Abstract
Objective
Primary hyperparathyroidism (PHPT) is a common endocrine disorder that can be cured only by parathyroidectomy. Cervical ultrasonography and scintigraphy are the imaging studies most widely used for preoperative localization of the affected glands. The aim of this retrospective comparative study was to define the respective roles of ultrasonography and parathyroid scintigraphy in these cases.
Materials and methods
We analyzed 108 patients who had undergone parathyroidectomies for PHPT following cervical ultrasonographic and scintigraphic examinations. The ultrasound examinations were carried out by an expert physician sonographer in 61 cases and by various physician sonographers with different levels of experience in 47 cases. Sonographic and scintigraphic findings were compared with surgical findings and the diagnostic performance of the two imaging methods was evaluated by means of statistical analysis.
Results
The operator dependency of ultrasonography was confirmed by marked variations in sensitivity related to the experience of the sonographer. When sonography was performed by an expert, the sensitivity of combined use of the two methods was not significantly higher than that of sonography alone.
Conclusions
In expert hands, the diagnostic yield of ultrasound is appreciably superior. It can therefore be used as the main and possibly sole method for preoperative localization of pathological parathyroid tissues. Combined use of ultrasound and scintigraphy is not cost-effective in these cases. Scintigraphy is indicated only when the ultrasound examination produces negative results.
Riassunto
Obiettivo
L’iperparatiroidismo primitivo (PHPT) è una frequente patologia endocrina che ha come unico trattamento risolutivo quello chirurgico (paratiroidectomia). L’ecografia del collo e la scintigrafia sono gli esami strumentali più comunemente utilizzati nella localizzazione preoperatoria delle paratiroidi patologiche. Scopo di questo studio retrospettivo è definire il ruolo dell’ecografia, confrontandolo con quello della scintigrafia paratiroidea.
Materiali e metodi
108 pazienti sottoposti a intervento di paratiroidectomia per PHPT che hanno eseguito pre-operatoriamente sia l’ecografia del collo che la scintigrafia. L’ecografia è stata eseguita in 61 pazienti da un medico ecografista esperto e in 47 pazienti da più medici ecografisti con esperienza eterogenea. Gli esiti dell’ecografia e della scintigrafia sono stati confrontati con le risultanze dell’atto operatorio e i dati relativi alle loro prestazioni diagnostiche sono stati elaborati mediante analisi statistica.
Risultati
L’ecografia si è confermata metodica operatore-dipendente, dipendendo la sua sensibilità in modo marcato dall’esperienza dell’operatore. Nel caso dell’operatore esperto, l’incremento di sensibilità ottenuto con il contemporaneo uso della scintigrafia si è dimostrato poco rilevante.
Conclusioni
Se eseguita da un operatore esperto, l’ecografia ha una resa diagnostica sensibilmente superiore e può assumere il ruolo di principale ed eventualmente unica metodica di localizzazione preoperatoria delle paratiroidi patologiche. L’uso parallelo dell’ecografia e della scintigrafia risulta in tal caso non giustificato e non cost-effective. Si ritiene invece corretto esclusivamente il loro uso seriale (esecuzione della scintigrafia solamente in caso di negatività dell’ecografia).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary hyperparathyroidism (PHPT) is an endocrine disorder caused by the excessive secretion of parathyroid hormone (PTH) secondary to hyperactivity of one or more of the parathyroid glands. It is the third most common endocrine disorder after diabetes and thyroid disease.
In most cases, onset occurs between 50 and 60 years of age, and the disorder is more common in women than in men [1]. In 90 % of the cases, the cause is a single adenoma (SA). The remaining 10 % is caused by multiglandular disease characterized by diffuse glandular hyperplasia and by double adenomas. Parathyroid carcinomas are extremely rare (<1 % of all cases) [2].
Excess PTH production by a hyperfunctioning parathyroid gland produces negative effects at the level of the bone tissue characterized by increased resorption secondary to enhanced osteoclast activity. The result is osteoporosis (sometimes quite severe) caused by reductions in both bone mass and bone mineral density.
Hypercalcemia––which is found in the majority of patients with PHPT––is the result of increased mobilization of bone calcium and increased intestinal absorption of calcium. It can affect the kidneys (nephrolithiasis, nephrocalcinosis), the central nervous system (apathy, depression, irritability, confusion), the gastrointestinal (nausea, vomiting, epigastric pain, constipation), cardiovascular (arterial hypertension), and the neuromuscular (proximal muscle weakness) systems.
Currently, around 80 % of all cases of PHPT are asymptomatic and are diagnosed as a result of incidental findings of mild hypercalcemia [3]. The less common symptomatic forms are manifested mainly by nephrolithiasis with renal colic and/or osteopenia associated with bone pain and pathological fractures. Severe hypercalcemia is a relatively uncommon finding [4].
The concomitant presence of a vitamin D deficiency can be associated with more severe forms of PHPT (larger adenomas, marked reductions in blood phosphorus levels, severe osteoporosis) that are plainly symptomatic.
Parathyroidectomy is the only definitive treatment available for PHPT. It is indicated for symptomatic patients [5] and those who are asymptomatic but have one or more specific conditions listed in current practice guidelines [6].
The diagnosis of PHPT is based essentially on laboratory findings of persistent hypercalcemia associated with plasma levels of PTH that are elevated or “inappropriately normal” [7]. The latter expression refers to plasma PTH levels in the presence of hypercalcemia that are in the upper third of the normal range. This is an important finding for differential diagnosis since forms of hypercalcemia that are unrelated to the parathyroid glands are characterized by low plasma PTH levels (i.e., in the lower half of the normal range).
The role of imaging studies in the work-up of PHPT is limited to preoperative localization of the pathological parathyroid tissue; the diagnosis itself is based on calcium and PTH levels [8]. These studies have become increasingly important since the introduction of less invasive, imaging-guided surgical techniques [9, 10]. The latter has largely replaced conventional surgical procedures (bilateral exploration of the neck) reducing operative times and hospital stay as well as the risk of complications [11].
Cervical ultrasonography and parathyroid scintigraphy are two of the imaging techniques most widely used to locate pathological parathyroid tissue, and they are also acknowledged as the “first-line localization methods” [12]. Their diagnostic accuracy has proved to be similar or even superior to those of second-line localization methods like computed tomography (CT) or magnetic resonance imaging (MRI) [13].
Cervical ultrasonography [14, 15] is performed with high-frequency linear transducers. The examination should include the entire anterior cervical region. Particular attention should be paid to the thyroid region (Fig. 1), since pathological parathyroid tissue is usually found along the posterior margin of the thyroid lobes or near their lower poles. Normal parathyroid glands, which are small and isoechoic relative to the thyroid parenchyma, cannot be visualized.
The pathological parathyroid tissue (or more simply “adenoma”) becomes visible by virtue of its increased size and altered echogenicity. The parathyroid adenoma appears as a well-circumscribed, oval or oblong mass whose echogenicity is clearly inferior to that of the adjacent thyroid gland (Fig. 2).
Adenomas of the superior parathyroid glands (Fig. 3), which are less common than those of the inferior glands, are typically located in the retrothyroid region, superior or middle-superior to the posterior margin of the thyroid lobe. Inferior parathyroid adenomas may be located behind the thyroid or adjacent to the lower pole of the thyroid lobe (Fig. 4).
Larger parathyroid adenomas sometimes present partially liquid echo structures, which reflect areas of cystic involution (Fig. 5).
In some cases, the use of color Doppler imaging [16, 17] reveals vascular patterns (Fig. 6), which can be helpful for differentiating parathyroid adenomas from enlarged lymph nodes and for identifying parathyroid adenomas within the thyroid gland (Fig. 7). The latter is relatively rare and often indistinguishable from common thyroid nodules [18].
Paratracheal adenoma (maximum diameter approx. 1.8 cm) of the right inferior parathyroid gland located near the inferior pole of the thyroid lobe. Axial scan (panel A), longitudinal scan (panel B), longitudinal scan with color Doppler imaging (panel C, monopolar vascular pattern referred to as the arc or rim sign)
Intrathyroidal adenoma of the right inferior parathyroid gland (maximum diameter approx. 1.5 cm circa) that developed within a multinodular goiter. Axial scan (panel A), longitudinal scan (panel B), longitudinal scan with color Doppler imaging (panels C and D). The vascular pattern is predominantly monopolar. Pulsed Doppler sampling of the afferent artery (panel D) revealed typical low-resistance flow (RI 0.52)
Parathyroid scintigraphy [19, 20] is a diagnostic method that involves the intravenous administration of technetium (99mTc) sestamibi, a tracer that is taken up by thyroid and parathyroid tissues.
Images are acquired with a gamma camera in the planar mode (matrix 256 × 256, HR parallel-hole collimator, 140 keV, window 20 %) 10 min (early phase) and 120 min (late phase) after tracer injection. This technique exploits the differential tracer washout between the thyroid and hyperactive parathyroid tissues (double-phase single-tracer washout technique [21]). In the images obtained, the pathological parathyroid tissue is reflected by a focus of uptake, which can already be identified in the early phase and become increasingly evident during the late phase (Fig. 8––panel A).
Two to three days later, a second scan can be done after intravenous administration of 99mTc-pertechnetate [99mTcO4], which is taken up exclusively by thyroid tissue. Using special software, the image of the thyroid gland can be precisely aligned with those of the previous scan to obtain a subtraction image in which the diseased parathyroid tissue is reflected by residual activity (dual-tracer subtraction technique) [22], (Fig. 8––panel B).
Compared with parathyroid scintigraphy, cervical ultrasound:
-
is easier to perform and less costly;
-
furnishes greater detail relative to the location of the pathological parathyroid tissues and their relation to the surrounding anatomic structures;
-
does not involve exposure to ionizing radiation;
-
is operator-dependent so its diagnostic yield is strongly related to the experience of the sonographer;
-
is less sensitive in identifying ectopic foci of pathological parathyroid tissue [23] and incapable of localizing a small percentage (<2 %) of parathyroid adenomas that are not accessible to surgical exploration of the neck (e.g., those located deep in the mediastinum).
The aim of this retrospective study was to evaluate and define the current roles of cervical ultrasonography and parathyroid scintigraphy in the preoperative localization of pathological parathyroid tissue. In particular, we:
-
assessed the diagnostic performance of the two methods in the identification and localization of single parathyroid adenomas (useful information for surgical planning);
-
looked at whether the use of both methods is necessary and if so, how each should be used;
-
investigated the possible operator-dependence of the results of the ultrasound examination with reference to the first two objectives.
Materials and methods
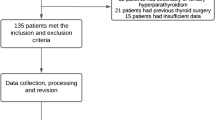
We examined a population of 108 patients (80 women, 28 men; F:M ratio 3:1; mean age approximately 59 years, range 24–83 years) who underwent parathyroidectomy for PHPT between 2001 and 2011.
Fifty-two (48.1 %) had a history of nephrolithiasis, and 18 of these (16.6 % of the total population) had documented osteopenia. In four patients (3,7 %) the hyperparathyroidism had presented with severe hypercalcemia.
The cause of the primary hyperparathyroidism (Table 1) was a solitary adenoma (SA) in 99 cases (91.7 %) and multiglandular disease (MGD) in the remaining 9 (8.3 %).
Eighteen (18.2 %) of the 99 single adenomas were ectopic (7 were located in the upper mediastinum, 4 were paraesophageal, 4 were intrathyroidal, 2 were retrotracheal, and 1 was retrotracheal and paraesophageal).
Six of the nine patients with multiglandular disease (66.6 %) had a double adenoma (DA), which was contralateral in four cases and ipsilateral in two. The other three had diffuse hyperplasia associated with multiple endocrine neoplasia type 1 (MEN 1) (n = 1), hyperplasia involving three of the four glands (n = 1), and adenoma plus hyperplasia involving two glands.
The dimensions/maximum diameter of the parathyroid glands that were removed ranged from 0.5 to 7.0 cm (weights 200 mg–30 g).
In all cases, intraoperative parathyroid hormone assays were performed to provide an immediate indication of the efficacy of the procedure [24, 25].
On the basis of the results of preoperative imaging studies aimed at localization of the lesion, the presence/absence of concomitant nodular disease of the thyroid, and the results of the intraoperative PTH assay, unilateral interventions were performed in 82 (75.9 %) cases and bilateral interventions in the remaining 26 (24.1 %).
In 17 (65.4 %) of the latter cases, bilateral intervention was necessary for reasons related to the parathyroid disease (confirmed or suspected MGD; inability to localize or erroneous localization of site of involvement with ultrasound and/or scintigraphy; insufficient reduction of PTH level on the intra-operative assay); in the remaining nine patients (34.6 %) it was necessitated by the need for concomitant intervention on the thyroid gland (partial or total thyroidectomy for nodular disease).
In only one case (1.6 % of the population), surgery was unsuccessful resulting in persistent biochemical evidence of hyperparathyroidism in the immediate postoperative period despite removal of the two diseased parathyroid glands.
Surgery was not associated with significant complications in any of the patients.
All of the patients had laboratory-based diagnoses of PHPT and all had cervical ultrasound scans and parathyroid scintigraphic examinations with sestamibi. In most cases, the studies were performed in no particular order with the aim of obtaining as much information as possible from each regarding the exact location of the parathyroid disease. The information obtained from the two imaging studies was considered sufficient. Therefore, none of the patients was subjected to ultrasound-guided fine-needle aspiration.
The ultrasound examinations were carried out and read by a physician sonographer in our hospital. The examinations were performed with an ATL HDI 3000 scanner or an Esaote MyLab 70 XVG scanner, both equipped with multifrequency linear array transducers and color/power Doppler modules.
The scintigraphic examinations (read by a nuclear medicine specialist) were done in our hospital (68 patients, approximately 63 % of the population) or in other diagnostic centers (40 patients, approximately 37 %).
In each case, the results of the ultrasound and scintigraphic examinations were compared with surgical findings.
The total study population (n = 108), referred to hereafter as Group A, was therefore divided into two groups; Group B, which included 61 (56.5 %) patients whose ultrasound examination was carried out by a single physician with expertise in neck sonography and Group C, which comprised 47 patients (43.5 %) whose sonographic examinations were done by various physicians with different levels of experience in neck pathology.
Classical analysis of the sensitivity and specificity (the so-called performance parameters) of the two methods was not possible since the population we studied did not include healthy subjects (i.e., all had pre-existing diagnoses of PHPT).
For this reason, the results of each imaging study were classified numerically as follows: 1 (one) if the operator identified a single parathyroid gland or the side of involvement (positive results); 0 (zero) if no identification was made (negative results).
In parallel, similar codes were assigned to the surgical findings: 0 (zero) if the site identified by ultrasound or scintigraphy as pathological was found to be healthy at surgery (absence of disease, D−) and 1 (one) when surgical exploration revealed parathyroid pathology (presence of disease, D+) (Table 2).
This allowed us to calculate and compare the performance parameters for ultrasound and for scintigraphy (sensitivity, specificity, accuracy, positive predictive value).
For both samples (groups B and C), the performance of the two methods was evaluated in terms of their abilities to localize single pathological parathyroid glands and to identify the side of involvement.
The patients of group B were examined by a single physician sonographer and by a nuclear medicine specialist; those of group C were examined by multiple physician sonographers (other than the one who examined patients in group B) and by the nuclear medicine specialist.
The analysis was completed by verification of the significance of differences that emerged between the two samples and between the two imaging methods (Student’s t test) regarding localization of single pathological parathyroid glands and the identification of the side of the gland involved.
In essence, the same analysis was carried out for non-independence within each single sample (groups B and C) and within the total population (group A).
Our aim was to determine whether use of both methods in parallel was justified (the additional cost being justified by an overall increase in sensitivity) or whether the method with superior performance should be used initially and the other only if the first study was negative (improving sensitivity at a cost that was more limited).
We also analyzed the sensitivity of ultrasound and scintigraphy in the localization of 18 single ectopic adenomas.
Further analysis was precluded by the small number of patients with multiglandular disease (9/108 patients).
Results
Table 3 shows the following information:
-
1.
Performance parameters for the two methods (sensitivity, specificity, accuracy, positive predictive value) in the localization of single pathological parathyroid glands and in the identification of the side of the gland involved;
-
2.
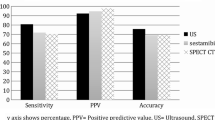
Overall sensitivity for the use of ultrasound and scintigraphy “in parallel” (for group A, 88 % in the identification of a single pathological parathyroid gland and 94 % in the identification of the side of involvement).
Statistical analysis did not reveal any significant differences in the diagnostic performance parameters (sensitivity, specificity, accuracy, positive predictive value) in groups B and C. Therefore, these two samples were pooled for cumulative analysis (carried out on group A).
Analysis of the data in this table showed that the sensitivity of ultrasound was strongly dependent on the experience of the operator. This relation was evident in the identification of single pathological parathyroid glands (sensitivity, 84 % in group B vs. 71 % in group C) and of the involved side of the gland (sensitivity, 91 % for group B vs. 78 % in group C).
As for the other performance parameters (specificity, etc.), the improvements observed do not require detailed explanations.
The superior diagnostic performance parameters in Group B (regarding the sonographic examination and its ability to identify single pathological parathyroid glands) are reflected in the conclusive results evaluated in Group A, where the positive predictive value of the sonographic examination was significantly higher than that of scintigraphy (82 vs. 74 %, p < 0.05).
In group B and to a lesser extent in group A, there was only a small increase in overall sensitivity (a few percentage points) when scintigraphy was also performed.
In contrast, in group C, the use of both methods was associated with clear improvement of the overall sensitivity not only in the localization of single pathological parathyroid glands (from 71 to 87 %) but also in the identification of the involved side of the gland (from 78 to 92 %).
The nuclear medicine study yielded comparable results regardless of the sample being examined.
It should be stressed that the high values of specificity, all of which are essentially similar, are related to the mechanism by which the healthy cases were identified (in both the localization of the parathyroid gland and the identification of the involved side); in fact, for every patient examined the sonographer and the nuclear medicine specialist were faced with the evaluation of four parathyroid sites and two sides to evaluate, thereby increasing the “true negatives.”
Table 4 summarizes the data on the sensitivity of the two first-line methods in the localization of the single ectopic adenomas. Analysis of these data shows that:
-
1.
in all three groups (A, B, C), the sensitivity of scintigraphy exceeded that of ultrasound (77.7 vs. 55 % in group A), which is consistent with data in the literature [26];
-
2.
the sensitivity of ultrasound was higher in group B (80 %, scintigraphy 90 %) than in group C (25 %, scintigraphy 62.5 %). In addition, the sensitivities of the two methods were even more markedly different in the latter sample, where the scintigraphic examination displayed obvious superiority.
In group B ultrasound was not capable of localizing one of the two adenomas in the upper mediastinum, but with the aid of color Doppler imaging it correctly identified three of the four intrathyroidal adenomas on the basis of their typical unipolar vascular patterns.
Discussion and conclusions
In accordance with data in the literature [27], the vast majority of cases of PHPT in our population (91.7 %) were caused by a single adenoma.
The increased diagnostic sensitivity observed in Group B confirms previous reports underlining the operator dependency of ultrasonography and the fact that its diagnostic yield is appreciably higher when performed by a more experienced examiner [28].
The results of the statistical analysis of data from group C confirm the findings of numerous other studies [29–32], that is that preoperative use of ultrasound and scintigraphy in parallel improves accuracy in the detection and localization of single pathological parathyroid glands In fact, the use of both methods was associated with higher diagnostic sensitivity (87 %) than use of ultrasound (71 %) or scintigraphy (73 %) alone. We nonetheless feel that, as reported in previous studies, the inferior sensitivity of ultrasonography observed in Group C is due to the absence of dedicated physician sonographers and/or to the relative lack of experience of some of the examiners.
Statistical analysis of the data for Group B supports the findings that have emerged from other recent studies [33–36]: when performed by expert operators, ultrasound can become the main and possibly the sole method for preoperative localization of pathological parathyroid glands. This conclusions supported by the limited increase in overall sensitivity associated with the use of ultrasound and scintigraphy (88 vs. 84 % with ultrasound alone) and by the higher positive predictive value of ultrasound in localizing single pathological parathyroid glands (85 vs. 74 % for scintigraphy).
As we have already noted, in the identification of single pathological parathyroid glands, ultrasound performed best in group B and these results are reflected in those for group A. As a result, the difference between the positive predictive values of ultrasound (82 %) and scintigraphy (74 %) is statistically significant (p < 0.05).
If expert physician sonographers are available, the use of the two methods in parallel is in our opinion not cost-effective, which is in line with previous findings [37], since it does not appreciably improve the overall diagnostic sensitivity.
In expert hands, ultrasound has proved to be an accurate and cost-effective method for preoperative localization [38]. We therefore feel that preoperative use of ultrasound alone offers the following advantages:
-
1.
it eliminates the need to expose patient to the ionizing radiation associated with parathyroid scintigraphy (approximately 6.5 mSv with dual-tracer subtraction imaging with 99mTc-sestamibi and 99 mTc-pertechnate);
-
2.
it reduces the costs of preoperative imaging without significantly diminishing the diagnostic accuracy. (Based on the current reimbursement schedule of the Piedmont Region of Italy, healthcare facilities receive €33,45 for an ultrasound examination of the neck and €347,80 for parathyroid scintigraphy using the dual-tracer subtraction technique);
-
3.
it allows an expert surgeon to perform targeted, low invasive surgery with the aid of rapid intraoperative PTH assays with success rates similar to those associated with bilateral procedures.
In these cases, the use of parathyroid scintigraphy is justified only when the ultrasound examination is negative (serial or sequential use of the two diagnostic methods) [39].
In our study, too, scintigraphy proved to be more sensitive for localizing ectopic adenomas.
Calculation of the Youden index with the basic performance parameters for the two methods (sensitivity + specificity −1), an expression of the overall diagnostic capacity, provided additional confirmation of ultrasound’s role as the main and possibly sole method for preoperative localization.
The results of these calculations (Table 5) confirm that expert physician sonographers can produce more accurate diagnoses than nuclear medicine.
In patients who have not been operated on, negative findings in the cervical ultrasound examination and in sestamibi scintigraphy should raise the suspicion of multiglandular disease (in the presence of which both methods displayed substantially lower sensitivity). This finding thus represents sufficient cause for performing bilateral exploration of the neck.
In these cases, second-line localization methods like CT or MRI cannot be justified, in fact they are not cost-effective [40] because:
-
1.
they are less sensitive and more expensive and cannot guarantee the correct localization of pathological parathyroid tissues in all cases;
-
2.
they expose the patient to additional risks related to ionizing radiation exposure (CT) and/or use of contrast medium (CT and MRI);
-
3.
the skill and experience of the surgeon and the use of rapid intraoperative PTH assays during bilateral neck exploration may be sufficient to ensure high rates of success.
On the basis of our experience in the present study, we propose the algorithm shown in Fig. 9 for preoperative localization and surgical treatment of all patients with primary hyperparathyroidism who have not already had surgery.
References
Silverberg SJ (2000) Natural history of primary hyperparathyroidism. Endocrinol Metab Clin North Am 29:451–464
Ruda JM, Hollenback CS, Stack BC (2005) A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 132(3):359–372
Silverberg SJ, Lewiecki EM, Mokesilde L, Peacock M, Rubin MR (2009) Presentation of asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. J Clin Endocrinol Metab 94:351–365
Bilezikian JP, Silverberg SJ (2000) Clinical spectrum of primary hyperparathyroidism. Endocr Metab Disord 1:237–245
Bilezikian JP (2000) Primary hyperparathyroidism: when to observe and when to operate. Endocrinol Metab Clin North Am 29(3):465–478
Bilezikian JP, Khan AA, Potts JT (2009) Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Third International Workshop. J Clin Endocrinol Metab 94:335–339
Davies M, Fraser WD, Hosking DJ (2002) The management of primary hyperparathyroidism. Clin Endocrinol 57(2):145–155
Hindié E, Ugur Ö, Fuster D, O’Doherty M, Grassetto G, Ureña P et al (2009) 2009 EANM (European Association of Nuclear Medicine) parathyroid guidelines. Eur J Nucl Med Mol Imaging 36(7):1201–1216
Baliski CR, Stewart JK, Anderson DW, Wiseman SM, Bugis SP (2005) Selective unilateral parathyroid exploration: an effective treatment for primary hyperparathyroidism. Am J Surg 189(5):596–600
Sosa JA, Udelsman R (2003) Minimally invasive parathyroidectomy. Surg Oncol 12(2):125–134
Berngelfelz A, Kanngiesser V, Zielcke A, Nies C, Rothmund M (2005) Conventional bilateral cervical exploration versus open minimally invasive parathyroidectomy under local anaesthesia for primary hyperparathyroidism. Br J Surg 92(2):190–197
Uruno T, Kebebew E (2006) How to localize parathyroid tumors in primary hyperparathyroidism? J Endocrinol Invest 29(9):840–847
Scheiner JD, Dupuy DE, Monchik JM, Noto RB, Cronan JJ (2001) Preoperative localization of parathyroid adenoma: a comparison of power and colour Doppler ultrasonography with nuclear medicine scintigraphy. Clin Radiol 56(12):984–988
AIUM practice guideline for the performance of a thyroid and parathyroid ultrasound examination. J Ultrasound Med 2003; 22(10):1126–1130
Reeder SB, Desser TS, Weigel RJ, Jeffrey RB (2002) Sonography in primary hyperparathyroidism: review with emphasis on scanning technique. J Ultrasound Med 21(5):539–552
Mohammadi A, Moloudi F, Ghasemi M (2012) The role of colour Doppler ultrasonography in the preoperative localization of parathyroid adenomas. Endocr J 59(5):375–382
Versamidis K, Versamidou E, Mavropoulos G (1999) Color-doppler sonography in detection of parathyroid adenomas. Head Neck 21(7):C648–C651
Yabuta T, Tsushima T, Masuoka H, Tomoda C, Fukushima M, Kihara M et al (2011) Ultrasonographic features of intrathyroidal parathyroid adenoma causing primary hyperparathyroidism. Endocr J 58(11):989–994
Palestro CJ, Tomas MB, Tronco GG (2005) Radionuclide imaging of the parathyroid gland. Semin Nucl Med 35(4):266–276
Smith JR, Oates ME (2004) Radionuclide imaging of the parathyroid glands: patterns, pearls and pitfalls. Radiographics 24:1101–1115
Taillefer R, Boucher Y, Potvin C, Lambert R (1992) Detection and localization of parathyroid adenomas in patients with iperparathyroidism using a single radionuclide imaging procedure with technetium-99 m-sestamibi (double-phase study). J Nucl Med 33(10):1801–1807
Giordano A, Rubello D et al (2001) New trends in parathyroid scintigraphy. Eur J Nucl Med 28:1409–1420
Uruno T, Kebebew E (2006) How to localize parathyroid tumors in primary hyperparathyroidism? J Endocrinol Invest 29(9):840–847
Bachar G, Mizrachi A, Hadar T, Feinmesser R, Shpitzer T (2011) Role of parathyroid hormone monitoring during parathyroidectomy. Head Neck 33(12):1754–1757
Sharma J, Milas M, Berber E, Mazzaglia P, Siperstein A, Weber CJ (2008) Value of intraoperative parathyroid hormone monitoring. Ann Surg Oncol 15:493–498
Haber RS, Kim CK, Inabnet WB (2002) Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99 m) technetium sestamibi scintigraphy. Clin Endocrinol 57:241–249
Ruda JM, Hollenback CS, Stack BC et al (2005) A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 132(3):359–372
Reeder SB, Desser TS, Weigel RJ, Jeffrey RB (2002) Sonography in primary hyperparathyroidism: review with emphasis on scanning technique. J Ultrasound Med 21:539–552
Johnson NA, Tublin ME, Ogilvie JB (2007) Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol 188(6):1706–1715
Kepbaci M, Entok E, Kepbaci N, Adapinar B (2004) Preoperative evaluation of parathyroid lesions in patients with concomitant thyroid disease: role of high resolution ultrasonography and dual phase technetium 99 m sestamibi scintigraphy. J Endocrinol Invest 27:24–30
De Feo ML, Colagrande S, Biagini C, Tonarelli A, Bisi G, Vaggelli L et al (2000) Parathyroid glands: combination of 99 mTc MIBI Scintigraphy and US for demonstration of parathyroid glands and nodules. Radiology 214(2):393–402
Lumachi F, Zucchetta P, Marzola MC, Boccagni P, Angelini F, Bui F et al (2000) Advantages of combined technetium-99 m-sestamibi scintigraphy and high-resolution ultrasonography in parathyroid localization: comparative study in 91 patients with primary hyperparathyroidism. Eur J Endocrinol 143(6):755–760
Tublin ME, Pryma DA, Yim JH, Ogilvie JB, Mountz JM, Bencheri B et al (2009) Localization of parathyroid adenomas by sonography and technetium tc 99 m sestamibi single-photon emission computed tomography before minimally invasive parathyroidectomy: are both study really needed? J Ultrasound Med 28(2):183–190
Grosso I, Sargiotto A, D’Amelio P, Tamone C, Gasparri G, De Filippi PG et al (2007) Preoperative localization of parathyroid adenoma with sonography and 99mTc Sestamibi scintigraphy in primary hyperparathyroidism. J Clin Ultrasound 35(4):186–190
Haber RS, Kim CK, Inabnet WB (2002) Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m) technetium sestamibi scintigraphy. Clin Endocrinol 57:241–249
Ulanovski D, Feinmesser R, Cohen M, Sulkes J, Dudkiew M, Shpitzer P (2002) Preoperative evaluation of patients with parathyroid adenoma: role of high-resolution ultrasonography. Head Neck 24(1):1–5
Grosso I, Sargiotto A, D’Amelio P, Tamone C, Gasparri G, De Filippi PG et al (2007) Preoperative localization of parathyroid adenoma with sonography and 99mTc Sestamibi scintigraphy in primary hyperparathyroidism. J Clin Ultrasound 35(4):186–190
Ulanovski D, Feinmesser R, Cohen M, Sulkes J, Dudkiew M, Shpitzer P (2002) Preoperative evaluation of patients with parathyroid adenoma: role of high-resolution ultrasonography. Head Neck 24(1):1–5
Haber RS, Kim CK, Inabnet WB (2002) Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m) technetium sestamibi scintigraphy. Clin Endocrinol 57:241–249
Lumachi F, Ermani M, Basso S, Zucchetta P, Borsato N, Favia G (2001) Localization of parathyroid tumors in the minimally invasive era: wich tecnique should be chosen? Population based analysis of 253 patients undergoing parathyroidectomy and factors affecting parathyroid glands detection. Endocr Relat Cancer 8:63–69
Conflict of interest
The authors of this paper Giovanni Mariano Vitetta, Pierluigi Neri, Andrea Chiecchio, Alessandro Carriero, Stefano Cirillo, Annalisa Balbo Mussetto, Alessandra Codegone declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). All patients provided written informed consent to enrollment in the study and to the inclusion in this article of information that could potentially lead to their identification.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vitetta, G.M., Neri, P., Chiecchio, A. et al. Role of ultrasonography in the management of patients with primary hyperparathyroidism: retrospective comparison with technetium-99m sestamibi scintigraphy. J Ultrasound 17, 1–12 (2014). https://doi.org/10.1007/s40477-014-0067-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-014-0067-8