Abstract
Rationale
Compared to men, the smoking behavior of women may be less responsive to nicotine and more responsive to nonpharmacological factors, perhaps including verbal information (e.g., dose instructions).
Objective
This study compared the influence of the presence vs absence of dose instructions on the subjective and reinforcing effects of nicotine via cigarette smoking in men and women.
Methods
Subjects (n=120) abstained overnight from smoking and were randomly assigned to one of four groups. Half of the subjects received nicotine cigarettes (Quest 1, yield of 0.6 mg), and the other half received denicotinized cigarettes (“denic”; Quest 3, yield of 0.05 mg). Furthermore, half of each subsample was accurately instructed they were receiving a “normal nicotine” or a “no nicotine” cigarette, while the other half received no instructions. Subjects completed baseline measures of craving and mood (positive and negative affect), took two puffs from the cigarette after receiving dose instructions or no instructions, and then rated the cigarette's “reward” value (liking, satisfying) and other characteristics. They also repeated the craving and mood measures. Subjects then smoked more of that same brand ad libitum over the next 30 min to measure reinforcement (puff number and latency to first puff).
Results
Overall, nicotine increased reward, other cigarette ratings, and positive affect, but did not affect craving or smoking behavior. However, results varied by sex. Dose instructions enhanced the effects of nicotine on smoking reward and reinforcement in women, while instructions tended to dampen or even reverse these effects of nicotine in men (i.e., interaction of sex×nicotine×instructions).
Conclusions
In women but not in men, the influence of nicotine on smoking reward and reinforcement is enhanced by accurate verbal information about the cigarette's nicotine dose. These results are consistent with the notion that the smoking behavior of women, relative to men, may be more responsive to nonpharmacological factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug responses are influenced by pharmacological and nonpharmacological factors. The most common procedure of testing pharmacological effects of a drug in human research is under “blind” conditions, in which subjects are given no explicit verbal information about the drug (or told the substance may or may not contain active drug). Blind conditions hold constant nonpharmacological factors, such as expectancies about drug (Kirsch 1997), allowing the isolation of the drug's pharmacological effects. Conversely, research on nonpharmacological factors in drug effects usually involves the opposite manipulation, holding constant pharmacological exposure while varying nonpharmacological factors. For example, verbal information provided to subjects regarding the drug content of a substance (e.g., instructions that it contains “drug” vs “no drug”) can influence the responses of subjects to consuming the substance (e.g., Fillmore and Vogel-Sprott 1992; Kirsch 1997). Such information can influence responses to cigarette smoking (Juliano and Brandon 2002), sometimes to a greater extent than actual nicotine dose (Perkins et al. 2003, 2004). Because accurate information about substances is usually available to drug users in the natural environment, testing drug effects in the presence of dose instructions may better capture drug responses as they occur under naturalistic conditions, compared to testing in the absence of instructions (Kirsch and Rodino 1993; Martin and Sayette 1993).
Several studies have directly compared responses to drug as a function of the presence vs absence of dose instructions to determine if providing such instructions enhances responding. A few studies assessing physiological and subjective arousal effects of caffeine failed to show differences due to the presence vs absence of instructions. However, these studies did not manipulate dose, administering only caffeine (Nash et al. 2002) or only a placebo beverage (i.e., no caffeine; Mikalsen et al. 2001, experiment 2; Walach et al. 2002). Yet, Kirsch and Rodino (1993) also manipulated dose and found that self-reported tension was greater for caffeine vs placebo only among those given instructions that the beverage contained caffeine and not among those given no instructions or those told it contained no caffeine. In a study with alcohol, Bradlyn and Young (1983) found greater intoxication ratings due to 0.3 g/kg alcohol in those given accurate dose instructions vs those given no instructions, but instructions had no effect on ratings among subjects given a drink with no alcohol or a higher dose (0.6 g/kg).
In a clinical study involving quitting smokers, Hughes et al. (1989) found that under accurate dose instructions, but not under blind conditions, nicotine vs placebo gum medication influenced the number of cigarettes smoked, the rated helpfulness of the gum, and gum self-administration (trend only). On the other hand, other measures, such as self-reported side effects of the gum and smoking abstinence, tended to show the opposite influence of instructions—smaller effects of drug when subjects were accurately instructed about dose relative to blind conditions—suggesting that dose instructions dampened, rather than enhanced, some drug effects. To our knowledge, no study has compared the influence of the presence vs absence of dose instructions on the acute effects of nicotine in cigarettes (i.e., an abused form of nicotine).
In addition, unexplored are individual differences in the influence of explicit dose instructions on drug responding. There is reason to believe that men and women may differ in responsivity to some instructional manipulations, at least when it comes to smoking or nicotine reinforcement (self-administration) and reward (i.e., hedonic characteristics of the drug, such as self-reported “liking”). We have found that, relative to men, these effects in women may be more sensitive to nonpharmacological factors such as nonverbal information about smoking, or “cues” (e.g., sensory stimuli from smoking; Perkins et al. 2001), and less sensitive to the pharmacological factor of actual nicotine dose (i.e., assessed under blind conditions; Perkins et al. 1996, 2002). Other research suggests that women are more susceptible than men to instructional manipulations when reporting on perceived internal states (Roberts and Pennebaker 1995; Tremblay et al. 2004).
Recently, we examined the influence of the type of verbal information about nicotine dose (accurate vs inaccurate dose instructions), as well as the actual dose, on responses to smoking (Perkins et al. 2004). This study used a modified 2×2 balanced-placebo design (see Martin and Sayette 1993), in which subjects were divided into groups receiving a cigarette containing nicotine or one containing no nicotine (i.e., placebo), and within each group, half of the subjects received accurate instructions and the other half inaccurate instructions about the nicotine content of the cigarette they received. Thus, all subjects got dose instructions of some kind; there was no blind condition (i.e., a “no instruction” condition). We found that under accurate, but not inaccurate, dose instructions, nicotine increased smoking reward more in women than in men. However, there was no sex difference in smoking reinforcement, measured in this study by a computer task with escalating response requirements (i.e., progressive ratio) for earning cigarette puffs.
Thus, it appears that the rewarding and reinforcing effects of smoking in women, compared to men, are less sensitive to cigarette nicotine dose under blind conditions (Perkins et al. 2002), while the rewarding effects may be more sensitive to nicotine under conditions of accurate dose instructions (Perkins et al. 2004). However, confirmation of this notion requires a direct test of the effects due to the presence vs absence of dose instructions within one study, given the variability in procedures between these studies. If confirmed, this finding would extend prior observations regarding sex differences in response to nonverbal information about smoking (i.e., cues; Perkins et al. 2001) to another type of nonpharmacological factor in drug reward and reinforcement, verbal information about smoking (i.e., nicotine dose instructions).
In the present study, we directly compared the influence of accurate dose instructions vs no dose instructions on the subjective and reinforcing effects of nicotine via cigarette smoking in men and women. We hypothesized that the presence of dose instructions would enhance the influence of nicotine on these effects, compared to no instructions (i.e., blind conditions). We also hypothesized that the impact of instructions on the rewarding and reinforcing effects of nicotine (but not other effects) would be greater in women than in men, consistent with the notion that smoking behavior of women is more responsive than that of men to nonpharmacological factors.
Method
Subject eligibility
Eligible subjects were healthy young men and women who smoked more than ten cigarettes per day for at least 1 year. Their preferred brand had to have a nicotine yield of at least 0.6 mg, so that the denicotinized brand used in the study would be distinctly different from their usual brand (see below). Subjects were excluded if they had ever smoked “ultra-light” brands (defined as nicotine yield <0.3 mg) for more than 2 weeks. Participants were recruited from ads placed in the surrounding community and were paid for participation.
Cigarettes
To manipulate nicotine exposure, the “normal” nicotine brand was Quest 1 (yield of 0.6 mg nicotine, 9 mg tar) and the denicotinized (“denic”) brand was Quest 3 (yield <0.05 mg nicotine, 9 mg tar), both sold commercially by Vector Group, Ltd. (Miami, FL). Menthol smokers received menthol Quest; nonmenthol smokers received nonmenthol Quest. All cigarettes had identifiable markings covered.
Subjective measures
Cigarette ratings
Smoking reward was assessed with two items from the Rose Sensory Questionnaire (see Westman et al. 1996): the ratings of “liking” and “satisfying.” The Rose measure also asks “how high in nicotine” and “how similar to own brand” the cigarette is, which were included to assess other perceptual responses to the cigarettes that might differ as a function of nicotine and dose instructions. Each item in the Rose Questionnaire was rated on a 0 (“not at all”) to 100 (“very much”) visual analog scale (VAS). This measure has been used in similar studies from this lab (Perkins et al. 2001, 2002).
Mood
Self-reported mood was assessed with the Diener and Emmons (1984) Mood Scale, consisting of nine items rated on 0 (not at all) to 100 (very much) VAS that yield a positive affect (PA) and negative affect (NA) score. PA scale items are happy, joyful, pleased, and enjoyment/fun, while NA scale items are depressed/blue, unhappy, frustrated, worried/anxious, and angry/hostile.
Craving
Craving was assessed with the 4-item version of the Questionnaire on Smoking Urges (QSU), a widely used clinical and laboratory measure of urges to smoke (Tiffany and Drobes 1991). The four items in this version, which is described by Carter and Tiffany (2001), are “Nothing would be better than smoking a cigarette right now,” “I have an urge for a cigarette,” “All I want now is a cigarette,” and “I crave a cigarette right now.”
Smoking behavior
Smoking reinforcement was determined by behavioral observation of the number of puffs and latency to first puff during the 30-min ad lib smoking period (see Procedure, below). These measures were obtained by videotaping the subjects during this period (see Perkins et al. 1997; Conklin and Perkins 2005). Inter-rater reliability of assessing smoking behavior in this manner exceeds 99% (Perkins et al. 1997).
Procedure
Subjects participated in one session, preceded by overnight abstinence from smoking (at least 14 hrs), verified by expired-air CO<13 ppm, as in previous research (e.g., Perkins et al. 2002). They provided written informed consent to participate in a study to “evaluate the characteristics of different types of cigarettes and how much smokers like them.” Subjects were randomly assigned to one of four conditions manipulating dose instructions and cigarette nicotine content in a 2×2 between-subjects design: accurate instructions about the cigarette's nicotine dose vs no dose instructions (i.e., kept blind) and given nicotine vs denic cigarettes. Groups were stratified by sex, so that each condition had 15 men and 15 women.
After a brief rest, subjects completed baseline measures of craving and mood. They then were given general instructions about the session, namely, that they would take a few puffs on a cigarette, rate it, and then be able to smoke more of that cigarette if they wanted. Subjects were introduced to the designated cigarette (nicotine vs denic) and, depending on their group assignment, given either no instructions or the following dose instructions:
-
Subjects given the nicotine brand (Quest 1) were told: “This cigarette has a normal amount of nicotine.”
-
Subjects given the denic brand (Quest 3) were told: “This cigarette has no nicotine.”
Subjects then took two puffs to sample the cigarette (exposure period), rated it on the Rose Questionnaire, and repeated the craving and mood measures. Then, subjects were given ten more of the same cigarettes and told they were free to smoke as much or as little as they wanted over the subsequent 30 mins. Subjects were given reading material and allowed to listen to the radio.
Upon completion of the session, subjects were debriefed and told that our interest was to examine conditions that affect smokers' preferences for different cigarettes. This study was approved by the University of Pittsburgh Institutional Review Board.
Data analysis
Analyses of variance (ANOVAs) were used to analyze Rose Questionnaire items after the exposure puffs, as well as the change in mood (negative, positive affect) and craving from baseline to after the exposure puffs. Instructions (presence vs absence of dose instructions), nicotine (nicotine vs denic cigarette), and sex were between-subjects factors. Time (baseline to postexposure) was an added within-subjects factor for mood and craving ANOVAs. The number of puffs during the 30-min ad lib smoking period and the latency to first puff were each analyzed by similar ANOVAs. Follow-up tests were conducted by Fisher's LSD t test (see Huitema 1980). We hypothesized an interaction of nicotine×instructions for all measures, indicating that the presence of instructions would enhance nicotine's effects. Based on prior research (see Introduction), we also hypothesized that this influence of instructions would be greater for women vs men in analyses of the smoking reward and reinforcement measures (i.e., significant interaction of sex×nicotine×instructions). Because men and women differed on cigarettes per day (see Subject characteristics, below), reward and reinforcement ANOVAs with significant interactions involving sex were repeated using cigarettes per day as a covariate to determine whether the interaction could be due to the difference in smoking frequency.
Results
Subject characteristics
Characteristics (mean±SE) for the 60 men and 60 women, respectively, were as follows: age, 32.0±1.6 vs 28.6±1.3; nicotine yield of preferred brand, 1.04±0.03 vs 0.99±0.03 mg; daily smoking rate, 19.7±1.0 vs 15.9±0.5; number of years smoking, 14.8±1.5 vs 11.7±1.2; and Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al. 1991) score, 4.9±0.3 vs 4.5±0.2. Men and women did not differ significantly on these characteristics, except daily smoking rate [t(118)=3.37, p<.001], as commonly observed in the general population of smokers (compare with means of 20.6 vs 17.0 cigarettes per day for American men and women daily smokers, respectively; Centers for Disease Control 2003).
ANOVA results for all measures
The ANOVA results are summarized for all measures in Table 1.
Cigarette ratings
Reward
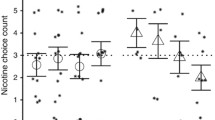
As expected, nicotine increased the cigarette reward items of liking and satisfying (see Table 1). More importantly, men and women differed in how they rated cigarette reward in response to the interaction of nicotine and dose instructions. For women, nicotine increased liking only in the presence of dose instructions and not in the absence of instructions, as hypothesized (see Fig. 1). Results were identical for the measure of satisfying (not shown). Unexpectedly, however, the opposite was seen for men, as nicotine increased each measure only in the absence of dose instructions and not in the presence of instructions. When examined separately by sex in follow-up tests, the interaction of nicotine × instructions significantly affected liking for women [t(59)=2.45, p<.05] and for men [t(59)=4.26, p<.001]. Results were similar for satisfying.
Ratings of reward (liking; top) and cigarette perceptual characteristics (similar to own brand, how much nicotine; bottom) as a function of nicotine, the presence (yes) vs absence (no) of dose instructions and, where relevant, subject's sex. Those presented with dose instructions were accurately informed whether the cigarette contained a normal amount of nicotine or no nicotine. Each rating was made using a visual analog scale (range=0–100). *p<0.05; **p<0.01; ***p<0.001 for comparisons between nicotine and denicotinized cigarettes
Perceptual characteristics
Nicotine increased the rating of “similar to own brand,” while sex interacted with dose instructions on this measure. In women, this rating was higher in the presence vs absence of instructions, regardless of nicotine, while the opposite was true in men (see Fig. 1). Nicotine also increased the rating of “how much nicotine” and interacted with instructions, such that the difference in ratings due to nicotine was greater in the presence vs absence of instructions (Fig. 1). There were no differences due to sex on this rating.
Mood and craving change
Baseline values (mean±SE) were not significantly different due to sex, nicotine, or instructions for negative affect (27.9±1.8), positive affect (43.5±1.8), and craving (76.6±1.7). As shown in Fig. 2, negative affect decreased from baseline to postexposure puffs [i.e., main effect of time; F(1,112)=18.83, p<.001], but was not significantly influenced by nicotine, sex, or instructions. Nicotine increased positive affect, but neither sex nor instructions had significant effects (see Fig. 2). Craving decreased from baseline to postexposure puffs [main effect of time; F(1,112)=9.89, p<.005], and this decrease was greater in men than women. Moreover, for men but not women, nicotine decreased craving in the presence of instructions but not in the absence of instructions (Fig. 2), opposite of the pattern of results for smoking reward (Fig. 1).
Change from presmoking baseline to postexposure puffs in negative and positive affect scores and in craving as a function of nicotine and, where relevant, sex and dose instructions. Asterisks as in Fig. 1
Smoking behavior
Similar to the results for cigarette reward ratings (above), smoking reinforcement during the ad lib smoking period differed between men and women in response to nicotine and dose instructions. As shown in Fig. 3, for women, nicotine increased the number of puffs and decreased latency to first puff only in the presence of dose instructions and not in the absence of instructions, as hypothesized, while these effects were not seen in men. Confirming this interpretation was the significant interaction of nicotine×instructions within the women alone for both puff number [t(59)=2.12, p<.05] and latency [t(59)=4.30, p<.001], but not within men [t's(59)=1.67 and 1.54, respectively, not significant]. Overall, smoking latency was slower in the presence vs absence of dose instructions, regardless of nicotine.
Smoking reinforcement (number of puffs, latency to first puff) during the 30-min ad lib smoking period due to nicotine, dose instructions, and sex. Asterisks as in Fig. 1
Covarying for cigarettes per day
Because men and women differed significantly on cigarettes per day, we repeated the analyses for the reward and reinforcement measures by conducting analyses of covariance (ANCOVA), with cigarettes per day as the covariate. Results were unchanged for the reward measures of liking and satisfying, and for the reinforcement measure of latency to first puff. However, the interaction of sex×nicotine×instructions was no longer significant for number of puffs during the ad lib period [F(1,111)=2.60, p=.11, vs p<.05 without covarying cigarettes per day; see Table 1].
Discussion
In women, nicotine increased smoking reward and reinforcement only in the presence of dose instructions and not in the absence of instructions. In men, by contrast, instructions did not increase nicotine's effects on smoking reward and reinforcement. The greater responsivity of women vs men to nonpharmacological factors in smoking has previously been shown in research manipulating nonverbal drug information (e.g., cues; Perkins et al. 2001). The present study extends this potential sex difference to verbal drug information (i.e., dose instructions). The robustness of the effects of these instructions is perhaps surprising in light of their brevity, a single short sentence among other instructions given to all subjects. More extensive instructions, or instructions about the likely effects of the drug (i.e., manipulation of response expectancies; see Kirsch 1997), may have an even more pronounced influence on responding in women vs men.
In general, our findings confirm results from prior studies showing that nicotine increases smoking reward and reinforcement less in women vs men under blind conditions (Perkins et al. 2002), while nicotine increases smoking reward (if not reinforcement) more in women vs men under conditions of accurate dose instructions (Perkins et al. 2004). However, these prior studies differed from each other in a number of ways (e.g., differences in samples, types of nicotine cigarettes, measures of reinforcement, within- vs between-subjects designs). By directly manipulating the presence vs absence of dose instructions within the same study, the current findings strengthen conclusions about these sex differences in response to dose instructions. Notably, because of the sex difference in response to nicotine as a function of dose instructions, the two-way interaction of nicotine × instructions was not significant for almost all measures (contrary to hypotheses). Thus, if we had not explicitly examined the possibility of sex differences in the influence of dose instructions, we may have concluded that providing dose instructions, a potentially important nonpharmacological factor in drug effects, has essentially no influence on the effects of nicotine during cigarette smoking. Clearly, such instructions do alter the effects of nicotine on smoking reward and reinforcement, but the influence differs markedly based on subject sex. Moreover, these results may have broader implications for pharmacology studies with women. As noted in the introduction, because most human pharmacology studies are conducted under blind conditions (without dose instructions), while drug users in the natural environment are not blind to drug, the results for women in pharmacology studies may not generalize well to drug effects for women in the natural environment.
In regard to possible explanations for these sex differences, differential perception of nicotine does not seem to be relevant, as men and women did not differ in ratings of “how much nicotine.” Affect and craving responses also would not seem to explain these sex differences, since men and women generally did not differ on these responses, except for the greater craving relief due to nicotine in men given dose instructions (which was opposite of the observed sex differences in nicotine reward and reinforcement). When the analyses for reward and reinforcement were repeated using as a covariate cigarettes per day, which differed between men and women, the sex differences were unchanged for the reward and smoking latency measures but were no longer significant for number of puffs. Therefore, sex differences in the amount of puffing in response to the instructional and nicotine manipulations could be related to the higher daily smoking rate in men vs women, but this is unlikely to account for the other sex differences.
An unexpected observation was the apparent dampening influence of dose instructions on the effect of nicotine on smoking reward in men. Under no instructions (i.e., blind conditions), smoking reward in men differed markedly between the nicotine and denic cigarettes (see Fig. 1), as previously observed (Perkins et al. 2002). Yet, when given accurate instructions about dose, smoking reward in men no longer differed between the nicotine and denic cigarettes. In addition, smoking reinforcement (number of puffs, latency) in men was nonsignificantly reduced by nicotine under the dose instruction conditions, relative to no instructions (see Fig. 3). These observations suggest that dose instructions may dampen or reverse the typical effects of nicotine per se on smoking reward and reinforcement in men. Such findings are not unprecedented and are similar to the previously noted results for side effects and smoking abstinence reported by Hughes et al. (1989) in a clinical study with nicotine gum. On the other hand, nicotine alleviated craving in men only in the presence of instructions and not in the absence of instructions (Fig. 2), suggesting that instructions do enhance some effects of nicotine in men.
In contrast with most other responses, dose instructions had no effects on mood following the exposure puffs. Of interest, however, is the fact that smoking either the denic or nicotine cigarette (regardless of instructions) reduced negative affect, which was modestly elevated due to overnight abstinence, while only the nicotine cigarette increased positive affect (Fig. 2). Conceivably, withdrawal-related negative affect may be alleviated by mere smoking behavior, with or without nicotine, perhaps suggesting that relief of this type of negative affect is a conditioned response to smoking. On the other hand, increases in positive affect may be a direct influence of nicotine and largely unaffected by conditioned effects of smoking (see Rose et al. 2003).
As noted, these findings are consistent with research indicating broader sex differences in the relative influence of pharmacological (i.e., nicotine) and nonpharmacological factors on acute reinforcing effects of smoking in humans (see reviews by Perkins 1996; Perkins et al. 1999). Compared with men, the rewarding and reinforcing effects of smoking in women generally are less responsive to nicotine per se and more responsive to nonpharmacological manipulations such as verbal (instructions) and nonverbal (cues) information about drug. Moreover, nicotine self-administration behavior in female rats (vs male rats) is similarly more responsive to the presence of nicotine-associated stimuli, although female rats do not appear to be less sensitive than male rats to nicotine per se (Chaudhri et al. 2005). Research is more limited in regard to sex differences in pharmacological and nonpharmacological factors in the acute effects of other drugs. However, women may be more sensitive than men on some subjective responses to cocaine-related visual stimuli (Robbins et al. 1999) but less sensitive to cocaine per se (Lukas et al. 1996). Thus, sex differences in these influences may reflect very basic biological factors that cut across species and generalize across drugs, although far more research is needed to confirm the generalizability of these differences.
A strength of this study is that, to our knowledge, it is the first study to explicitly compare the influence of the presence vs absence of dose instructions on the acute subjective and reinforcing effects of cigarette smoking. It is also the first study with any drug to examine sex differences in response to this type of instructional manipulation. Limitations of this study include the very modest level of smoking exposure on which the reward and other ratings were based (two puffs). This amount was chosen because greater exposure could lead virtually any smoker to detect interoceptive effects of nicotine from the nicotine cigarette, overwhelming the instructional manipulation. We also wanted subjects to remain sufficiently smoke-deprived as to allow an adequate test of smoking reinforcement during the subsequent ad lib smoking period. Another limitation is the relatively young sample of smokers; results with older and more dependent smokers may differ (see Brauer et al. 2001).
In conclusion, the presence of accurate dose instructions enhanced the effects of nicotine on smoking reward and reinforcement in women, but not in men. These findings are consistent with past research indicating that, compared to men, smoking behavior of women may be influenced less by nicotine and more by nonpharmacological factors. Previous studies showed that such factors included nonverbal information of smoking cues (sight and smell of smoke; Perkins et al. 2001), and this study extends these factors to include verbal information about nicotine dose. Future research should examine sex differences in the rewarding and reinforcing effects of other drugs following similar manipulations of verbal information.
References
Bradlyn AS, Young LD (1983) Parameters influencing the effectiveness of the balanced placebo design in alcohol research. In: Pohorecky LA, Brick J (eds) Stress and alcohol use. Elsevier Science, New York, pp 87–103
Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE (2001) Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine Tob Res 3:101–109
Carter BL, Tiffany ST (2001) The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Exp Clin Psychopharmacol 9:183–190
Centers for Disease Control (CDC) (2003) Cigarette smoking behavior of adults: United States, 1997–98. Advance Data, no. 331
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA (2005) Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180:258–266
Conklin CA, Perkins KA (2005) Subjective and reinforcing effects of smoking during negative mood induction. J Abnorm Psychol 114:153–164
Diener E, Emmons RA (1984) The independence of positive and negative affect. J Pers Soc Psychol 47:1105–1117
Fillmore M, Vogel-Sprott M (1992) Expected effect of caffeine on motor performance predicts the type of response to placebo. Psychopharmacology 106:209–214
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127
Hughes JR, Gulliver SB, Amori G, Mireault GC, Fenwick JF (1989) Effect of instructions and nicotine on smoking cessation, withdrawal symptoms, and self-administration of nicotine gum. Psychopharmacology 99:486–491
Huitema BE (1980) Analysis of covariance and alternatives. Wiley, New York
Juliano LM, Brandon TH (2002) Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnorm Psychol 111:88–97
Kirsch I (1997) Specifying nonspecifics: psychological mechanisms of placebo effects. In: Harrington A (ed) The placebo effect: an interdisciplinary exploration. Harvard University Press, Cambridge MA, pp 166–186
Kirsch I, Rodino MJ (1993) Do double-blind studies with informed consent yield externally valid results? Psychopharmacology 110:437–442
Lukas SE, Sholar MB, Fortin M, Wines J, Mendelson JH (1996) Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology 125:346–354
Martin CS, Sayette MA (1993) Experimental design in alcohol administration research: limitations and alternatives in the manipulation of dosage-set. J Stud Alcohol 54:750–761
Mikalsen A, Bertelsen B, Flaten MA (2001) Effects of caffeine, caffeine-associated stimuli, and caffeine-related information on physiological and psychological arousal. Psychopharmacology 157:373–380
Nash JM, Holroyd KA, Rokicki LA, Kvaal S, Penzien DB (2002) The influence of placebo awareness on stimulant drug response in a double-blind trial. Psychopharmacology 161:213–221
Perkins KA (1996) Sex differences in nicotine versus non-nicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol 4:166–177
Perkins KA, Grobe JE, D'Amico D, Fonte C, Wilson A, Stiller RL (1996) Low-dose nicotine nasal spray use and effects during initial smoking cessation. Exp Clin Psychopharmacol 4:157–165
Perkins KA, Grobe JE, Caggiula AC (1997) Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav 56:235–241
Perkins KA, Donny E, Caggiula AR (1999) Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1:301–315
Perkins KA, Gerlach D, Vender J, Grobe JE, Meeker J, Hutchison S (2001) Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res 3:141–150
Perkins KA, Jacobs L, Sanders M, Caggiula A (2002) Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology 163:194–201
Perkins KA, Sayette M, Conklin CA, Caggiula AR (2003) Placebo effects of tobacco smoking and other nicotine intake. Nicotine Tob Res 5:695–709
Perkins KA, Jacobs L, Ciccocioppo M, Conklin CA, Sayette M, Caggiula A (2004) The influence of instructions and nicotine dose on the subjective and reinforcing effects of smoking. Exp Clin Psychopharmacol 12:91–101
Robbins SJ, Ehrman RN, Childress AR, O'Brien CP (1999) Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend 53:223–230
Roberts TA, Pennebaker JW (1995) Gender differences in perceiving internal states: toward a his-and-hers model of perceptual cues. Adv Exp Soc Psychol 27:143–175
Rose JE, Behm FM, Westman EC, Bates JE, Salley A (2003) Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav 76:243–250
Tiffany ST, Drobes DJ (1991) The development and initial validation of a questionnaire of smoking urges. Br J Addict 86:1467–1476
Tremblay L, Elliott D, Starkes JL (2004) Gender differences in perception of self-orientation: software or hardware? Perception 33:329–337
Walach H, Schmidt S, Dirhold T, Nosch S (2002) The effects of a caffeine placebo and suggestion on blood pressure, heart rate, well-being and cognitive performance. Int J Psychophysiol 43:247–260
Westman EC, Behm FM, Rose JE (1996) Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav 53:309–315
Acknowledgements
This research was supported by Grants DA12655 and DA16483 from the National Institute on Drug Abuse. The authors thank Carolyn Fonte for her helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perkins, K.A., Doyle, T., Ciccocioppo, M. et al. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology 184, 600–607 (2006). https://doi.org/10.1007/s00213-005-0103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0103-7