [2••, 21]. While these findings are not specific to TRPTSD, they may nevertheless be useful for conceptualizing the disorder, and addressing intervention [22]. After briefly reviewing standard treatments, and presenting a practical approach to treatment resistance, this chapter focuses on options for managing TRPTSD.
Treatment
Review of first-line treatments for PTSD
A number of treatment goals in the management of PTSD are useful to keep in mind: relieving core symptoms, treating comorbidity, reducing functional impairment, preventing relapse, and improving quality of life [23]. Towards these ends, it is important that evidence-based first-line therapies have been tried, before turning to next-step options for those with resistant symptoms.
Many individuals with PTSD are inadequately treated [6, 24, 25], meaning that a substantial proportion with chronic and seemingly resistant symptoms, may not in fact be treatment resistant, but may instead not have received efficacious treatment. In the absence of adequate past treatment with evidence-based therapies, treatment resistance should not be inferred, and instead appropriate first-line therapy should be initiated.
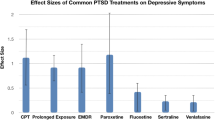
Based on increasingly robust evidence, trauma-focused cognitive-behavioral therapies (such as prolonged exposure—PE—and eye movement desensitization and reprocessing—EMDR), and a selection of antidepressants (specifically the SSRIs sertraline, paroxetine, and fluoxetine, and the SNRI venlafaxine) are efficacious evidence-based therapies for PTSD [8••, 9, 26,27,28,29]. Effect sizes for psychotherapy are greater than for medication [27••], but this may reflect the infrequent use of good active comparators in psychotherapy trials [8••]. Thus, depending on availability of adequately trained psychotherapists, and individual patient factors (such as comorbid illness), any first-line evidence-based treatment option can be initiated, and symptom response monitored over an adequate period. The use of standardized rating scales such as the Clinician-Administered PTSD Scale (CAPS) is a systematic means of assessing treatment response, which can guide treatment and should be encouraged.
Having initiated evidence-based treatment, some choose response as an acceptable goal; this being defined as a 30% or greater reduction in CAPS score, or a score of 1 (very much improved) or 2 (much improved) on the Clinical Global Impressions Scale-Improvement item [10•, 11]. However, for severe PTSD, even a 30% reduction in symptoms can still mean a substantial ongoing burden (Berger et al., 2009). For this reason, and in both clinical and research settings, the goal of treatment should be remission [2••], which would be a reduction in CAPS score to ≤ 20 [10•, 11]. Not achieving remission arguably indicates some degree of treatment resistance.
An approach to treatment resistance
For the 70% of patients who do not achieve full remission despite use of first-line treatments, a number of important factors should be considered before determining the best next-step—please see the algorithm in Fig. 1 which summarizes a practical approach.
The first is to review the diagnosis and clarify problematic target symptoms. While the characteristic trauma and well-defined symptoms of PTSD should make the diagnosis relatively straightforward, mistakes may have occurred. Methodical review of the history and mental state examination, as well as gathering collateral information, will confirm the diagnosis, while also identifying comorbid disorders and other factors influencing treatment-planning. Important differential diagnoses include adjustment disorders, acute stress disorder, anxiety disorders and obsessive-compulsive disorder, major depressive disorder, personality disorders, dissociative disorders, psychotic disorders, and neurocognitive disorders [1]. Comprehensive assessment of sleep disturbance (including the extent of nightmares) is essential, given that it is a common and refractory symptom, which contributes to the persistence of illness and functional impairment [14•, 30].
The majority of those with PTSD have at least one comorbid mental disorder, and many have three or more [4]. Psychiatric comorbidity is important because of the additional symptom burden, effects on adherence or engagement with treatment (e.g., due to low motivation from depression), and the possibility of drug interactions with pre-existing medication. The most commonly encountered psychiatric comorbidities are depressive, anxiety, and substance use disorders [4, 5], and there can be considerable symptom overlap with PTSD, making diagnosis more challenging. Comorbid physical disorders, including chronic pain, are also more likely [2••]. Missed or inadequately managed comorbid conditions can easily be part of the explanation for apparent treatment resistance and chronic functional impairment, and recognizing this guides successful individualized care.
The second step is thoroughly reviewing past treatment response and assessing whether there was adequate adherence for it to have been effective. [33•] suggest that 80% adherence, equating to correct medication use on 6 out of 7 days of the week, would ensure adequate exposure to therapy. There are multiple reasons for poor adherence, including poor insight, problems relating to access or financial constraints, impaired understanding of the medication regime, problematic side effects, or other unacceptable consequences of treatment (such as extreme distress experienced by some during exposure therapy) [31•]. Several of these may be relevant at once, and by successfully addressing barriers to adherence, the clinician may facilitate patients gaining substantial benefit from treatment without needing to resort to second-line or augmentation strategies. Ongoing monitoring of adherence, and education about correct medication use, should remain a basic component of clinical care plans.
Finally, it is important to ensure that the dose and duration of treatment have been adequate [10•, 12]. While high dose prescribing is found in clinical practice, and a wide range of doses have been used in clinical trials, there is no clear evidence to support high-dose antidepressants in PTSD [9, 32], but at least a minimum effective dose should have been used [33•]. The ideal duration of treatment trials is not clear; and while [33•] recommended that at least 8 weeks of pharmacological treatment, and six psychotherapy sessions should be adequate, others have recommended medication trials of up to 12 weeks [34].
Having followed this systematic approach, the clinician should have clarified if a patient is genuinely treatment resistant, and whether associated factors such as comorbid conditions and specific symptoms are problematic. This provides an important foundation for individualized treatment, as the evidence base for next-step approaches is limited [8••, 10•].
Next-step and augmentation strategies for treatment resistance
Managing TRPTSD requires balancing the desire to reduce the symptom burden and functional impairment endured by sufferers with a degree of uncertainty about the best treatment due to the relatively limited evidence base [8••, 27••]. A rational approach should involve detailed assessment as already described, followed by an individualized treatment plan. The intervention choices in any individual case should depend on particular target symptoms and recovery goals identified by the patient and the clinician through a process of shared decision-making [8••]. Treatment should be regularly monitored, and if there is no clear benefit, it should be modified. In particular, if there is no response to a new medication after an adequate period, it should be tapered and discontinued, so as to avoid unnecessary side effects.
Next-step strategies employing first-line therapies
As an initial step, three relatively simple options may be worth considering: dosage increase of an existing SSRI or SNRI antidepressant, sequential monotherapy with first-line antidepressants, or the combination of first-line pharmacological and psychotherapeutic treatments.
As mentioned, there is no clear evidence for high-dose antidepressants in PTSD, and minimally effective doses, or low to moderate doses, are usually recommended [9, 33•]. However, when an agent is well-tolerated, and there has been a partial response, dose titration within the established safe range of that agent could provide further relief. This approach might be particularly worthwhile in the context of comorbid depressive and anxiety disorders, which if also partially treated, may respond further with higher doses [34, 35]. The extended neurotransmitter effects of venlafaxine at higher doses is also noteworthy [36]. Higher dosing, and which antidepressants would offer benefit when used in this way, has not been rigorously studied in TRPTSD however, so this approach remains speculative pending further evidence.
The idea of sequential monotherapy also originates from the mood and anxiety disorder literature, which indicates that non-response to one SSRI does not necessarily mean that another will not work, and there may be a good response with an alternative agent [29, 37]. For example, a case report of TRPTSD noted that switching to venlafaxine resulted in improvement even after several SSRIs failed [38]. More robust confirmation of this encouraging finding is needed, but as this strategy carries little additional risk, and SSRIs and venlafaxine remain the most evidence-based pharmacological treatment options for PTSD [8••, 9, 28•], empirical trials seem acceptable pending further investigation as to the most effective sequence of antidepressants [12, 26].
The combination of a first-line antidepressant, such as an SSRI, with a first-line psychotherapy, such as PE, after a poor response to either alone, is a third approach with empirical support. Even when not able to produce remission, antidepressants can reduce the intensity of symptoms [12], particularly anxiety, which may allow patients to successfully complete exposure therapy if it has previously been intolerable. It is attractive to imagine that combining two efficacious treatments results in an even more effective overall treatment, but the evidence for this approach is mixed. In patients who failed to adequately respond to PE, the addition of paroxetine did not produce significant further improvement [39], while when PE was added for those already on sertraline, there was further improvement, though only in those who had shown a partial response to medication [40]. In research on those with explicitly defined TRPTSD, combining sertraline and CBT was more effective than sertraline alone [41], an encouraging finding that awaits replication in larger trials. As this is again a relatively low-risk strategy, it may be warranted before progression to riskier alternatives, though recent recommendations emphasize the poverty of the evidence base [8••].
Pharmacological augmentation of first-line antidepressants
The addition of medication from other classes to ongoing treatment with antidepressants is a frequently used strategy for TRPTSD [13]. The evidence is inconsistent, however, as to the benefits of this approach, and both meta-analyses and clinical practice guidelines can be divided into those which support augmentation [9, 26, 29, 34, 42], and those which do not [8••, 27••, 28•, 43]. These conflicting reports indicate the need for more definitive studies.
From a neurobiological perspective, augmentation of antidepressants with agents targeting different neurotransmitter systems would appear justifiable, especially given the complexity of the underlying abnormalities [17, 18, 22]. In particular, targeting dopaminergic and noradrenergic processes augments the predominantly serotonergic effects of the antidepressants, which may assist in more effectively targeting all symptom clusters [2••, 13].
The most common pharmacological augmentation in PTSD is with second-generation antipsychotics (SGAs). Risperidone has been the most thoroughly investigated in RCTs and is probably the most frequently prescribed, though the evidence is inconsistent. Two meta-analyses determined that risperidone is effective as an augmenting agent [9, 26], while others have judged the evidence inadequate, emphasizing concerns about study bias, and the high risk of metabolic derangement [27••, 28•, 44]. Risperidone augmentation is, however, currently recommended in several clinical practice guidelines [29, 34, 42].
Olanzapine augmentation has been investigated in TRPTSD in one RCT, which found statistically significant improvements in PTSD symptoms including sleep measures, though the effects were judged not clinically significant, given that clinical global impression scores were unchanged [45]. There have also been open-label trials of quetiapine augmentation, and while these were positive, methodological weaknesses, including the absence of placebo controls and blinding procedures, are concerns [46, 47]. As with risperidone, metabolic derangement is common with olanzapine and quetiapine and should be carefully evaluated. Clozapine and aripiprazole have also been investigated, and while there have been encouraging findings, especially for aripiprazole in TRPTSD [48], the effect sizes are small and the quality of evidence low [10•].
The inconsistent results and low quality of evidence for SGA augmentation in PTSD have resulted in increasing caution regarding their use [27••], and while still included in some treatment guidelines [29, 42], they have been excluded from some recent recommendations [8••, 43]. Pragmatically, the clinician may still employ SGAs in certain circumstances, the most obvious indication being the presence of psychotic symptoms, present in up to 40% of patients with PTSD [13]. They may also be valuable for comorbid treatment-resistant anxiety or mood disorders [29, 49], as well as for associated insomnia or aggression [11, 13, 50, 51•]. Given the inconsistent evidence-base, initiating SGAs should be approached as a carefully monitored, time-limited treatment trial with specific goals. Metabolic monitoring is essential, and if significant derangement occurs, or if there is no symptomatic improvement, these medications should be discontinued.
In contrast to the concerns relating to SGA use, a more straightforward augmentation strategy is the addition of the alpha-1 adrenergic antagonist prazosin. Given that adrenergic hyperactivity may underlie hyperarousal symptoms [18], and the finding of abnormal adrenergic processing in the amygdala in PTSD [2••], this approach is consistent with the neurobiological evidence. Prazosin produces significant improvements in sleep and nightmares—two very common and treatment-resistant symptoms [52, 53]. There is also evidence that it improves PTSD symptoms overall in civilian and combat-veteran populations [53,54,55], and adding a daytime dose increases the benefit [56]. The benefits of prazosin are supported by two recent meta-analyses [9, 27••], with one concluding that it was the only augmentation agent to show significant benefit vs. placebo [27••]. So, while the use of prazosin is still not uniformly recommended [8••], patients with prominent nightmares may deserve a trial of prazosin [2••]. Given that it is well-tolerated [54] and that it helps significantly for two very problematic PTSD symptoms; it has even been suggested that prazosin be regarded as a first-line treatment in PTSD with disturbed sleep [2••]. Important practical points include regular blood pressure monitoring and keeping in mind that the dosage range is wide (1—45 mg/day), and titration to effect over several weeks may be required.
A final augmentation strategy is with the anticonvulsant topiramate, which has broad effects potentially relevant in PTSD, including increasing gamma-aminobutyric acid (GABA) levels, enhancing GABA receptor function, and glutamate NMDA receptor antagonism [57]. Anticonvulsants as a class may not be efficacious in PTSD [26, 27••], and the evidence on topiramate is inconsistent. Topiramate has shown benefit for reducing overall symptoms in a clearly defined group of combat-related TRPTSD patients [58], but there is also a negative RCT in combat-veterans [59]. While additional investigation of topiramate is needed in PTSD, given the encouraging effect size in the larger of the two trials [58], support from meta-analysis [26, 44], and the potential value of topiramate for comorbid alcohol and substance use disorders [60], it may warrant a treatment trial in select cases.
Other medication approaches
A wide range of other pharmacological options has been investigated for the treatment of PTSD, including other antidepressants as monotherapy or augmentation (such as mirtazapine, nefazodone, tricyclic antidepressants, monoamine oxidase inhibitors, or trazodone); SGA monotherapy (with risperidone, olanzapine, and quetiapine); anticonvulsants as monotherapy or augmentation (notably divalproex, tiagabine, lamotrigine, topiramate, and levetiracetam); anxiolytic-hypnotics (specifically benzodiazepines, and buspirone); other adrenergic agents (clonidine, guanfacine, and propranolol); and the mood stabilizer lithium.
Of the antidepressants, there are conflicting meta-analytic findings relating to nefazodone [26, 27••], and this agent is also associated with hepatotoxicity [8••]. Mirtazapine has been investigated as an adjunct to sertraline in a single RCT, with the study showing significantly greater remission rates [61], albeit not in TRPTSD. There is some low-quality evidence suggesting benefit from trazodone for sleep difficulty, but again, this was not exclusively in TRPTSD [62, 63]. Duloxetine has been studied in TRPTSD with comorbid major depressive disorder and led to significant improvements in PTSD symptoms and depression [64]. Apart from these four agents, which may reasonably be considered as second- or third-line choices [8••, 42], other antidepressants have not been systematically evaluated in TRPTSD or the quality of evidence is low [2••], and they are not recommended in recent practice guidelines [8••, 43], although they could be considered for comorbid depression or anxiety disorders.
In addition to their use as augmenting agents, SGAs have also been investigated as PTSD monotherapy, though research has not necessarily been in TRPTSD. While there is some support for risperidone, olanzapine, and quetiapine monotherapy, effect sizes are modest [27••, 65], and whether these agents can help in clearly defined TRPTSD is untested. Thus, they are currently recommended as third-line strategies [42]. If a treatment trial is planned, metabolic side effects must be carefully monitored.
Collectively, anticonvulsants are not efficacious treatments for PTSD [26, 27••, 66], and there is insufficient supporting evidence to recommend divalproex or tiagabine [8••, 29, 43]. However, topiramate may provide benefit as monotherapy in addition to its value as augmentation of antidepressant treatment [67, 68]. That said, it has not been studied in a TRPTSD sample. Lamotrigine monotherapy has also shown benefit in PTSD, but again, this was not in a TRPTSD sample [69]. Levetiracetam improved symptoms in TRPTSD [70], in a retrospective series, but has not been studied in a rigorous RCT. Closely monitored trials of topiramate or lamotrigine monotherapy may be justifiable as third-line options in certain patients [42], though any potential benefits must be balanced with the known risk of side effects [18].
Benzodiazepines are not efficacious in PTSD [9, 26], and apart from concerns about dependence, may disrupt psychotherapy due to their negative effect on cognitive processing [11, 18]. They are therefore not recommended in TRPTSD [8••]. Buspirone has shown value in an open-label trial as an augmentation agent in TRPTSD [71], but given the lack of subsequent replication, is not yet recommended [8••].
The benefits of prazosin, noted earlier, are not indicative of a class effect for adrenergic agents, as while guanfacine, clonidine, and propranolol have all been investigated, including in RCTs and open-label studies [2••, 11, 18], there is no evidence to suggest benefit in TRPTSD [2••, 10•, 72].
Finally, lithium has been shown, based only on evidence from case reports, to reduce anger, irritability, and suicidality in PTSD, albeit having no direct effect on core PTSD symptoms [2••, 73]. This is a promising finding, but needs to be confirmed in a rigorous RCT before lithium can routinely be recommended.
Novel and emerging treatments
Complementary and alternative medicine
Alongside conventional treatment, there is some evidence that complementary and alternative medicine (CAM) may be valuable in PTSD [74, 75]. While there are important criticisms of this evidence base, in particular the weak methodologies of many of the studies [76], the potential benefits of CAM include low cost, reduced side effects, and empowering self-management skills [74]. Yoga, meditation, and acupuncture are three of the most widely used interventions for not only reducing PTSD symptoms, but also contributing to significant improvements in daily functioning and well-being [74, 75].
When offered in the context of TRPTSD in women, yoga improved PTSD symptoms [77, 78], a benefit which was sustained if yoga was practiced over the long term [79]. Mindfulness meditation also relieved PTSD symptoms in a small study which applied a modified mindfulness-based cognitive therapy approach [80]. Finally, acupuncture resulted in significant improvements in PTSD symptoms, as well as improving depression, pain, and physical and mental health functioning [81], with a systematic review and meta-analysis concluding that the evidence for acupuncture was encouraging [82].
These approaches, while not yet sufficiently evidence-based to form part of standard guidelines, may nevertheless be valuable in TRPTSD and should be considered for patients who are willing to engage with alternative routes to symptom reduction and improvement in overall well-being. Methodologically robust research will help clarify the role of these treatments [76].
Emerging pharmacological options
Based on increasing understanding of the neurobiology of PTSD, several novel pharmacological treatments are being investigated [16•, 22]. Initially used for treatment-resistant depression [83], the glutamate NMDA receptor antagonist ketamine given as a single infusion produced rapid and significant improvements in PTSD symptoms, though these were relatively short-lived [84]. Questions relating to dose, timing, and the need for repeated infusions need to be resolved, but ketamine could be useful in acute or initial treatment, including in TRPTSD, and further studies are warranted [2••, 22].
Motivated in part by preclinical research which highlights HPA-axis dysregulation in PTSD [85], glucocorticoids have been studied both as preventative agents, and for augmentation of psychotherapy due to their role in memory reconsolidation processes relevant for fear acquisition and extinction [86, 87]. Though they have not yet been systematically investigated in TRPTSD, promising findings from two RCTs suggest they could become a valuable adjunctive treatment [88, 89].
Apart from glucocorticoids, two novel pharmacological agents have recently attracted attention because of their potential to enhance the effects of exposure therapy. D-cycloserine (DCS), a glutamate NMDA receptor partial agonist, may enhance fear extinction, provided this has occurred during the exposure sessions [17]. There is currently mixed evidence on the benefits of DCS, with both a positive [90•] and a negative [91•] meta-analysis. In addition, there are questions about patient selection, dosage, and timing of administration which remain to be answered [8••]. As a result, while some patients may benefit [92], and there is a neurobiological framework for the use of DCS [20], it is not currently recommended outside of research settings [8••, 22].
Similarly, though by quite different mechanisms, the stimulant-hallucinogen methylenedioxymethamphetamine (MDMA) has also been shown to enhance the effects of exposure therapy [31•]. MDMA-assisted psychotherapy has shown particular value in TRPTSD [93•, 94]. MDMA acts on multiple brain systems relevant to PTSD, including serotonergic, noradrenergic, and dopaminergic receptors, and the HPA axis, and promotes the release of the pro-social hormone oxytocin [31•]. Functional neuroimaging also indicates that it may alleviate amygdala hyperactivity, a core dysfunction of PTSD [95]. It improves mood, reduces anxiety, and enhances cognitive flexibility and social engagement, bringing patients into an “optimal arousal zone” for intensive psychotherapeutic work relating to their trauma [96]. Though research on MDMA-assisted psychotherapy is still preliminary, a meta-analysis suggests it may have equivalent effect sizes to PE and be better tolerated [97•]. These results are promising, and larger trials are under way [96].
Lastly, the endocannabinoid system is involved in multiple processes which could underlie PTSD symptoms and which could be harnessed as a component of treatment, particularly given its role in fear extinction learning [98, 99]. Though there are important safety concerns relating to the use of natural and synthetic cannabinoids, specifically addiction, cognitive impairment, and psychosis risk [17, 99], there is also evidence that natural and synthetic cannabinoids can relieve symptoms in TRPTSD [2••, 98, 100]. The evidence base at this stage remains weak, and given evidence that illicit cannabis use in PTSD worsens symptoms and functional outcomes [101•]; cannabinoids cannot be recommended at this time [8••, 98, 99].
Device-based approaches
Spurred on by neurobiological research indicating regional brain abnormalities in PTSD, including amygdala and dorsal anterior cingulate hyperactivity, and hippocampal and vmPFC hypoactivity [2••], various brain stimulation methods are being investigated [21]. Repetitive transcranial magnetic stimulation (rTMS), already FDA-approved for depression [102], is a non-invasive intervention that produces significant and sustained reductions in PTSD symptoms when compared to a placebo treatment [103,104,105]. rTMS is attractive because of its safety and low side effect profile, and while it is not yet part of standard practice guidelines [8••], it is an accessible intervention. Deep brain stimulation (DBS) is a surgical approach with established benefit in movement disorders, depression, and OCD [21]. Results from a case report in PTSD indicated substantial benefit in a patient with severe TRPTSD [106], which may be mediated via effects on fear extinction and BDNF-mediated neural plasticity [21]. Vagal nerve stimulation (VNS), which requires a surgical procedure, has been shown to potentiate fear extinction in animal models and reduced PTSD symptoms in a small case series [21, 107]. Finally, transcranial direct current stimulation (tDCS) is a non-invasive option that is attractive because of its low cost, portability, and ease of use [21]. Preclinical research suggests that it may be able to modulate memory consolidation, which could be a useful adjunct to other PTSD treatments [21], and a small case series showed some symptomatic improvement in PTSD [2••].
Finally, it is important to mention electroconvulsive therapy (ECT), whereby an electrical stimulus is applied to localized areas, either bitemporal, bifrontal, or right unilateral [108], with a resulting generalized seizure that represents a “whole-brain” stimulus as opposed to the more focused stimuli administered with rTMS, DBS, VNS, and tDCS. Though there remains ongoing debate about the mechanism of action of ECT [109], it is an established treatment in mood and psychotic disorders [110] and has been used as a treatment for PTSD, including in treatment-resistant cases [2••]. The evidence is of low quality, however, with no RCTs [2••], and while several case reports, a case series, and a single prospective uncontrolled trial indicate benefit for PTSD symptoms [2••, 111], a recent meta-analysis concluded that the apparent benefits may have been due to changes in depression rather than significant benefit for PTSD symptoms directly [112]. Further large, well-designed, controlled trials may clarify the benefits of ECT, but at present, it is not a recommended treatment for PTSD [8••].
For all these approaches, important questions still need to be answered about frequency, site, and duration of stimulation, but they may represent a new wave of therapeutic options for those suffering with TRPTSD.
Conclusions and recommendations
The management of treatment-resistant PTSD is a complex challenge, requiring thorough clinical assessment, and a willingness to offer flexible and individualized care [2••, 10•, 113]. Though TRPTSD has not been sufficiently investigated, and there are major gaps and weaknesses in the existing evidence-base, progress is being made [2••, 10•]. Having confirmed the presence of treatment resistance, there is some evidence of benefit from pharmacological augmentation, most notably with risperidone and prazosin [9, 13, 27••]. Other medication options are less well-researched, but other antidepressants and anticonvulsants may offer value [2••].
Combining pharmacological agents such as DCS and MDMA with exposure therapy to augment fear extinction is a novel treatment avenue, and encouraging beneficial effects have been reported from preliminary studies [2••, 31 •, 89, 90•]. A range of other approaches are being investigated, and promising results for ketamine, glucocorticoids, and device-based brain stimulation are emerging [2••, 21, 22]. Finally, given the frequently chronic nature of symptoms and impairment in TRPTSD, complementary and alternative medicine approaches deserve consideration [74, 75].
Given the associated disease burden, TRPTSD deserves to be prioritized on mental health care and neuroscience research and funding agendas. To move forward, systematic investigation is needed, starting with clear definitions of treatment resistance that are used consistently in research [33•]. Methodologically robust studies which determine treatment resistance prospectively and then evaluate the effects of psychotherapeutic and pharmacological options, including head to head comparisons and treatment sequencing, are indicated in various subpopulations, including civilians vs. combat-veterans, and across the age range [2••, 27••, 28•, 66]. As evidence accumulates, the clinician will be further empowered to make the safest and most beneficial treatment decisions to relieve the suffering associated with this disorder.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Psychiatric Association. The Diagnostic and Statistical Manual (5th ed.). 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
•• Koek RJ, Schwartz HN, Scully S, Langevin JP, Spangler S, Korotinsky A, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuro-Psychopharmacol Biol Psychiatry. 2016;70:170–218. Comprehensive review of the literature relating to treatment resistant PTSD.
Rodriguez P, Holowka DW, Marx BP. Assessment of posttraumatic stress disorder-related functional impairment: a review. J Rehabil Res Dev. 2012;49(5):649–65.
Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J clin psychiatry. 2000;61(Suppl 7):22–32.
Dorrington S, Zavos H, Ball H, McGuffin P, Rijsdijk F, Siribaddana S, et al. Trauma, post-traumatic stress disorder and psychiatric disorders in a middle-income setting: prevalence and comorbidity. Br J Psychiatry. 2014;205(5):383–9.
Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med 2017;47(13):2260-74.
Milliken CS, Auchterlonie JL, Hoge CW. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007;298(18):2141–8.
VA/DOD. VA/DOD Clinical Practice Guideline for the management of posttraumatic stress disorder and acute stress disorder. Washington (DC): Department of Veterans Affairs, Department of Defense; 2017. Available from: https://www.healthquality.va.gov/guidelines/MH/ptsd/. Comprehensive guideline for PTSD, including treatment resistance, detailed discussion of evidence behind recommendations.
Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD). Int J Neuropsychopharmacol. 2012;15(6):825–40.
Katz C, Stein M, Richardson JD, Seedat S, Sareen J. A review of interventions for treatment-resistant posttraumatic stress disorder. In: Selek S, editor. Different views on anxiety disorders: InTech; 2011, p. 251-69. Useful overview chapter on treatment resistant PTSD.
Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33(2):169–80.
Bernardy NC, Friedman MJ. Psychopharmacological strategies in the management of posttraumatic stress disorder (PTSD): what have we learned? Curr psychiatry rep. 2015;17(4):564.
Ahearn EP, Juergens T, Cordes T, Becker T, Krahn DA. Review of atypical antipsychotic medications for posttraumatic stress disorder. Int Clin Psychopharmacol. 2011;26(4):193–200.
• Pruiksma KE, Taylor DJ, Wachen JS, Mintz J, Young-McCaughan S, Peterson AL, et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol trauma : theory res prac policy. 2016;8(6):697–701. Helpful review regarding sleep disturbance as a refractory PTSD symptom.
Schmidt U. A plea for symptom-based research in psychiatry. Eur J Psychotraumatol. 2015;6(1).
• Kelmendi B, Adams TG, Yarnell S, Southwick S, Abdallah CG, Krystal JH. PTSD: from neurobiology to pharmacological treatments. Eur J Psychotraumatol. 2016;7:31858. Summarises the translational research relating to pharmacology in PTSD.
Steckler T, Risbrough V. Pharmacological treatment of PTSD—established and new approaches. Neuropharmacology. 2012;62(2):617–27.
Ravindran LN, Stein MB. Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res. 2009;1293:24–39.
Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: evidence from human studies. Neurosci Lett. 2017;649:147–55.
Myers KM, Carlezon WA Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacol : off pub Am College Neuropsychopharmacol. 2011;36(1):274–93.
Marin MF, Camprodon JA, Dougherty DD, Milad MR. Device-based brain stimulation to augment fear extinction: implications for PTSD treatment and beyond. Depress Anxiety. 2014;31(4):269–78.
Thomas E, Stein DJ. Novel pharmacological treatment strategies for posttraumatic stress disorder. Expert Rev Clin Pharmacol. 2017;10(2):167–77.
Ursano RJ, Bell C, Eth S, Friedman M, Norwood A, Pfefferbaum B, et al. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am J Psychiatry. 2004;161(11 Suppl):3–31.
Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, Wilk JE. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatric services (Washington, DC). 2014;65(8):997–1004.
Meltzer EC. Discrepancy in diagnosis and treatment of post-traumatic stress disorder (PTSD): Treatment for the wrong reason. J Behav Health Serv Res. 2012;39(2):190–201.
Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J clin psychiatry. 2013;74(6):e541–50.
•• Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta-analyses to determine first-line treatments. Depress Anxiety. 2016;33(9):792–806. Comprehensive systematic review and meta-analysis with balanced discussion of role of pharmacotherapy.
Hoskins M, Pearce J, Bethell A, Dankova L, Barbui C, Tol WA, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93–100. Recent meta-analysis with a useful discussion on quality of evidence
Baldwin DS, Anderson IM, Nutt DJ, Allgulander C, Bandelow B, den Boer JA, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403–39.
Brownlow JA, Harb GC, Ross RJ. Treatment of sleep disturbances in post-traumatic stress disorder: a review of the literature. Current psychiatry rep. 2015;17(6):41.
• Sessa B. MDMA and PTSD treatment: “PTSD: from novel pathophysiology to innovative therapeutics”. Neurosci Lett. 2017;649:176–80. Useful introduction to the potential role of MDMA as an adjunct to psychotherapy in PTSD.
Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatr. 2001;158(12):1982–8.
• Dunlop BW, Kaye JL, Youngner C, Rothbaum B. Assessing treatment-resistant posttraumatic stress disorder: the Emory treatment resistance interview for PTSD (E-TRIP). Behavi Sci. 2014;4(4):511–27. Discusses a standardised approach to the evaluation of treatment resistance in PTSD.
Bandelow B, Sher L, Bunevicius R, Hollander E, Kasper S, Zohar J, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16(2):77–84.
Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173(2):174–83.
Debonnel G, Saint-Andre E, Hebert C, de Montigny C, Lavoie N, Blier P. Differential physiological effects of a low dose and high doses of venlafaxine in major depression. Int J Neuropsychopharmacol. 2007;10(1):51–61.
Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231–42.
Hamner MB, Frueh BC. Response to venlafaxine in a previously antidepressant treatment-resistant combat veteran with post-traumatic stress disorder. Int Clin Psychopharmacol. 1998;13(5):233–4.
Simon NM, Connor KM, Lang AJ, Rauch S, Krulewicz S, LeBeau RT, et al. Paroxetine CR augmentation for posttraumatic stress disorder refractory to prolonged exposure therapy. J clin psychiatry. 2008;69(3):400–5.
Rothbaum BO, Cahill SP, Foa EB, Davidson JR, Compton J, Connor KM, et al. Augmentation of sertraline with prolonged exposure in the treatment of posttraumatic stress disorder. J Trauma Stress. 2006;19(5):625–38.
Otto MW, Hinton D, Korbly NB, Chea A, Ba P, Gershuny BS, et al. Treatment of pharmacotherapy-refractory posttraumatic stress disorder among Cambodian refugees: a pilot study of combination treatment with cognitive-behavior therapy vs sertraline alone. Behav Res Ther. 2003;41(11):1271–6.
Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
American Psychological Association. Clinical practice guideline for the treatment of posttraumatic stress disorder in adults. 2017. Available from: http://www.apa.org/about/offices/directorates/guidelines/clinical-practice.aspx.
Jonas DE, Cusack K, Forneris CA, Wilkins TM, Sonis J, Middleton JC, et al. AHRQ comparative effectiveness reviews: psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD). Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry. 2002;159(10):1777–9.
Ahearn EP, Mussey M, Johnson C, Krohn A, Krahn D. Quetiapine as an adjunctive treatment for post-traumatic stress disorder: an 8-week open-label study. Int Clin Psychopharmacol. 2006;21(1):29–33.
Hamner MB, Deitsch SE, Brodrick PS, Ulmer HG, Lorberbaum JP. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psychopharmacol. 2003;23(1):15–20.
Naylor JC, Kilts JD, Bradford DW, Strauss JL, Capehart BP, Szabo ST, et al. A pilot randomized placebo-controlled trial of adjunctive aripiprazole for chronic PTSD in US military Veterans resistant to antidepressant treatment. Int Clin Psychopharmacol. 2015;30(3):167–74.
Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403.
Monnelly EP, Ciraulo DA, Knapp C, Keane T. Low-dose risperidone as adjunctive therapy for irritable aggression in posttraumatic stress disorder. J Clin Psychopharmacol. 2003;23(2):193–6.
Krystal JH, Pietrzak RH, Rosenheck RA, Cramer JA, Vessicchio J, Jones KM, et al. Sleep disturbance in chronic military-related PTSD: clinical impact and response to adjunctive risperidone in the Veterans Affairs cooperative study #504. J clin psychiatry. 2016;77(4):483–91. A valuable discussion on sleep disturbance in PTSD
Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371–3.
Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629–32.
Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928–34.
Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003–10.
Taylor FB, Lowe K, Thompson C, McFall MM, Peskind ER, Kanter ED, et al. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59(7):577–81.
Stafstrom CE. Mechanisms of action of antiepileptic drugs: the search for synergy. Curr Opin Neurol. 2010;23(2):157–63.
Akuchekian S, Amanat S. The comparison of topiramate and placebo in the treatment of posttraumatic stress disorder: a randomized, double-blind study. J Res Med Sci. 2004;9(5):240–4.
Lindley SE, Carlson EB, Hill KA. Randomized, double-blind, placebo-controlled trial of augmentation topiramate for chronic combat-related posttraumatic stress disorder. J Clin Psychopharmacol. 2007;27(6):677–81.
Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress. Addict Behav. 2014;39(2):428–33.
Schneier FR, Campeas R, Carcamo J, Glass A, Lewis-Fernandez R, Neria Y, et al. Combined mirtazapine and SSRI treatment of PTSD: a placebo-controlled trial. Depress Anxiety. 2015;32(8):570–9.
Hertzberg MA, Feldman ME, Beckham JC, Davidson JR. Trial of trazodone for posttraumatic stress disorder using a multiple baseline group design. J Clin Psychopharmacol. 1996;16(4):294–8.
Warner MD, Dorn MR, Peabody CA. Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry. 2001;34(4):128–31.
Walderhaug E, Kasserman S, Aikins D, Vojvoda D, Nishimura C, Neumeister A. Effects of duloxetine in treatment-refractory men with posttraumatic stress disorder. Pharmacopsychiatry. 2010;43(2):45–9.
Villarreal G, Hamner MB, Canive JM, Robert S, Calais LA, Durklaski V, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205–12.
Friedman MJ. Pharmacotherapy. In: Benedek DM, GHW, editors. Clinical manual for management of PTSD. Washington, DC: American Psychiatric Publishing, Inc.; 2011. p. 131–82.
Yeh MS, Mari JJ, Costa MC, Andreoli SB, Bressan RA, Mello MF. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNS neurosci ther. 2011;17(5):305–10.
Tucker P, Trautman RP, Wyatt DB, Thompson J, SC W, Capece JA, et al. Efficacy and safety of topiramate monotherapy in civilian posttraumatic stress disorder: a randomized, double-blind, placebo-controlled study. J clin psychiatry. 2007;68(2):201–6.
Hertzberg MA, Butterfield MI, Feldman ME, Beckham JC, Sutherland SM, Connor KM, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;45(9):1226–9.
Kinrys G, Wygant LE, Pardo TB, Melo M. Levetiracetam for treatment-refractory posttraumatic stress disorder. J clin psychiatry. 2006;67(2):211–4.
Hamner M, Ulmer H, Horne D. Buspirone potentiation of antidepressants in the treatment of PTSD. Depression and Anxiety. 1997;5(3):137–9.
Neylan TC, Lenoci M, Samuelson KW, Metzler TJ, Henn-Haase C, Hierholzer RW, et al. No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry. 2006;163(12):2186–8.
Gupta MA, Knapp K. Lithium carbonate decreases acute suicidality in posttraumatic stress disorder. Aust N Z j psychiatry. 2013;47(12):1217.
Pradhan B, Kluewer D'Amico J, Makani R, Parikh T. Nonconventional interventions for chronic post-traumatic stress disorder: ketamine, repetitive trans-cranial magnetic stimulation (rTMS), and alternative approaches. J trauma dissociation : off j Int Soc Study of Dissociation (ISSD). 2016;17(1):35–54.
Wynn GH. Complementary and alternative medicine approaches in the treatment of PTSD. Curr psychiatry rep. 2015;17(8):600.
McLay RN, Loeffler GN, Wynn GH. Research methodology for the study of complementary and alternative medicine in the treatment of military PTSD. Psychiatr Ann. 2013;43(1):38–43.
Price M, Spinazzola J, Musicaro R, Turner J, Suvak M, Emerson D, et al. Effectiveness of an extended yoga treatment for women with chronic posttraumatic stress disorder. J altern complement med (New York, NY). 2017;23(4):300–9.
van der Kolk BA, Stone L, West J, Rhodes A, Emerson D, Suvak M, et al. Yoga as an adjunctive treatment for posttraumatic stress disorder: a randomized controlled trial. J clin psychiatry. 2014;75(6):e559–65.
Rhodes A, Spinazzola J, van der Kolk B. Yoga for adult women with chronic PTSD: a long-term follow-up study. J altern complement med (New York, NY). 2016;22(3):189–96.
Pradhan B, Gray R, Parikh T, Akkireddi P, Pumariega A. Trauma interventions using mindfulness based extinction and reconsolidation (TIMBER©) as monotherapy for chronic PTSD: a pilot study. Adolesc Psychiatry. 2015;5(2):125–31.
Engel CC, Cordova EH, Benedek DM, Liu X, Gore KL, Goertz C, et al. Randomized effectiveness trial of a brief course of acupuncture for posttraumatic stress disorder. Med Care. 2014;52(12 Suppl 5):S57–64.
Kim Y-D, Heo I, Shin B-C, Crawford C, Kang H-W, Lim J-H. Acupuncture for posttraumatic stress disorder: a systematic review of randomized controlled trials and prospective clinical trials. Evid Based Complement Alternat Med. 2013;2013:12.
Lee EE, Della Selva MP, Liu A, Himelhoch S. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen Hosp Psychiatry. 2015;37(2):178–84.
Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–8.
Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur neuropsychopharmacol : j Eur College of Neuropsychopharmacol. 2011;21(11):796–809.
Drexler SM, Wolf OT. The role of glucocorticoids in emotional memory reconsolidation. Neurobiol Learn Mem. 2017;142(Pt A):126–34.
Delahanty DL, Gabert-Quillen C, Ostrowski SA, Nugent NR, Fischer B, Morris A, et al. The efficacy of initial hydrocortisone administration at preventing posttraumatic distress in adult trauma patients: a randomized trial. CNS Spectr. 2013;18(2):103–11.
Suris A, North C, Adinoff B, Powell CM, Greene R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Ann clin psychiatry : off j Am Acad Clin Psychiatrists. 2010;22(4):274–9.
Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–97.
• Mataix-Cols D, Fernandez de la Cruz L, Monzani B, Rosenfield D, Andersson E, Perez-Vigil A, et al. D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: a systematic review and meta-analysis of individual participant data. JAMA Psychiatry. 2017;74(5):501–10. In depth evaluation of d-cycloserine as a potential adjunctive treatment.
• McGuire JF, Wu MS, Piacentini J, McCracken JT, Storch EA. A meta-analysis of d-cycloserine in exposure-based treatment: moderators of treatment efficacy, response, and diagnostic remission. J clin psychiatry. 2017;78(2):196–206. A further consideration of d-cycloserine.
Ori R, Amos T, Bergman H, Soares-Weiser K, Ipser JC, Stein DJ. Augmentation of cognitive and behavioural therapies (CBT) with d-cycloserine for anxiety and related disorders. Cochrane Database Syst Rev 2015(5):CD007803. https://doi.org/10.1002/14651858.CD007803.pub2.
• Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Martin SF, Yazar-Klosinski B, et al. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. J Psychopharmacol. 2013;27(1):28–39. Describes one of the major trials carried out on MDMA-assisted psychotherapy.
Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25(4):439–52.
Carhart-Harris RL, Murphy K, Leech R, Erritzoe D, Wall MB, Ferguson B, et al. The effects of acutely administered 3,4-methylenedioxymethamphetamine on spontaneous brain function in healthy volunteers measured with arterial spin labeling and blood oxygen level-dependent resting state functional connectivity. Biol Psychiatry. 2015;78(8):554–62.
Sessa B, Nutt D. Making a medicine out of MDMA. Br J Psychiatry. 2015;206(1):4–6.
Amoroso T, Workman M. Treating posttraumatic stress disorder with MDMA-assisted psychotherapy: a preliminary meta-analysis and comparison to prolonged exposure therapy. J Psychopharmacol. 2016;30(7):595–600. A meta-analysis of studies using MDMA in PTSD
Passie T, Emrich HM, Karst M, Brandt SD, Halpern JH. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug test anal. 2012;4(7–8):649–59.
Wilkinson ST, Radhakrishnan R, D'Souza DCA. Systematic review of the evidence for medical marijuana in psychiatric indications. J clin psychiatry. 2016;77(8):1050–64.
Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–8.
• Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J clin psychiatry. 2015;76(9):1174–80. Discusses reasons to be cautious with the use of marijuana in PTSD.
O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–16.
Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanhã C, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J clin psychiatry. 2010;71(8):992–9.
Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161(3):515–24.
Watts BV, Landon B, Groft A, Young-Xu YA. Sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain stimul. 2012;5(1):38–43.
Langevin JP, Koek RJ, Schwartz HN, Chen JW, Sultzer DL, Mandelkern MA, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82–4.
George MS, Ward HE Jr, Ninan PT, Pollack M, Nahas Z, Anderson B, et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul: Basic Trans Clin Res Neuromodulation. 2008;1(2):112–21.
Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT. Br J Psychiatry. 2010;196(3):226–34.
Fosse R, Read J. Electroconvulsive treatment: hypotheses about mechanisms of action. Front Psychiatry 2013;4:1-10. https://doi.org/10.3389/fpsyt.2013.00094.
Baghai TC, Möller HJ. Electroconvulsive therapy and its different indications. Dialogues Clin Neurosci. 2008;10(1):105–17.
Margoob MA, Ali Z, Andrade C. Efficacy of ECT in chronic, severe, antidepressant- and CBT-refractory PTSD: an open, prospective study. Brain stimulation. 2010;3(1):28–35.
Youssef NA, McCall WV, Andrade C. The role of ECT in posttraumatic stress disorder: a systematic review. Ann clin psychiatry : off j Am Acad Clin Psychiatrists. 2017;29(1):62–70.
Bernardy NC, Friedman MJ. Psychopharmacological strategies in the management of posttraumatic stress disorder (PTSD): what have we learned? Curr psychiatry rep. 2015;17(4):20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
In the past 3 years, Dr. Stein has received research grants and/or consultancy honoraria from Biocodex, Lundbeck, Servier, and Sun. Dr. Starke declares no conflict of interest.
Human and Animal Rights and Informed Consent
With regard to the authors’ research cited in this paper, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. In addition, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
This article is part of the Topical Collection on Anxiety, Obsessive Compulsive, and Related Disorders
Rights and permissions
About this article
Cite this article
Starke, J.A., Stein, D.J. Management of Treatment-Resistant Posttraumatic Stress Disorder. Curr Treat Options Psych 4, 387–403 (2017). https://doi.org/10.1007/s40501-017-0130-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40501-017-0130-0