Abstract
High-resolution ultrasound is the most common imaging technique used to supplement the physical examination of scrotum and penis with great accuracy in assisting the diagnosis of the various pathologies of male genital system, with the highest diagnostic potential in emergency conditions. Technical advancements in real-time high-resolution, color flow Doppler sonography and contrast enhanced ultrasonography (CEUS) have led to an increase in the clinical applications of scrotal and penile sonography. In this pictorial review we focus on common and uncommon male genitalia emergency with special emphasis on the role of ultrasound assessment and its specific findings to improve diagnostic accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-resolution ultrasound (US) is the most common imaging technique used to supplement physical examination of scrotum and penis with great accuracy in assisting the diagnosis of the various pathologies of male genital system, with the highest diagnostic potential in emergency conditions.
In this pictorial review we focus on common and uncommon male genitalia emergency with special emphasis on the role of US assessment and its specific findings starting from an overview of testicular and appendage torsion, discussing testicular and epididymis inflammations as well as their complications and Fournier's gangrene; also representing pictorial-cases of post-surgical testicular ischemia, hypertensive hydrocele, testicular compartment syndrome, then discuss in more details about trauma and post-traumatic complications both of the testicle and penis and finally, analysing and differentiating the high-flow and low-flow priapism, identifying the Mondor disease and discussing the inflammatory complications of the penis too.
Technique and anatomy
Scrotum and penis are examined in the supine position, better with a towel placed under the scrotum and with the penis dorsiflex on the patient’s abdomen, with high-frequency (7.5–15 MHz) linear probe, if possible in virtual convex mode to widen the field of view, and in some cases recurring to the use of low-frequency probes (5 MHz or lower) if greater penetration is required [1, 2]. Color- or power flow and pulsed Doppler US are also adopted to evaluate testicular and penis blood flow that is a crucial finding in the emergency setting [1, 2].
Scrotum
The scrotum is assessed through longitudinal and transverse planes starting with B-mode scans and comparing testis and epididymis to the contralateral side, scanning first the asymptomatic side to set the gray-scale and color—Doppler gains. The split-screen mode is useful for side-to-side comparison. Color- and pulsed-Doppler should be optimized to depict low-flow velocities with pulse repetition frequency (PRF) setting to the lowest velocity possible (Fig. 1a, b); three spectral Doppler recordings should be obtained in each testicle (upper, middle and lower thirds). Power—Doppler may be used to visualize the intratesticular blood flow as well as specific technologies that enhance microvascular flow (e.g. superbe microvascular imaging—SMI) (Fig. 1c, d, e). Testis shows homogeneous echogenicity with a mildly coarse echotexture. The tunica albuginea appears as an echogenic outline of the testicle and forms the mediastinum testis (Fig. 1. f). The epididymal head is a round structure isoechoic or mildly hypoechoic (thickened 5–12 mm), the epididymal body extends down the posterior aspect of the testicle (thickness 2–4 mm) and the epididymal tail curve at the inferior pole, becoming ductus deferens (thickness 2–5 mm) (Fig. 1g) [1, 2]. The velocity waveform of normal capsular and intra-testicular arteries shows low vascular resistance of the testis represented by high levels of antegrade diastolic flow throughout the cardiac cycle (Fig. 1h, i) [1]. In the investigation of the acute scrotum the diagnostic goal is the differentiation between hypovascular and avascular lesions, identifying the residual healthy parenchyma after twisting or derotation, after trauma or inflammatory events, and characterizing some complications, such as abscesses, which can be equivocal and misinterpreted at B-mode and color-Doppler too. Further to this point, contrast enhancement US (CEUS) provides a practical solution, increasing the confidence in the interpretation of lesion vascularity and of scrotal and cord vessels, allowing for appropriate clinical management [3]. CEUS is usually performed with a linear array transducer and a low mechanical index (MI = 0.07–0.08). The ultrasound contrast-medium consists of inert filling gas, such as sulfur hexafluoride, encapsulated by phospholipid shells (SonoVue, Bracco) injected intravenously (2.4–4.8 mL) through a 20-gauge cannula and followed by 10 mL saline flush. After contrast agent administration, testis and epididymis enhance rapidly: the arteries enhance first followed by complete and progressive fill-in of the parenchyma within a few seconds; after that, the enhancement declines over a variable period of time such that there is minimal residual enhancement by three minutes (Fig. 1l, m, n) [3].
a, b. PRF setting. High (a) and low (b) PRF setting show a better evidence of intra-testicular flow at low setting (white box; a, b). c, d, e Trans-mediastinal testicular vessels. Longitudinal scans of testis with power-Doppler (c), color-Doppler (d) and superb microvascular imaging (SMI) (e) mode show an artery branching from the mediastinum testis and running within the parenchyma. f Mediastinum testis and albuginea. Longitudinal B-mode scan of testis shows a normal homogeneous echotexture of testis with a mediastinum testis (arrow) as a linear echogenic band of fibrofatty tissue; a thin echoic line surrounds the testis and corresponds to the tunica albuginea (arrowheads). g Head of epididymis. Longitudinal B-mode scan of testis with virtual convex field of view shows the head of epididymis (arrows) with a normal homogeneous echotexture. T: testis. h, i Flow of trans-testicular arteries. Longitudinal scan of testis shows normal testicular arteries representation at color-Doppler mode (h) with low-impedance waveform with large amount of end diastolic flow at pulse-Doppler mode as well as low resistance pattern (i). l, m, n Normal testicular anatomy at CEUS. Longitudinal scans of testis after contrast agent administration show: Testicular arteries enhance first (l) followed by a complete fill-in of the parenchyma and a progressive late washout (n). (white box: time of the scans; l, m, n). o Normal penis anatomy. Transverse B-mode ventral scans of the penis at the basis shows two hypoechoic images (C) corresponding to the corpora cavernosa with a thin echoic line that surrounds them and corresponds to the tunica albuginea (arrowheads); the corpus spongiosum (S) is adjacent to the corpora cavernosa. p Cavernous artery. Longitudinal, ventral scan of the penis, with color-Doppler and pulsed mode shows a normal flow pattern of the cavernous artery (arrow) in a flaccid penis
Penis
Penis examination starts from the glans to the base through longitudinal and transverse scans with B-mode at first. The vascular pattern is evaluated by color-Doppler; filters are set at their lowest levels and the Doppler gain just below the noise threshold; PRF is set to the lowest velocity setting possible. The corpora cavernosa are homogeneous and relatively hypoechoic cylindrical structures lined with tunica albuginea, a thin echogenic membrane that has a thickness of approximately 2 mm when the penis is flaccid (Fig. 1o); medial corpus spongiosum is more echoic than the corpora cavernosa (Fig. 1o). Velocity waveforms of the cavernous arteries normally vary from high velocity and low resistance to a progressive increase of the resistance according to erection stages (Fig. 1p) [1, 2]. In Table 1 we have summarized some advice to the US setting with normal testis and penis US findings.
Testicular pathology
Testicular torsion
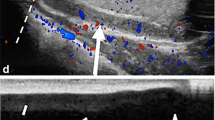
Torsion of testis is among the major causes of acute scrotal pain as a primary disease of adolescents and neonates. It is the most common cause of testicular loss in these age groups. However, torsion may occasionally occur in men 40–50 years old. Torsion can occur with sports or physical activity; it can be related to trauma in 4–8% of cases or can spontaneously develop [4]. At US, varying degrees of heterogeneous parenchymal echotexture might be seen in testicular torsion within 1 to 6 h [1]. In the early phase, immediately after the onset of the torsion, US reveal no abnormality, becoming progressively heterogeneous and hypoechoic, compared with the contralateral normal testis, due to the edematous infiltration (Fig. 2b–e). Significant heterogeneity and enlargement are suggestive for late torsion and testicular non-viability (Fig. 2f). The asymmetrical dampened spectral waveform in the affected testis and absence or reversal of diastolic flow suggests testicular torsion in the proper setting [5]. Both the intensity of the symptoms and the degree of testicular ischemia are closely associated to the timing of the torsion, the number of twists of the spermatic cord, and the grade of the compressed spermatic cord vessels. To a complete arterial occlusion is necessary a torsion at least 540 degrees with its characteristic US “whirlpool pattern” [1]. The presence of testicular blood flow in a patient with a typical clinical presentation for torsion does not exclude the diagnosis of a partial testicular torsion. The latter could be, in inexperienced hands, a confounding factor since on color-Doppler, in a partial torsion of fewer than 360 degrees, the arterial flow may still be represented; however, in this condition a diminished diastolic arterial flow with high resistance pattern on spectral Doppler examination, due to venous outflow obstruction, in addition to a twisted spermatic cord, may help to establish the correct diagnosis (Fig. 2g) (video 1) [1, 6]. The use of CEUS may also improve the sensitivity of detecting blood flow in the scrotum and to better identify segmental infarction or sub-acute segmental infarctions (Fig. 2h, i, l) [1, 3]. In Table 2 are summarized the US features of testicular torsion degree.
a Acute torsion. Longitudinal color-Doppler scan of the right and left testis shows no flow in the right testis (arrow; R: right) compared to the left (L: left). However, the parenchyma still shows a preserved ecostructure. b Sub-acute torsion. Longitudinal B-mode scan of testis shows a fine inhomogeneity of the parenchyma with poor evidence of the mediastinum testis (arrow). c, d Sub-acute torsion. Transverse B-mode scan (c) and color-Doppler scan (d) of testis show an enlarged hypoechoic testis (c) and absent flow within the testis with surrounding hyperemia. e Intermittent torsion. Longitudinal color-Doppler scan of testis shows a widely uneven eco-structure as a result of intermittent twisting but with represented flow. f Chronic torsion. Longitudinal B-mode scan of both testis shows heterogenous echotexture of the torsed right testis (arrow) which also appears increased in volume compared to the left as a result of hemorrhagic infarction. g Flow pattern in incomplete torsion. Longitudinal color-Doppler scan of testis with pulse mode shows increase in flow’s resistance of the intra-testicular arteries with reversal of flow in diastole caused by edema impeding venous flow. h, i, l After detorsion parenchymal testis viability. Longitudinal B-mode (h) and transverse scans with superbe microvascular imaging (i) and after contrast agent administration (l) show an inhomogeneously hypoechoic area (arrows; h) not vascularized at superbe microvascular imaging (arrows; i) which however appears much more extensive at CEUS (arrow; l) with peritesticular hyperemia
Torsion of the testicular appendage
Torsion of the testicular appendage is a common cause of acute scrotal pain and may clinically mimic a testicular torsion [1]. At US, an appendix testis with spherical shape and size larger than 5–6 mm with no internal blood flow and increased peri-appendiceal vascular signals is strongly suggestive of the torsion of appendix testis (Fig. 3a, b) [4]. It may be either hypoechoic or hyperechoic in comparison with the testis or epididymis echo texture and is often associated with a reactive hydrocele and skin thickening. The classical physical examination finding is the “blue dot” sign, referring to a palpable, small, and firm nodule on the upper pole of the testis (Table 3) [5].
Epididymitis/epididymo-orchitis
Epididymitis is the most common cause of acute scrotal pain in post-pubertal men, causing 75% of all acute intra-scrotal inflammatory processes of which the most frequent pathogens are Escherichia coli, Pseudomonas, and Klebsiella [1]. Usually, it is due to lower urinary tract infections but there are also other causes such as hematogenous or post-traumatic spread [1]. In up to 20% of patients the direct extension of epididymal inflammation to the testicle, causing the so-called epididymal-orchitis [1]. At US an inflamed epididymis may appear enlarged and heterogeneous focally or diffusely affected with varying degrees of echogenicity which can be respectively: hypoechoic relative to the testicle in case of edema, otherwise hyperechoic in case of hemorrhagic findings [7]. In the context of a diffusely swollen testicle with low reflectivity, well-defined patchy areas of low reflectivity may later appear (Fig. 4a, b) [8]. An occasional finding is a reactive hydrocele with thickening of the overlying skin. A focal hypoechoic area in the testicular hilum may mimic a testicular tumor but the pattern of vascular distribution on color-Doppler may demonstrate a linear pattern with the hyperemia associated with inflammation, suggesting orchitis. Color-Doppler shows increased peri-testicular perfusion and maybe the only finding of acute epididymitis (Fig. 4c–f) [9]. In the acute phase, there is an increased flow within the tunica vasculosa, radiating from the mediastinum testis out to the periphery, as lines of color flow. The PRF and filtration must be set low and a small color-sampling box should be used. To exclude technical reasons as the cause of absent flow is essential the comparison with the contralateral, asymptomatic side, is achieved by including both testes on the screen at the same time. This allows, also, the diagnosis of eventual unilateral increased flow to be made. A typical striated testicular pattern is seen in the case of fibrosis secondary to an inflammatory process and goes in differential diagnosis with infiltrative process such as lymphoma or leukemia [10]. In Table 3 we have summarized the clinical findings and US key features of epididymo-orchitis.
a, b Acute epididymitis. Longitudinal B-mode (a) and color-Doppler mode (b) scans of the tail of the epididymis show an enlargement and a heterogeneous echotexture (arrows; a) with an increased flow. c, d, e, f Acute epididymitis with spermatic cord extension. Longitudinal color-Doppler mode scans (c, d) of the head (c) and tail of epididymis and longitudinal B-mode (e) and color-Doppler mode scans (f) of the spermatic cord show an enlargement and increased flow of the epididymis extended to the spermatic cord that appears congested (arrows; e). g, h Orchitis. Transverse B-mode (g) and color-Doppler mode (h) scans of the testis show an enlargement and a heterogeneous echotexture of testis (g) with an increased flow (h). i, l Epididymal abscess. Transverse B-mode (i) and color-Doppler mode (l) scans of the tail of the epididymis (stars, i, l) shows a focal low reflective area (arrows; i, l) without vascularity within and with increased surrounding vascularity
Primary orchitis
Isolated orchitis may also occur especially in the pediatric population and are often associated with viral mumps infections [1, 11]. At the US, the testis appears usually hypoechoic, sometimes heterogeneous, and hyperemic with the increased flow at color—Doppler (Fig. 4g, h); in one-third of patients, the epididymis is enlarged and hyperemic too (Table 3) [10].
Epididymis/testicular abscess
An epididymis or testicular abscess may develop secondary to severe epididymo-orchitis, or may be secondary to mumps, trauma, or infarction. The US may depict a corpuscular fluid collection, or a markedly hypoechoic mass with unclear edges, surrounded by a hypoechoic halo, in rare cases gas bubbles are observed in the abscess cavity and they appear as focal hyperechoic spots with posterior shadowing; color-Doppler shows a lack of flow centrally with peripheral hypervascularity (Fig. 4i, l). CEUS may be able to determine the development of an abscess at an earlier stage, or the complete extent of a large abscess (unenhanced area surrounded by a hyper enhanced rim) and allow for prompt treatment (Table 3) [3, 12, 13].
Epididymitis-related testicular infarction
Epididymitis-related testicular infarction is uncommon and may be due to the acute onset of edema of epididymitis, which compromises the venous drainage of the testes with the interruption of the arterial flow and subsequently thrombosis formation, or maybe due to bacterial toxins that cause endothelial damage of vessels, which results in thrombus formation [14]. At the US, testis appears diffusely or partially hypoechoic with segmental areas wedge shape with no detectable flow within it and without enhancement on CEUS (Fig. 5a–c) (Table 3).
a, b, c Epididymitis-related testicular infarction. Longitudinal (a) and transverse (b) color-Doppler mode scans of testis show a focal avascularized area of low reflectivity (arrows, b) at the upper aspect of the testis surrounded by a perilesional hyperemia (star; b). The transverse scans obtained after administration of contrast medium (c) confirms that the area is completely avascular (arrows; c) and identifies a perilesional rim of enhancement (stars; c)
Testicular ischemia after inguinal hernia repair
Complications after inguinal hernia repair occur in 1.7–8% of all cases [15]. One of these complications is testicular infarction. US appearance of testicular ischemia results in volume increase during acute-subacute phases and volume reduction during the chronic phase. Testicular parenchyma appears hypoechoic, with no detectable flow within (Fig. 6a–e) (Table 3) [15]. In subacute or chronic phases peritesticular hyperemia occurs, due to the anastomoses with epididymal and deferential arteries. Hypertrophy of cremasteric vessels can be seen in chronic phases.
a, b, c, d, e Testicular ischemia after inguinal hernia repair. Longitudinal color-Doppler mode scans of testis (a, b, c) and the spermatic cord (d) show a focal low reflective necrotic area (star; a, b, c) into a diffuse avascularised testis and haemorrhagic infarction of the cord (arrows; d) after inguinal hernia repair. The operative exploration confirms the US finding and haemorrhagic infarction of the cord (arrows; e—case of dr. Antonio Brillantino)
Fournier’s gangrene
Fournier’s gangrene is a rapidly progressing necrotizing fasciitis involving the perineal, perianal, or genital regions, caused by both aerobic and anaerobic bacterial flora [16]. Usually, there is thickened and swollen scrotal wall and multiple hyperechoic–hyperreflective spots with posterior acoustic “dark” shadowing, in relation to gas bubbles, associated with a typical crackling during the compression of the probe (Fig. 7a–c) (Table 3); an increased flow at color-Doppler is also typical [17].
a, b, c, d Fournier’s gangrene. Longitudinal B-mode scan of the scrotal sac (a) shows scrotal wall thickening with multiple areas of high reflectivity (arrows; a) in the subcutaneous tissue representing gas formation. Longitudinal color-Doppler scan (b) of the onside testis shows normal parenchymal echotexture with flow pattern within. Computed tomography on axial view and lung window (c) confirms the scrotal wall thickening and subcutaneous gas (arrows; c), consistent with Fournier’s gangrene. Perineal and scrotal region after necrosectomy (d—case of dr. Giuseppe Romano). From Di Serafino et al. [16]
Compartment syndrome
The compartment syndrome is a condition in which the vasculature of the testis is compromised due to increased venous resistance (varicocele hypertension, testicular torsion, hernia compression of veins, or intratesticular arteriovenous fistula) or extraluminal vessels compression [18]. The latter may be intravaginal (hemorrhage/hematoma, hematocele, hydrocele, chylocele, lymphocele, pneumoscrotum, orchitis/epididymo-orchitis) or extravaginal (fasciitis, cryptorchidism) too. Between these, the hypertensive hydrocele is the main cause of testicular compartment syndrome since the pressure within the hydrocoele exceed tissue perfusion pressures with decreased microcirculation perfusion until to complete absence of blood flow which leads to hypoxia and reperfusion injury risk (Fig. 8a–d) (Table 3) [18].
a, b, c, d Compartment syndrome. Longitudinal B-mode (a), color-Doppler (b) and power-Doppler (c) scans of right testis (arrows; a, b, c) in neonate with hypertensive hydrocele (star; a) show homogenous echotexture of testis without any vascular flow within compared to the left side (b); the testis is also marginalized. Transverse color-Doppler (e) scan after decompression shows an increased blood flow within right testis (arrow; d)
Testicular trauma
Testicular trauma is the third most common cause of acute scrotal pain. Timely diagnosis is crucial to avoid therapeutic failure. Approximately 90% of ruptured testicles can be saved if surgery is performed within the first 72 h, whereas only 45% may be saved after 72 h [1]. The US is an irreplaceable diagnostic tool since the clinical diagnosis is often made difficult by the intense scrotal pain and swelling [1]. US findings may vary from a small hematocele to a testicular rupture. Findings of a heterogeneous echotexture within the testis, testicular contour abnormality, and disruption of the tunica albuginea are considered very sensitive and specific for the diagnosis of testicular rupture in an appropriate clinical setting [19]. Evidence of rupture could be suggested by the abnormality in the contour of the testis, resulting from the extrusion of testicular parenchyma after disruption of the tunica albuginea, obscured by a large extratesticular hematocele or large scrotal wall hematoma (Fig. 9a–d). When the tunica is ruptured, the underlying testicular parenchyma is almost always injured. However, a heterogeneous echotexture also may be seen in the presence of intratesticular hematomas without a tunica albuginea rupture (Fig. 9e, f). Suspected acute hematomas are re-examined within 12–24 h after the initial US evaluation because hyperacute and acute hematomas may appear isoechoic to the surrounding testicular parenchyma or may have a diffusely heterogeneous echotexture [19]. Chronic hematomas appear more hypoechoic to anechoic and tend to decrease in size as they resolve. The absence of vascularity at color-Doppler in a focal area of the testis as well as at CEUS may be secondary to an intratesticular hematoma. The tunica vasculosa and the tunica albuginea are in close apposition, so the rupture of tunica albuginea is almost always associated with a disruption of the tunica vasculosa. As a result, the rupture of the testis results in a loss of vascularity to a portion or the entirety of the testis, depending on the grade of injury [19]. A testicular fracture refers to a break or discontinuity in the normal testicular parenchyma. A testicular fracture line is identified as a linear hypoechoic and avascular area within the testis, a finding that may be or may not be associated with a tunica albuginea rupture [2]. Besides integrity or interruption of the tunica albuginea, the most important information for the surgeon is the extent of viable testicular tissue, which is a difficult data interpretation at B-mode and color-Doppler exam as well, because of a post-traumatic parenchyma often appears hypovascular due to edema infiltration; fracture lines often can not be seen directly. In this regard CEUS allows the best differentiation between viable vascularized tissue from nonviable devascularized tissue, enabling organ-sparing treatment; fracture lines and intra-testicular hematomas are also better defined by CEUS (Fig. 9g, h, i, l) [3, 20]. In Table 3 we have summarized the clinical findings and US key features of testicular trauma.
a, b Injury of the scrotal wall. Transverse B-mode (a) and color-Doppler (b) scans show diffuse, inhomeogeneous thickening of the scrotal wall (arrows; a, b), consistent with blood extravasation. The testis (T; a, b) is intact with normal blood flow within (b). c, d Extra-testicular hematoma. Longitudinal B-mode (c) and color-Doppler (d) mode scans in two different patient show a hyperechoic subacute hematoma on sub-albuginea side (arrows; c) and chronic hypoechoic hematoma on extra-albuginea side (arrows; d) without albuginea tears (arrowheads; c, d). e Intra-testicular hematoma without tunica tears. Longitudinal color-Doppler mode scan (e) of upper pole of the testis shows an avascularized hypoechoic heterogeneous area referred to hematoma (arrows) with tunica intact (arrowheads). f Intra-testicular hematoma with tunica tear. Longitudinal color-Doppler mode scan (f) of the anterior surface of the testis shows an avascularized hypoechoic heterogeneous area referred to hematoma (arrows) with disruption of the tunica (arrowheads). Surgery confirmed testicular rupture. g, h, i, l Testicular rupture. Longitudinal B-mode scans of the right (R; g) and left testis (L; h) after a motorcycle accident, a well defined hematoma of the right testis (arrows; g) and diffuse parenchymal distortion of the left testis with contour irregularity referred to rupture (arrows; h) is shown; the corresponding CEUS findings of the right (i) and left testis (l) define better the areas of avascular traumatic impairment (caliper; i, l) with a large part of the right parenchyma vital (star; i) unlike the left. The right testis was rescued at surgery while the left has been removed
Penile pathology
Penile trauma
Penile fracture is described as the rupture of the tunica albuginea and/or corpus spongiosum in the erect penis caused by rapid blunt force. Most commonly, penile fractures involve one of the corpora cavernosa. In 10–20% of cases, the rupture of the albuginea is associated with a urethral injury [21, 22]; when the Buck fascia is intact, the hematoma is confined to the penis, while when the Buck fascia is injured, it tends to extend to the scrotum, pubis and perineum, delimited exclusively by the Colles fascia. The US can show the integrity of the tunica albuginea as well as the extent and location of a tunical tear or any hematoma on either side of the tunica. The timing of the trauma influences the US aspects of the hematoma which could be iso/hyperechoic or hypoechoic/anechoic respectively in acute or in sub-acute/chronic phase according to hemoglobin degradation. US has the potential to better localize the hematoma within the corpus cavernosum, into the septal area, or sometimes outside the tunica albuginea (Fig. 10a–d). Injury to the sub-tunical venous plexus, or the smooth muscle trabeculae in the absence of complete tunica disruption, can lead to cavernosal hematomas. If an arterial lesion is present, a post-traumatic arterio-venous fistula with increased venous pressures and dilatations of the injured vessel may result [23]. When trauma occurs with the penis in a flaccidity state, is most commonly observed the development of intracavernous or extracavernal hematomas, but the albuginea remains unharmed. Color-Doppler allows defining hematomas, to confirm the integrity of the albuginea tunic and to evaluate the penile vessels (Fig. 10e, f). In Table 4 we have summarized the clinical findings and US key features of penile trauma.
a Extra-albuginea hematoma. Transverse B-mode scan shows a hematoma (arrows) adjacent to the left corpus cavernosum (C); no fracture was identified in the tunica albuginea (arrowheads). b Intra-cavernosus hematoma without tunica tears. Longitudinal color-Doppler mode scan on right lateral view shows an avascularized intra-cevernosus hematoma (arrow) without tunica albuginea tear (arrowhead). c Intra-cavernosus hematoma with tunica tear. Longitudinal B-mode scan on left lateral view shows rupture of the tunica albuginea (arrowhead) with an adjacent hematoma (arrow) due to trauma. d Urethral leak. Transverse B-mode scan at the basis of the penis shows a large corpuscolate collection suggestive for hemo-urinoma (arrows) due to post-traumatic urethral leak. (U: urethra). e, f Pseudoaneurysm. Transverse B-mode and color-Doppler mode (e) scan show a hypoechoic collection (star; e) referred to hematoma with pseudoaneurysm (arrows; e) secondary to traumatic injury of the penis. Digital subtraction angiogram (f) shows an area of abnormal blush at the penile base that corresponds to the pseudoaneurysm (arrow; f—case of dr. Giuseppe de Magistris)
Priapism
Priapism is a prolonged tumescence or erection unrelated to sexual stimulation that lasts more than 6 h [23]. The erection is limited to the corpora cavernosa without affecting the corpus spongiosum. The two different classes of priapism are low-flow priapism (LFP) and high-flow priapism (HFP) (Table 4) [23]. LFP is the most common type and results from failure of the detumescence mechanism. Prolonged low-flow priapism leads to a painful ischemic state, which can cause fibrosis of the corporeal smooth muscle and cavernosal artery thrombosis. The degree of ischemia is a function of the number of emissary veins involved and the duration of occlusion. Color-Doppler demonstrates the absence of flows in the cavernous arteries or flows with low speed and high resistances (Fig. 11a) (Table 4). The fibrosis appears hyperechoic compared to the normal echogenicity of the corpora cavernosa [24]. High-flow priapism is the result of uncontrolled arterial inflow from a fistula between the cavernosal artery and the corpus cavernosum. At US, the area of laceration of the artery and of the cavernous tissue appears hypoechoic in the context of the cavernous tissue that presents intense color signal on Doppler. At color-Doppler fistula sampling documents turbulent flows with high peak velocity (Fig. 11b) (Table 4) [23].
a Low flow priapism. Transverse color-Doppler mode scan shows the corpora cavernosa (C) with a coarsened heterogeneous echotexture, a finding consistent with congestion without blood flow in the substance of the corpora. b High flow priapism. Transverse color-Doppler mode with pulse mode scan shows a right arterio-venous cavernous fistulas (arrow) with turbulent high-flow pulse pattern. c, d Mondor disease. Transverse (c) and longitudinal (d) dorsal color-Doppler mode scan of the penis at the basis show an increased calibre of the superficial dorsal vein (arrows; c, d), which is filled with echogenic material (arrows; d) without blood flow referred to thrombosis in leukemic patient
Mondor disease
Penile Mondor disease also known as penile superficial venous thrombosis, is a rare, self-limiting process, characterized by a lack of flow to and non-compressibility of the superficial dorsal vein of the penis referred to thrombosis that typically affects sexually active young men [21]. On US the lumen of the superficial dorsal vein is occupied by echogenic material corresponding to the thrombosis; the vein appears as an echogenic cord with no color filling and flow spectrum at color-Doppler US mode [21]. The latter is an important tool in the diagnosis and in the follow-up therapy (Fig. 11c, d) (Table 4).
Penile abscess
A cavernosum abscess is a rare condition. It can be idiopathic or with an underlying cause such as intracavernosal injection therapy, foreign bodies, perineal abscesses extension, priapism or trauma [25]—US permits to detect, in patients with penile inflammation extended to erectile tissues, intra-cavernous hypoechogenic areas, with no definite edges and stratified thickness, similar to onion skin. Abscess presents as a hypoechoic pool with an irregular profile including floating echoes inside, located in erectile bodies or between the lining bands (Fig. 12a–d) (Table 4).
a, b, c, d Penis abscess. Transverse ventral B-mode scan of the penis at the basis shows an extensive collection (arrows; a) with a mixed ecostructure engaging the extra-albugine space, imprinting the left cavernous body. Longitudinal color-Doppler mode (b) scans shows the perineal extension of the collection (arrows; b) without any blood flow within. Magnetic resonance images on axial T2w (a) and T1w post-contrast media on axial (d) and coronal (e) view confirm the extent and heterogeneity of the collection (arrows; c, d, e) which appears non-vascularized and suggestive for extra-albugine abscess. C corpora cavernosa. S corpus spongiosum
Conclusion
The US is the main imaging modality for the evaluation of male-genital emergency pathologies. Technical advancements in high-resolution real-time, color flow Doppler sonography, and CEUS have led to an increase in the clinical applications of scrotal and penile sonography and make it a fundamental tool in emergency diagnosis.
References
Gorman B (2011) The scrotum. In: Rumack CM (ed) Diagnostic ultrasound, 4th edn. Elsevier, Philadelphia, pp 840–877
Pozniak MA, Allan P (2014) Clinical Doppler Ultrasound—ClinicalKey. In: Churchill Livingstone Elsevier 3rd edn, pp261–294
Sidhu PS, Cantisani V, Dietrich CF et al (2017) The EFSUMB Guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (Long Version). Ultraschall Med 39(2):e2–e44. https://doi.org/10.1055/a-0586-1107
Dogra VS, Bhatt S, Rubens DJ (2006) Sonographic evaluation of testicular torsion. Ultrasound Clin 1(1):55–66
Prando D (2009) Torsion of the spermatic cord: the main gray-scale and Doppler sonographic signs. Abdom Imaging 34(5):648–661. https://doi.org/10.1007/s00261-008-9449-8
Esposito F, Di Serafino M, Mercogliano C et al (2014) The “whirlpool sign”, a US finding in partial torsion of the spermatic cord: 4 cases. J Ultrasound 17:313–315. https://doi.org/10.1007/s40477-014-0095-4
Kühn AL, Scortegagna E, Nowitzki KM, Kim YH (2016) Ultrasonography of the scrotum in adults. Ultrasonography (Seoul, Korea) 35(3):180–197. https://doi.org/10.14366/usg.15075
Cook JL, Dewbury K (2000) The changes seen on high-resolution ultrasound in orchitis. Clin Radiol 55(1):13–18. https://doi.org/10.1053/crad.1999.0372
Patiala B (2009) Role of color doppler in scrotal lesions. Indian J Radiol Imaging 19(3):187–190. https://doi.org/10.4103/0971-3026.54874
Casalino DD, Kim R (2002) Clinical importance of a unilateral striated pattern seen on sonography of the testicle. AJR Am J Roentgenol 178(4):927–930. https://doi.org/10.2214/ajr.178.4.1780927
Tarantino L, Giorgio A, De SG et al (2001) Echo color Doppler findings in postpubertal mumps epididymo-orchitis. J Ultrasound Med 20(11):1189–1195. https://doi.org/10.7863/jum.2001.20.11.1189
Pavlica P, Barozzi L (2001) Imaging of the acute scrotum. Eur Radiol 11(2):220–228. https://doi.org/10.1007/s003300000604
Sudakoff GS, Quiroz F, Karcaaltincaba M et al (2002) Scrotal ultrasonography with emphasis on the extratesticular space: anatomy, embryology, and pathology. Ultrasound Q. https://doi.org/10.1097/00013644-200212000-00004
Hsu PC, Huang WJ, Huang SF (2017) Testicular infarction in a patient with spinal cord injury with epididymitis: a case report. J Rehabil Med 49(1):88–90. https://doi.org/10.2340/16501977-2174
Dellabianca C, Bonardi M, Alessi S (2011) Testicular ischemia after inguinal hernia repair. J Ultrasound 18(4):255–273. https://doi.org/10.1016/j.jus.2011.10.004
Di Serafino M, Gullotto C, Gregorini C et al (2014) A clinical case of Fournier’s gangrene: imaging ultrasound. J Ultrasound 17:303–306. https://doi.org/10.1007/s40477-014-0106-5
Douglas JW, Hicks JA, Manners J et al (2008) A pressing diagnosis—a compromised testicle secondary to compartment syndrome. Ann R Coll Surg Engl 90:1–3. https://doi.org/10.1308/147870808X257184
Gandhi J, Dagur G, Sheynkin YR, Smith NL, Khan SA (2016) Testicular compartment syndrome: an overview of pathophysiology, etiology, evaluation, and management. Transl Androl Urol 5(6):927–934. https://doi.org/10.21037/tau.2016.11.05
Bhatt S, Ghazale H, Dogra VS (2007) Sonograhic evaluation of scrotal and penile trauma. Ultrasound Clin 2(1):45–56. https://doi.org/10.1016/j.cult.2007.01.003
Trinci M, Cirimele V, Ferrari R et al (2019) Diagnostic value of contrast-enhanced ultrasound (CEUS) and comparison with color Doppler ultrasound and magnetic resonance in a case of scrotal trauma. J Ultrasound. https://doi.org/10.1007/s40477-019-00389-y
Avery L, Scheinfeld MH (2013) Imaging of penile and scrotal emergencies. RadioGraphics 33:721–740. https://doi.org/10.1148/rg.333125158
Wilkins CJ, Sriprasad S, Sidhu PS (2003) Colour Doppler ultrasound of the penis. Clin Radiol 58(7):514–523. https://doi.org/10.1016/s0009-9260(03)00112-0
Acampora C, Borzelli A, Di Serafino M et al (2020) High-flow post-traumatic priapism: diagnostic and therapeutic workup. J Ultrasound. https://doi.org/10.1007/s40477-020-00449-8
Fernandes M, de Souza L, Cartafina LP (2018) Ultrasound evaluation of the penis. Radiologia Brasileira 51(4):257–261. https://doi.org/10.1590/0100-3984.2016.0152
Moussa M, Abou Chakra M (2019) Spontaneous cavernosal abscess: a case report and review of literature. J Surg Case Rep 4:rjz108. https://doi.org/10.1093/jscr/rjz108
Acknowledgments
We thank dr. Antonio Brillantino, Emergency Surgery Department “Antonio Cardarelli” Hospital—Naples, for providing the Fig. 6e; dr. Giuseppe Romano, Urology Department “S. Maria alla Gruccia” Hospital—Montevarchi (AR), for providing the Fig. 7d; dr. Giuseppe de Magistris, Interventistic Radiology Department “Antonio Cardarelli” Hospital—Naples, for providing the Fig. 10f.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MS, CA, MLS, FI, and AB. The first draft of the manuscript was written by MS and MLS. All authors contributed to review the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments.
Informed consent
Informed consented was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 Longitudinal B-mode scan with acute spermatic cord torsion shows the “torsion knot” complex of epididymis and spermatic cord (MOV 5188 kb)
Rights and permissions
About this article
Cite this article
Di Serafino, M., Acampora, C., Iacobellis, F. et al. Ultrasound of scrotal and penile emergency: how, why and when. J Ultrasound 24, 211–226 (2021). https://doi.org/10.1007/s40477-020-00500-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-020-00500-8