Abstract
Cigarette smoking continues to be the leading cause of preventable morbidity and mortality. Similar to other addictive substances, the prevalence of cigarette smoking is greater among men than women, yet women are less successful at quitting smoking. Preclinical and clinical research suggests that ovarian hormones (i.e., estradiol and progesterone), which fluctuate over the course of the menstrual cycle, may contribute to these sex differences. Specifically, research suggests that progesterone may protect against cigarette smoking and nicotine addiction, whereas estradiol may underlie enhanced vulnerability. In this review, we discuss new research on ovarian hormone and menstrual cycle phase effects on smoking-related responses and behavior in women, including studies examining neural responses to smoking cues, hormonal influences on medication-assisted smoking cessation, and acute smoking abstinence. We highlight innovative studies with strong research methodology and provide suggestions for future research that may allow evidence-based knowledge for immediate translation to the clinic to guide novel, hormonally informed treatment strategies. Thus, rigorous scientific study holds the potential to reduce relapse rates, thus improving the health and saving the lives of the many thousands of women who unfortunately do not respond to current treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cigarette smoking is the number one cause of preventable death and is responsible for more than 480,000 deaths per year in the USA [1]. Long-term cigarette smoking leads to a wide range of deleterious health consequences, including cancer, emphysema, and cardiovascular diseases [1], and the total economic cost of smoking is more than $300 billion annually [2]. Thus, cigarette smoking is a major economic burden and health problem that continues to be a challenge for prevention and treatment efforts.
In line with the literature on other addictive substances, the prevalence of cigarette smoking is somewhat greater among men than women [3], yet women may be more vulnerable to some of the consequences of cigarette smoking than men [4–7]. For example, women who smoke cigarettes report greater positive mood effects following cigarette use [8] and appear to be more sensitive to the rewarding effects of nicotine than men [9, 10]. Women also exhibit lower odds of successfully quitting smoking [11, 12] and respond less favorably to certain smoking cessation approaches than men [12–15]. Furthermore, women who smoke cigarettes are at a higher risk of developing tobacco-related diseases and experience more severe tobacco-related health consequences than men, including increased incidence of lung-cancer-related deaths [16] and increased risk of coronary heart disease [17]. These findings demonstrate clear sex differences in smoking-related behaviors and consequences, yet our understanding of the factors contributing to these sex differences remains unclear. Recent preclinical and clinical research, however, suggests that ovarian hormones (i.e., estradiol and progesterone), which fluctuate over the course of the menstrual cycle [18], may underlie these sex differences. Thus, the purpose of this review is to provide an update on the clinical research (2012–2015) examining the influence of ovarian hormones (and/or menstrual cycle phase) on smoking-related responses and behavior in women. We discuss convergences in the literature and recommendations for future directions.
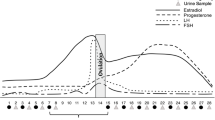
The Menstrual Cycle
When discussing ovarian hormone influences, one is often referring to the fluctuations in levels of the two major ovarian hormones, estradiol and progesterone, across the menstrual cycle. Although considerable individual variability exists, the menstrual cycle is commonly described by specific events and phases (Fig. 1). The first day of the menstrual cycle is defined by the onset of menses (blood flow), which coincides with the beginning of the follicular phase [18]. During the follicular phase, progesterone levels remain very low, whereas estradiol is very low during the early follicular phase (menses) followed by a rise and eventual peak during the late follicular phase, which coincides with the onset of ovulation followed by the luteal phase. During the early luteal phase, progesterone levels increase and peak at the midpoint of the phase when estradiol levels reach a secondary, yet less pronounced peak. Following these mid-luteal phase peaks, both estradiol and progesterone levels decrease rapidly, and this part of the late luteal phase is commonly described as the premenstrual phase. There are several different approaches to determining menstrual cycle phase that are reviewed elsewhere [19]. For the purposes of this review, we refer to the follicular phase as the phase when estradiol levels are increasing and greater than progesterone, whereas the luteal phase is the phase of the cycle when progesterone levels are increasing and greater than estradiol.
Ovarian Hormones and Smoking: an Evolving Theory
Although progesterone and estradiol are primarily involved in sexual maturation and reproductive functions, they also produce direct effects on the reward-related mesolimbic dopaminergic system and influence addition-related processes [20–22]. Recent research suggests that estradiol may underlie enhanced vulnerability to continued smoking and relapse in women, whereas progesterone may have protective effects on vulnerability to smoking behavior [23]. For example, preclinical research indicates that estradiol increases dopamine release in the ventral striatum [24] and differentially modulates nicotine-evoked dopamine release in response to nicotine administration [25]. Further, during smoking abstinence, women smokers report enhanced nicotine craving and increased relapse rates during the follicular phase of the menstrual cycle when estradiol levels are higher than progesterone levels [26]. There are also studies reporting that women who are pregnant or taking oral contraceptives, and consequently have enhanced levels of estradiol, metabolize nicotine more quickly than those who are not pregnant [27, 28] and those not taking oral contraceptives [29]. Progesterone, however, appears to have an opposite effect and protects against vulnerability to smoking. Specifically, when progesterone levels are high, nicotine self-administration (i.e., motivation) is decreased [23]. Similarly, exogenously administered progesterone decreased the positive subjective effects of cigarette smoking [30] and reduced urges to smoke in female smokers [30, 31]. Together, these studies provide support for the theory that estradiol may promote smoking behaviors through increased reward, yet progesterone may protect against smoking behavior by decreasing nicotine-related reward.
Neural Responses and Subjective Craving to Smoking Cues
Most smoking lapses and relapses occur in the context of smoking cue exposure and smoking cue-elicited craving [32], and as such, there has been great interest in uncovering the neural substrates that may underlie smoking cue-elicited relapse. Researchers have just begun to explore the influence of the hormonal milieu on smoking cue neural responses. Our literature search identified two studies that examined neural responses to smoking-related cues and subjective craving following smoking cue exposure by menstrual cycle phase in sated smokers [33•, 34]. One study, conducted by Mendrek et al. [34], explored potential sex differences (between-group comparison: 15 men, 13 women) and potential differences across the women’s menstrual cycle (within-subject comparison: follicular versus luteal phase) in neural responses following exposure to smoking cue images compared to neutral images during blood oxygenation level–dependent (BOLD) functional magnetic resonance imaging (fMRI). Although no sex differences were observed, greater neural activity to smoking cues was observed in a small cluster within the angular gyrus during the follicular phase compared to the luteal phase. The angular gyrus has been associated with guiding attention to relevant information about reward [35]. However, it is unclear whether the smoking images from the Mendrek et al. study elicited a desire/craving to smoke a cigarette because a post–cue exposure craving score was not acquired, and consequently, associations between neural responses and subjective cue-elicited craving could not be assessed. Ratings of smoking-related and neutral images shown during fMRI were acquired following scanning and did not differ across menstrual cycle phase. These issues could account for the lack of sex differences and minimal menstrual cycle phase effects.
In another fMRI study, Franklin et al. [33•] used arterial spin labeling (ASL) perfusion fMRI to examine neural responses to smoking cues compared to nonsmoking cues in cigarette-dependent women who were in the follicular phase compared to cigarette-dependent women who were in the luteal phase of their menstrual cycle. Similar to the Mendrek et al. study [34], participants smoked to satiety prior to the scanning session; however, the Franklin et al. study [33•] used 10-min audio–visual clips of smoking or nonsmoking cue videos presented during perfusion fMRI and assessed subjective craving prior to and following smoking cue exposure. Follicular phase women showed greater activation to smoking cues relative to nonsmoking cues in a large area that extended from the middle frontal gyrus to the anterior cingulate. Further, women in the follicular phase reported smoking cue-elicited craving, which correlated with smoking cue-elicited neural activation in the insula; however, those in the luteal phase did not. These findings suggest that females in the follicular phase (when estradiol levels are higher than progesterone) show greater neural responses to smoking cues in brain regions commonly associated with reward processes. Further, the insula is a brain region commonly associated with craving and incentive salience, and as such, the finding that follicular females showed correlations between smoking cue-elicited neural activity within this region and self-reported craving provide a potential neural mechanism through which menstrual cycle and ovarian hormones influence smoking behavior. Given these promising neuroimaging findings suggesting potential mechanisms through which smoking-related factors may contribute to continued smoking and relapse, additional research incorporating ovarian hormone measurement levels and neuroimaging approaches is needed and could help identify treatment targets.
Stress Cue Reactivity
Stress promotes the development and maintenance of smoking behavior [36–38]. Given that stress contributes to smoking behavior and men and women respond to stress differently [39], Saladin et al. [40] examined sex differences among sated smokers in response to negative affect and stress and found that women who smoke cigarettes showed greater subjective craving for nicotine and stress/emotional reactivity to personalized stress cues than males. Using the same dataset, Saladin et al. [41••] assessed whether menstrual cycle phase may have influenced the previous findings by stratifying women by menstrual cycle phase, and no effect of menstrual cycle phase was observed. Comparing follicular and luteal females to males, however, revealed that women in the luteal phase reported greater craving than males, whereas women in the follicular phase reported greater stress and arousal than males and perceived the stress cues as more emotionally aversive than males. These findings suggest that menstrual cycle phase may influence response to stress cues, and as such, women who smoke cigarettes may face different challenges during smoking cessation depending on the menstrual phase. Based on these findings, one could speculate that women in the follicular phase experiencing low levels of progesterone and higher levels of estradiol may have to contend with heightened emotional responsiveness and respond more favorably to a cessation treatment focused on mindfulness and coping skills, whereas women in the luteal phase experiencing higher levels of progesterone and lower levels of estradiol may respond more favorably to a treatment focused on coping with craving. It is important to note, however, that the finding of increased craving during the luteal phase is somewhat surprising given that progesterone levels are higher during the luteal phase, and progesterone is thought to decrease reward and craving. Thus, additional research is warranted.
Medication-Assisted Smoking Cessation
Although substantial evidence supports the theory of the enhancing effects of estradiol on reward-related behavior and the protective effects of progesterone on nicotine reinforcement and relapse risk, the smoking cessation literature typically stratifies women who smoke cigarettes according to menstrual cycle phase rather than measuring hormone levels directly during a quit attempt. Saladin et al. [42] recently conducted the first study to examine the effects of naturally occurring variation in ovarian hormone levels on medication-assisted smoking cessation. Briefly, female smokers were randomized into a 5-week, double-dummy, placebo-controlled cessation trial that involved a 1-week titration period followed by a target quit date and 4-weeks of study drug (i.e., either varenicline tablets and placebo patches or placebo tablets and nicotine patches). Throughout the course of the trial, female smokers completed weekly clinic visits and associated assessments, including providing blood samples for plasma measurements of progesterone and estradiol levels, self-report of smoking behavior, and expired carbon monoxide (CO). Consistent with previous research, findings indicated that increases in progesterone levels were associated with an increase in the odds for being abstinent within each week of the 4-week treatment.
The Saladin et al. [42] article is of particular interest and importance, as the study is innovative and timely, the research methodology is strong, and the findings have important clinical and research implications. First, as aforementioned, it is the first study to measure ovarian hormone levels directly while women smokers are making a quit attempt during a medication-assisted smoking cessation trial. By directly measuring estradiol and progesterone levels throughout the monthly cycle and while making a quit attempt, Saladin and colleagues avoid the pitfalls associated with stratifying women according to menstrual cycle phase (e.g., assumptions about relative levels of hormones when there are within- and between-cycle hormone variations both within the individual and from woman to woman). Although the duration of the pharmacotherapy was brief and did not contain a placebo control, the research design was innovative and carefully reasoned to address the research goals. Finally, the Saladin et al. study [42] extends upon human laboratory study findings of progesterone’s protective effects on smoking behavior [30, 31, 43] and identifies an association between increasing progesterone levels and abstinence outcomes in women smokers participating in a medication-based treatment. Research examining whether exogenous progesterone is safe and effective in augmenting cessation interventions is underway and could lead to significant public health benefits (S. Allen, personal communication).
Acute Smoking Abstinence Studies
Given that the vast majority of individuals relapse within the first few days of a quit attempt [44], most of the research published on ovarian hormones and smoking over the past 3 years focuses on risk factors for smoking relapse within the first 4 days of abstinence. Specifically, S. Allen et al. [45••] conducted a controlled cross-over trial to explore the differences in smoking-related symptomatology by menstrual cycle phase and depressive symptoms during acute smoking abstinence, which was defined as 4 days of biochemically verified smoking abstinence. Briefly, the large, controlled cross-over trial involved two testing weeks (i.e., 1 week during follicular phase and the other week during the luteal phase of the menstrual cycle) that included serum hormone level measurements, daily symptomatology ratings, and depression assessments. During each testing week, women smoked ad libitum for the first 2 days followed by 4 days of biochemically verified smoking abstinence. The two testing weeks occurred approximately 2 weeks apart in order to obtain measures during each of the menstrual cycle phases. We highlight research methodology of the S. Allen et al. [45••] study because the controlled cross-over design has several strengths, including limiting confounds, multimodal assessments, between- and within-subject data, and information on both follicular and luteal phases within the same participant. As such, several publications have resulted from using this strong research approach [26, 45••, 46–51].
In the main article, S. Allen et al. [45••] found that women showed a stronger association between depressive symptoms and negative affect during the follicular phase than the luteal phase. That said, S. Allen et al. [49] also examined depressive symptom status (i.e., with or without depressive symptoms) and menstrual phase influences on response to nicotine following acute smoking abstinence in a subsample of the larger, main study. Women smokers were classified in the “with depressive symptoms” group if they had a history of major depressive disorder (MDD) and/or current depressive symptoms but did not meet criteria for current MDD, whereas those who did not have depressive symptoms were classified as women “without depressive symptoms.” Findings indicated that women who smoke cigarettes without depressive symptoms had a greater menstrual phase difference in response to nicotine following acute smoking abstinence than those with depressive symptoms. Specifically, women without depressive symptoms showed greater physiological response to nicotine following acute smoking abstinence. An additional article from the controlled cross-over trial explored whether menstrual phase influenced subjective response to nicotine during acute smoking abstinence and found that the luteal phase was associated with a greater increase in stimulation and greater decrease in urge to smoke after the first dose of nicotine following acute smoking abstinence [48]. It should be noted that 13 subjective responses to nicotine were examined in this study, but only two significant findings emerged, providing minimal evidence for menstrual cycle phase influences on subjective response to nicotine during acute smoking abstinence. Overall, findings from these studies provide important information on risk factors for relapse during acute smoking abstinence indicating that depressive symptoms and menstrual phase influence smoking-related symptomatology; however, further study is needed to determine whether these effects influence smoking cessation outcomes.
Allopregnanolone
In addition to examining how depressive symptoms and menstrual phase influence nicotine response during acute smoking abstinence, two additional articles describe how allopregnanolone, a neuroactive steroid metabolized from progesterone, may also fluctuate by menstrual phase and influence response to nicotine [46, 47]. Findings revealed that change in allopregnanolone levels during acute smoking abstinence varied by menstrual phase such that allopregnanolone decreased by 10 % in the follicular phase and increased by 31 % in the luteal phase [47]. The second study [46] found that during acute smoking abstinence before nicotine administration, allopregnanolone was positively associated with cardiovascular and subjective physical state; however, after nicotine nasal spray administration, allopregnanolone levels were associated with changes in cognition. Together, these findings suggest that allopregnanolone may also play a role in smoking-related symptomatology, and this role might vary by menstrual phase.
Exogenous Hormones/Oral Contraceptives
Typically, clinical and human laboratory research on ovarian hormones and smoking is conducted in naturally cycling women who are not taking exogenous hormones; however, research is now exploring how oral contraceptives may influence hormonal fluctuations and smoking-related symptomatology. In a secondary-data analysis on a subset of women smokers that participated in the controlled cross-over trial, Hinderaker et al. [50] explored differences in smoking-related symptomatology during acute smoking abstinence between women who were taking a standardized oral contraceptive (OC) and women who were not taking an OC. In addition, menstrual cycle timing was also examined between OC women and no-OC women by comparing symptomatology during low progesterone week (first week of OC pills) and the follicular phase of no-OC women and high progesterone week (third week of OC pills) and the luteal phase. During acute smoking abstinence, the OC group reported lower levels of positive affect compared to the no-OC group. In menstrual cycle timing comparisons, the OC group and no-OC group showed differences in smoking satisfaction and changes in symptomatology as progesterone levels changed. Overall, findings provide preliminary evidence suggesting that women taking oral contraceptives may have different patterns of smoking-related symptomatology during acute smoking abstinence compared to naturally cycling women; however, additional research examining how oral contraceptive use may influence smoking cessation efforts is warranted.
Influences on the Effects of Nicotine
In our review of the past 3 years of literature on ovarian hormones and smoking, we identified four studies examining menstrual cycle phase influences on the effects of nicotine [51–54]. In one study, intravenous (IV) nicotine was administered to men and women smokers after biochemically verified overnight abstinence, which resulted in men reporting greater subjective reactivity to nicotine, whereas women showed greater physiological reactivity to nicotine [52]. Further, women in the luteal, relative to follicular, phase reported lower subjective reactivity to nicotine, fewer negative affect symptoms, and enhanced cognitive task performance [52]. These results are similar to the findings reported by S. Allen et al. [49] showing that women without depression showed greater menstrual phase differences in nicotine response, with more pronounced responses to nicotine during the follicular compared to the luteal phase. Similarly, in a study examining associations between ovarian hormone levels and smoking behavior, Schiller et al. [53] found that women with lower levels of progesterone relative to estradiol, which corresponds to the hormonal milieu of the follicular phase, took more puffs of their cigarettes and smoked more of their cigarettes during the laboratory topography session. Further, larger decreases in estradiol and progesterone over time were associated with greater puff intensities. Together, these studies, which used different laboratory approaches to examine responses to nicotine following overnight abstinence, report similar findings of greater nicotine reactivity and cigarette use when progesterone levels are lower than estradiol levels (i.e., follicular phase) and, conversely, lower nicotine response and cigarette use when progesterone levels are higher than estradiol levels (i.e., luteal phase).
Given that anxiety and stress have been identified as key factors in smoking-related behavior [36], recent research has focused on the potential role of ovarian hormones and hypothalamic-pituitary-adrenal (HPA) axis hormones on the effects of nicotine [51, 54]. Using a subset of women smokers from the S. Allen et al. [45••] controlled cross-over trial, Huttlin et al. [51] assessed the association between menstrual phase and salivary cortisol with subjective responses to nicotine and found that associations between cortisol levels and subjective response to nicotine varied by menstrual phase. Specifically, during the follicular phase, higher morning cortisol levels and greater diurnal cortisol variation were associated with greater declines in negative affect and withdrawal following nicotine administration. A different pattern emerged during the luteal phase where higher morning cortisol was associated with a decrease in head rush and urge to smoking following nicotine administration [51]. HPA axis hormones were also examined in a pilot study conducted by Goletiani et al. [54], which explored the effects of cigarette smoking on HPA hormones ((i.e., adrenocorticotropin hormone (ACTH), cortisol, and dehydroepiandrosterone (DHEA)) and subjective responses by menstrual cycle phase after overnight abstinence. Findings revealed that smoking increased levels of HPA hormones, increased subjective ratings of high and rush, and decreased subjective craving; however, there were no significant differences in HPA hormones or subjective ratings between follicular and luteal groups after smoking. The lack of significant differences between follicular and luteal groups is surprising and might be a ceiling effect on physiological and subjective responses as smoking relieved the withdrawal state induced by overnight abstinence. In other words, as women in both groups were in a highly vulnerable, deprived state, all women experienced smoking-related responses. However, an alternative explanation was explored by the authors. After reviewing the data, Goletiani et al. [54] found that the luteal group was comprised of women smokers with differing levels of progesterone. As such, post hoc analyses examined differences between groups after the luteal group was divided into two groups (i.e., high progesterone, low progesterone) and found that the high progesterone luteal group showed blunted subjective responses and ACTH response to nicotine. Although these post hoc findings are consistent with the idea that progesterone is protective and reduces the reward value of nicotine, conclusions are limited due to the small sample size. Together, findings from these two articles provide additional evidence for the effects of nicotine on HPA hormones, but the interactions between ovarian hormones and HPA hormones are complicated and additional research in this area is clearly warranted.
Conclusion
The literature on the influence of ovarian hormones and menstrual cycle phase on smoking-related responses and behavior continues to grow, and the recent research reviewed here provides additional support for the differential effects of estradiol and progesterone on vulnerability to smoking and relapse. Unfortunately, the specific influence of ovarian hormones and menstrual cycle phase on smoking behaviors remains unclear due to differences in research methodologies across studies and inconsistencies in research findings. As such, we suggest that the field moves toward standardizing the research approach by incorporating several aspects of the research reviewed here. Specifically, we encourage researchers to collect direct measures of ovarian hormones across multiple time points, at least weekly, throughout an entire menstrual cycle (at least one full menstrual cycle, but longer is ideal) to allow for a full characterization of the hormonal influences on smoking-related responses and behaviors. Importantly, the direct measures, preferably estradiol to progesterone ratios, can be used in analyses as a covariate or dependent variable, rather than a tool for stratifying women into groups or confirming whether a woman is in the follicular or luteal phase. We also suggest that researchers avoid stratifying women by phase and subsequently using menstrual phase as the proxy for the hormonal milieu because there are several important hormonal events that occur throughout the entire menstrual cycle that might alter and confound research findings. Further, smoking assessments should be standardized to include urine cotinine levels, time since last cigarette, and carbon monoxide (CO) measures, as relying on just one method does not provide enough data to fully assess whether an individual is actively smoking or abstaining, which can also affect results. Future studies that include such design features could help improve consistency across studies and allow judicious extrapolations thereby improving our understanding of the influences of the hormonal milieu on smoking among women.
If rigorous scientific study continues to demonstrate estradiol-mediated vulnerabilities and protective effects of progesterone, immediate translation to the clinic and smoking cessation outcomes could be realized. Simply timing quit dates to coincide with times of high progesterone levels during treatment interventions could significantly reduce relapse rates for women. Further, and perhaps more importantly, ongoing and future studies examining the safety and efficacy of exogenous progesterone as a smoking cessation treatment could improve treatment outcomes for both men and women.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
United States Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014.
Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF. Annual healthcare spending attributable to cigarette smoking: an update. Am J Prev Med. 2015;48(3):326–33. doi:10.1016/j.amepre.2014.10.012.
Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current Cigarette Smoking Among Adults - United States, 2005-2013. Morb Mortal Wkly Rep (MMWR) 2014.
Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addict Behav. 2013;38(2):1527–31. doi:10.1016/j.addbeh.2012.06.002.
Perkins KA, Giedgowd GE, Karelitz JL, Conklin CA, Lerman C. Smoking in response to negative mood in men versus women as a function of distress tolerance. Nicotine Tob Res. 2012;14(12):1418–25. doi:10.1093/ntr/nts075.
Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Womens Health (Larchmt). 2007;16(8):1211–8. doi:10.1089/jwh.2006.0281.
Vogel RI, Hertsgaard LA, Dermody SS, Luo X, Moua L, Allen S, et al. Sex differences in response to reduced nicotine content cigarettes. Addict Behav. 2014;39(7):1197–204. doi:10.1016/j.addbeh.2014.03.021.
Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl). 2006;184(3-4):600–7. doi:10.1007/s00213-005-0103-7.
Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr Symp Motiv. 2009;55:143–69.
Van Voorhees EE, Mitchell JT, McClernon FJ, Beckham JC, Kollins SH. Sex, ADHD symptoms, and smoking outcomes: an integrative model. Med Hypotheses. 2012;78(5):585–93. doi:10.1016/j.mehy.2012.01.034.
Smith PH, Kasza KA, Hyland A, Fong GT, Borland R, Brady K, et al. Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17(4):463–72. doi:10.1093/ntr/ntu212.
Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99(11):1462–9.
Schnoll RA, Patterson F. Sex heterogeneity in pharmacogenetic smoking cessation clinical trials. Drug Alcohol Depend. 2009;104 Suppl 1:S94–9. doi:10.1016/j.drugalcdep.2008.11.012.
Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–57. doi:10.1093/ntr/ntq067.
Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72(4):712–22.
Printz C. Lung cancer new leading cause of death for women in developed countries: data reflects increased rates of smoking. Cancer. 2015;121(12):1911–2. doi:10.1002/cncr.28995.
Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–305. doi:10.1016/S0140-6736(11)60781-2.
Yen SSC, Jaffee RB, Barbieri RL. Reproductive endocrinology: physiology, pathophysiology and clinical management. Philadelphia: W.B. Saunders Company; 1999.
Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL et al. Determining menstrual phase in human biobehavioral research: a review with recommendations. Exp Clin Psychopharmacol. in press.
Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, et al. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology. 2009;34(3):548–54. doi:10.1038/npp.2008.3.
Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl). 2008;197(2):237–46. doi:10.1007/s00213-007-1028-0.
Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl). 2002;164(2):121–37. doi:10.1007/s00213-002-1183-2.
Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–61. doi:10.1037/a0021265.
Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62(5):1750–6.
Dluzen DE, Anderson LI. Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett. 1997;230(2):140–2.
Allen SS, Allen AM, Lunos S, Hatsukami DK. Patterns of self-selected smoking cessation attempts and relapse by menstrual phase. Addict Behav. 2009;34(11):928–31. doi:10.1016/j.addbeh.2009.05.013.
Dempsey D, Jacob 3rd P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–8.
Weinberger AH, Smith PH, Allen SS, Cosgrove KP, Saladin ME, Gray KM, et al. Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res. 2015;17(4):407–21. doi:10.1093/ntr/ntu249.
Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob 3rd P. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–8.
Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69(1-2):299–304.
Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum Psychopharmacol. 2009;24(7):559–64. doi:10.1002/hup.1055.
Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64(2):366–79.
Franklin TR, Jagannathan K, Wetherill RR, Johnson B, Kelly S, Langguth J, et al. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob Res. 2015;17(4):390–7. doi:10.1093/ntr/ntu183. This study showed that women in the follicular phase of their menstrual cycle exhibited greater neural activiation to smoking cues relative to nonsmoking cues) in brain regions commonly associated with reward processes. Further, follicular phase women reported smoking cue-elicited craving, which correlated with smoking cue-elicited neural activation in the insula; whereas, luteal women did not.
Mendrek A, Dinh-Williams L, Bourque J, Potvin S. Sex differences and menstrual cycle phase-dependent modulation of craving for cigarette: an FMRI pilot study. Psychiatry J. 2014;2014:723632. doi:10.1155/2014/723632.
Studer B, Cen D, Walsh V. The angular gyrus and visuospatial attention in decision-making under risk. NeuroImage. 2014;103:75–80. doi:10.1016/j.neuroimage.2014.09.003.
Torres OV, O’Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry. 2015. doi:10.1016/j.pnpbp.2015.04.005.
Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24(2):247–55. doi:10.1177/0269881108095716.
McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25(4):490–502. doi:10.1177/0269881110376694.
Schmaus BJ, Laubmeier KK, Boquiren VM, Herzer M, Zakowski SG. Gender and stress: differential psychophysiological reactivity to stress reexposure in the laboratory. Int J Psychophysiol. 2008;69(2):101–6. doi:10.1016/j.ijpsycho.2008.03.006.
Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21(3):210–20. doi:10.1111/j.1521-0391.2012.00232.x.
Saladin ME, Wray JM, Carpenter MJ, McClure EA, LaRowe SD, Upadhyaya HP, et al. Menstrual cycle phase effects in the gender dimorphic stress cue reactivity of smokers. Nicotine Tob Res. 2015;17(5):607–11. doi:10.1093/ntr/ntu203. Very important study with innovative and strong research methodology demonstrating the direct effects of progesterone on medication-assisted smoking cessation.
Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, et al. Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob Res. 2015;17(4):398–406. doi:10.1093/ntr/ntu262.
Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36(1):123–32. doi:10.1016/j.psyneuen.2010.07.005.
Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008;103(5):809–21. doi:10.1111/j.1360-0443.2008.02146.x.
Allen SS, Allen AM, Tosun N, Lunos S, al’absi M, Hatsukami D. Smoking- and menstrual-related symptomatology during short-term smoking abstinence by menstrual phase and depressive symptoms. Addict Behav. 2014;39(5):901–6. doi:10.1016/j.addbeh.2014.01.029. Important study using controlled cross-over design to examine the effects of menstrual phase, depressive symptoms, and the combined effect of menstrual phase and depressive symptoms on smoking- and menstrual-related symptomatology during acute smoking abstinence. Several secondary analyses have been published from this main study.
Allen AM, al’Absi M, Lando H, Allen SS. Allopregnanolone association with psychophysiological and cognitive functions during acute smoking abstinence in premenopausal women. Exp Clin Psychopharmacol. 2015;23(1):22–8. doi:10.1037/a0038747.
Allen AM, al’Absi M, Lando H, Hatsukami D, Allen SS. Menstrual phase, depressive symptoms, and allopregnanolone during short-term smoking cessation. Exp Clin Psychopharmacol. 2013;21(6):427–33. doi:10.1037/a0034075.
Allen AM, Lunos S, Heishman SJ, al’Absi M, Hatsukami D, Allen SS. Subjective response to nicotine by menstrual phase. Addict Behav. 2015;43:50–3. doi:10.1016/j.addbeh.2014.12.008.
Allen SS, Allen AM, Kotlyar M, Lunos S, Al’absi M, Hatsukami D. Menstrual phase and depressive symptoms differences in physiological response to nicotine following acute smoking abstinence. Nicotine Tob Res. 2013;15(6):1091–8. doi:10.1093/ntr/nts236.
Hinderaker K, Allen AM, Tosun N, al’Absi M, Hatsukami D, Allen SS. The effect of combination oral contraceptives on smoking-related symptomatology during short-term smoking abstinence. Addict Behav. 2015;41:148–51. doi:10.1016/j.addbeh.2014.10.018.
Huttlin EA, Allen AM, Tosun NL, Allen SS, al’Absi M. Associations between adrenocortical activity and nicotine response in female smokers by menstrual phase. Addict Behav. 2015;50:135–9. doi:10.1016/j.addbeh.2015.06.026.
DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–40. doi:10.1038/npp.2013.339.
Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ. Association between ovarian hormones and smoking behavior in women. Exp Clin Psychopharmacol. 2012;20(4):251–7. doi:10.1037/a0027759.
Goletiani NV, Siegel AJ, Lukas SE, Hudson JI. The effects of smoked nicotine on measures of subjective States and hypothalamic-pituitary-adrenal axis hormones in women during the follicular and luteal phases of the menstrual cycle. J Addict Med. 2015;9(3):195–203. doi:10.1097/ADM.0000000000000117.
Acknowledgments
Reagan R. Wetherill would like to gratefully acknowledge the National Institutes of Health/National Institute on Drug Abuse grants (R01DA030394 and R01DA029845 awarded to Teresa R. Franklin; R01DA008075 and R21DA034840 awarded to Sharon S. Allen) and National Institute on Alcohol Abuse and Alcoholism grant (K23AA023894 awarded to Reagan R. Wetherill), in support of writing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Reagan R. Wetherill, Teresa R. Franklin, and Sharon Allen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by any of the authors. All studies conducted by the authors using human subjects were in accordance with each author’s respective institution IRB standards. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
This article is part of the Topical Collection on Tobacco
Rights and permissions
About this article
Cite this article
Wetherill, R.R., Franklin, T.R. & Allen, S.S. Ovarian Hormones, Menstrual Cycle Phase, and Smoking: a Review with Recommendations for Future Studies. Curr Addict Rep 3, 1–8 (2016). https://doi.org/10.1007/s40429-016-0093-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40429-016-0093-z