Abstract
Several theories have been proposed to explain the raised richness and diversity of species in native tropical forests, with an emphasis on those that invoke the niche characteristics. In the present study, we sample the woody vegetation in four enclaves of Deciduous Forests and investigate whether environmental variables can explain the floristic and structural differences among the sampled fragments. The studied areas are located the “Cerrado” biome core zone and in “Cerrado”–Atlantic Forest and “Cerrado–Caatinga” transition zones. The woody vegetation (diameter at breast height—DBH ≥ 5 cm) was sampled in 100 plots of 20 × 20 m, 25 plots in each enclaves. The investigated environmental variables were chemical and textural properties of the soil, rockiness, declivity and altitude. We found significant differences for the floristic variables (richness, diversity), among all the environmental variables, as well as for the density, height and for the DBH first class. We noticed strong influence of the environmental variables, and the nutrients availability, texture, soil acidity and the land relief are the most responsible for the floristic-cultural difference. We propose that the gradient existence of rainfall has influenced the edaphic characteristics, creating variations in the habitats, which may have favored the arrival and establishment of different species in each study area and the distinctive development of the woody vegetation at the enclaves to the Deciduous Forest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tropical forests are geographically distributed near the equator line, and it is more specifically located between the Tropic of Cancer and the Tropic of Capricorn, between the latitudes 23.5°N and 23.5°S (Whitmore 1990). Those forests are recognized for their high richness and diversity of species, and several theories have been proposed to explain this pattern (Waide et al. 1999; Chown et al. 2003). Most of those theories are related to the high environmental heterogeneity (Wilson 2000; Kolb and Diekmann 2004). Thus, it is supposed that the spatial distribution of the species and the structure of the woody vegetation in tropical forests are related to the discontinuous distribution of biotic and abiotic factors along the space (Ricklefs 1977; Denslow 1980; Shmida and Wilson 1985) and of its complex chain of interactions.
Historical and biogeographical aspects are also fundamental to determine the distribution standards and vegetation structuration in the entire world (Kellman and Tackaberry 1997). However, this relation is difficult to measure. On the other hand, the local environmental variables, because of its ease to measure, are commonly most investigated mainly the association with composition and structure of the regional communities, especially the ones from edaphic order (fertility, pH, texture and water availability) and climatic order (rainfall and temperature) (Oliveira Filho et al. 1994; Oliveira Filho and Fontes 2000; Fagundes et al. 2007; Gonzaga et al. 2008; Machado et al. 2008; Apgaua et al. 2014; Neves et al. 2015).
The contribution of environmental variables to the distribution of species changes considerably in space, among vegetation types (Oliveira Filho and Fontes 2000) and in the same physiognomy, especially when it occurs in a wide geographical distribution, as in the Brazilian Deciduous Seasonal Forests (Santos et al. 2012). Because the vegetation is present in great extension in Brazil (Rizzini 1977; Ribeiro and Walter 2008) and in the form of enclaves along the “Cerrado”, Atlantic Forest and Amazon rainforest, in general distributed over limestone outcrops (Rizzini 1977) is submitted to a large environmental amplitude like the variation in the edaphic conditions (Fagundes et al. 2007) and climatic conditions (Santos et al. 2007), for example. The Neotropical Dry Forests have high diversity (alpha), high endemism and high species turnover (beta diversity) (Dryflor et al. 2016), but are highly threatened by human activities and have few protected areas (Miles et al. 2006).
Thus, we investigate the differences in chemical and textural properties of the soil, rockiness, declivity and altitude and we evaluate their relationships with the structural variables, and also the richness, the diversity, the equability and the rarity of species of the woody vegetation in four Deciduous Forest enclaves, located in the core zone of the “Cerrado” biome, in the “Cerrado”–Atlantic Forest transition zone and in the “Cerrado–Caatinga”. We evaluated the hypothesis that there are differences in the floristic and structural composition among the studied enclaves and that such differences in Deciduous Forests are the reflection of local abiotic factors.
Materials and methods
We sampled four fragments of the Deciduous Forest spread in four enclaves of this phytophysiognomy located in the core zone of the “Cerrado” biome (Paracatu, Minas Gerais; from now on denominated CE), in the “Cerrado”–Atlantic Forest transition zone (Arcos, Minas Gerais; denominated ZTMA) and two in the “Cerrado–Caatinga” (Peruaçu, Minas Gerais and Coribe, Bahia; denominated ZTCA 1 and ZTCA 2, respectively). Thus, the geographical extension of the work comprises the coordinates 13°29′–20°17′S and 44°14′ and 46°49′W (Fig. 1; Table 1).
Location of the Deciduous Forest enclaves sampled in different Brazilian phytogeographical domains with detail of biomes (light gray) “Caatinga”; (medium gray) “Cerrado”; (dark gray) Atlantic Forest. CE Core zone of the “Cerrado” biome, ZTMA “Cerrado”–Atlantic Forest transition zone and ZTCA 1 and ZTCA 2 = “Cerrado–Caatinga” transition zone. See description of the areas in Table 1
To sample the woody vegetation, we divided each fragment in 20-m wide strips, perpendicular to the land declivity, and subdivided those strips into plots of 20 × 20 m. Then, we randomly selected the sampling lines and then the plots in the lines. Thus, to each fragment, we randomly selected 25 plots, which totalized one hectare of sampling area. We inventoried all of the tree individuals which had the diameter at breast height (DBH) ≥ 5 cm, except dead individuals, lianas and climbers. Individuals with multiple stems were measured when the square root of the sum of the perimeters was >15.71 cm (square root of the sum of the diameters’ squares), which implies an equivalent basal area (Moro and Martins 2011).
To perform chemical, physical and textural analyses of the soil, we collected five simple samples which result in soil compound samplings (0–20 cm deep) in all the plots (Rodrigues et al. 2007). To analyze the samplings, we used the EMBRAPA Protocol (EMBRAPA 1997).
We had a rocky coverage percentage on the surface of the plots by means of the adapted method of Braun Blanquet (1979). The evaluation consisted in the assignment of nominal values: 0 = absence of superficial rocks; 1 = 0–25%; 2 = 26%; 3 = 51–75%; and 4 = 76–100%.
We evaluated the declivity of the land by measuring the inclination of the microtopography in each plots with the help of the hypsometer (Oliveira Filho et al. 1998; Espírito Santo et al. 2002). We measured the microtopography from the central line sight of each plots, and basing on their vertices, we calculated the declivity, obtained by the difference between maximum and minimum elevation.
The richness and diversity of tree species in the four enclaves were evaluated by the following parameters: number of species, Shannon diversity (H) and evenness (J) (Brower and Zar 1984) (see Gonzaga et al. 2013). The species richness was compared among the enclaves through rarefaction curves (Gotelli and Colwell 2001) using the PAST program (Hammer et al. 2001) and 1000 randomisations, besides that the diversity was compared by means of diversity profiles, Obtained from the Rényi exponential series (Hill 1973). With this analysis, it was possible to compare both richness and fairness of species in each enclave, and the arbitrary choice of diversity index is not necessary (Melo 2008). The rarity of the species in each enclave is used as a criterion for numerical rarity of the species that had only one individual per hectare (Rabinowitz 1981).
We performed Kruskal–Wallis test (Zar 1996) to compare the possible differences in soil variables in relief, hypsometric quota and structural descriptors of vegetation parameters (total minimum and maximum diameter class density and basal area and height) between the enclaves. For variables that showed significant differences was applied, retrospectively, the Dunn test (Zar 1996). We process and analyze the BioEstat software version 5.0 (Ayres et al. 2007) and adopted as a criterion of significance P ≤ 0.05. For the frequency distribution of individuals, calculate diameter classes and used constant intervals of 5.0 cm diameter class (5–10, 10–15, 15–20 and >20 cm).
In order to check the formation of clusters according to the similarity in floristic and structural composition of the enclaves, we have used classification by TWINSPAN (two-way indicator species analysis) (Kent and Coker 1992). To do so, we prepared an array of quantitative data (density) of species in enclaves, using the following cutoff levels 0, 1, 2, 4, 8, 16, 32, 64 and 128. Consider satisfactory eigenvalues above 0.5 because this limit produces strong divisions and significant ecological significance (Kent and Coker 1992). We have processed the TWINSPAN analysis with the use of PC-ORD software version 6.0 for Windows (McCune and Mefford 2011).
Results
The physical properties of the soils among the enclaves were significantly different (Kruskal–Wallis; P < 0.001) (Table 2). ZTCA 1 has presented soils characteristics better drained, with high levels of sand and low levels of clay, statistically different from the other enclaves (Table 2). In opposite position, we recorded in ZTMA the highest values of clay and lower values of sand, both significantly different from the other areas (Table 2).
In relation to the acidity of the soil, we have had a higher pH in ZTCA that was considered the most neutral within the four enclaves (7.78 ± 0.27), which statistically differed it from the others. However, all of the other enclaves showed tendency to the neutral acidity (pH ≈ 7) and did not differed significantly (Table 2). If considering the potential soil acidity (H + Al), ZTMA was the one that showed significantly superior values, considering the most acid enclave, while the others showed intermediary to neutral values (>3.0 cmolc dm−3) and did not differed statistically (Table 2).
Basing on variables that summarize the availability of nutrients in the soils, we observed that ZTCA 2 and CE showed higher levels of Ca, SB, V, CTC and Na, all statistically similar between the two enclaves. In addition to these variables, ZTCA 2 showed significantly higher values for potassium (K: 0.51 ± 0.2 mg dm−3) (Table 2). This situation was similar to that recorded in CE which also showed significantly higher values for phosphorus (P: 524.44 ± 397.02 mg dm−3) and organic matter (OM: 124.54 ± 64.33 dag kg−1). In summary, ZTCA 1 showed fertility levels ranging from intermediate to low, while ZTMA presented only magnesium with high levels (Table 2).
The highest rock coverage was found in ZTCA 2 (68.50 ± 27.27%) and the lowest in ZTMA (25.92 ± 33.65%) and was the only ones that differed significantly in relation to other fragments (Table 2). The enclaves with higher proportion of rock cover were considered to have the lowest availability and depth of the soil, and thus, ZTCA 2 showed more shallow soils, while soils ZTMA be deeper (Table 2).
The altitudes ranged from 819.6 ± 29.12 m in the ZTMA to 533 ± 25.76 m in ZTCA 2 and were statistically different between all the enclaves (Table 2). Regarding the slope, ZTCA 2 only differentiated for showing the highest slope (19.04 ± 6.52 m), almost four to five times greater than the value recorded in other enclaves (Table 2).
The rarefaction curves (Fig. 2) showed higher potential richness in species for ZTCA 1 and CE. On the other hand, ZTCA 2 was the enclave with the lowest number of species. Except between CE and ZTCA1, the other curves showed significant differences by analysis of the confidence intervals (95%) of the curves. However, for clarity the confidence intervals are not shown in the curves of stress collector (Fig. 2).
Considering the diversity profile of the parameter α = 0, where diversity is equal to the number of species in the sample, the largest enclaves of richness were CE (65 species) and ZTCA1 (64), which did not differ; however, ZTMA (46) and ZTCA (43) have differed that were also significantly different from each other (Fig. 3). As for α tending to 1, where diversity value is equivalent to the Shannon diversity (Euler number’s base), all enclaves differed significantly, following the gradient from highest to lowest diversity ZTCA 1—CE—ZTMA—ZTCA 2, similar to that observed for the index of Pielou (Table 3). The same pattern occurred for α = 2, where the value is the inverse of Simpson index and higher values of α, in other words, those that evaluate only equability and despise evenness and species richness (Fig. 3). Considering the infrequency of species, a pattern similar to the previously found by following the gradient CE (27% of rare species)—ZTCA 1 (20%)—ZTMA (19%)—ZTCA 2 (16%).
Profiles diversity of tree species sampled in Deciduous Forest enclaves. CE Core zone of the “Cerrado” biome, ZTMA “Cerrado”–Atlantic Forest transition zone and ZTCA 1 and ZTCA 2 = “Cerrado–Caatinga” transition zone. When α = 0, the diversity is expressed by the richness or the number of species in the sample; α → 1, it is equivalent to the Shannon diversity (Naperian base); α = 2, it is the inverse of the Simpson index (1/D)
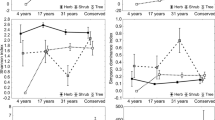
In all, 3,504 individuals were sampled in the four enclaves. The densities were significantly lower in ZTMA (635 ind ha−1) and ZTCA 2 (778 ind ha−1) and higher in ZTCA 1 (1,046 ind ha−1) and CE (1,045 ind ha−1) (Table 3). No significant differences were observed in terms of basal area due to the high internal variation in the enclaves, which ranged from 15.98 ± 5.90 m2 ha−1 (ZTCA 1) to 26.29 ± 30.17 m2 ha−1 (ZTCA 2) (Table 3). In terms of heights, significant differences were found with ZTMA recording the lowest average peak height (15:28 ± 1:57 m) and the highest average value of minimum height (5.1 ± 1.63 m), whereas in CE there was the highest average maximum height (20:44 ± 3.3 m) (Table 3). ZTCA 2 was the enclave with the highest biomass (34% of total sample) and one of lower abundance (22%) (Table 3).
Comparisons among the four enclaves allowed to detect statistical differences in the total number of individuals, as well as the height (maximum and minimum) (Table 3). However, the basal area differed significantly among the areas (P > 0.05). The CE and ZTCA 1 enclaves had higher values of total density and were statistically similar among themselves and different from the other areas (Table 3). The maximum total heights of trees in the sampled differed statistically (Table 3). CE showed the highest average value of maximum height (20.44 ± 3.3 m), while ZTMA recorded the lowest maximum height 15.28 ± 1.57 m. For the minimum height, it is noticed that ZTMA had the highest value (5.10 ± 1.63 m), and this was significantly different from the others (Table 3).
The frequency distributions in the diameter classes in all the enclaves showed high concentration of individuals in the smaller classes and marked decrease toward the largest ones, characterized as negative exponential distribution or reverse—J (Fig. 4). The first two classes (5–20 cm), when added together, accounted for over 64% of sampled individuals, which emphasizes the importance of these classes in the structure of woody vegetation of the enclaves. The comparison of the distribution of frequencies in diameter classes adopted in the four enclaves has shown statistical differences only for the first class (5–10 cm of DBH). Thus, CE, ZTCA1 and ZTCA2 showed high concentration of individuals in the initial class and are considered statistically similar, while ZTMA density was lower in this class, and the difference between this and the others was significant (Table 3).
The analysis classification by TWINSPAN has shown strong divisions in all the levels, with values higher than 0.68 (Fig. 5). That suggests that the four investigated enclaves had strong floristic-structural differentiation, since according to Felfili et al. (2011) values over 0.3 characterize the distinction among the species. On the first division (eigenvalue: 0.6815), the ZTMA enclave was separated from the other areas (Fig. 5), while on the second hierarchic level (eigenvalue: 0.7315) the ZTCA 2 and ZTCA 1 were grouped and the CE area was separated. And on the last group divisions (eigenvalue: 0.6958), the ZTCA 2 and ZTCA 1 enclaves were separated.
Discussion
The variations in edaphic and geomorphological factors were determinants in the characterization of woody vegetation in the Deciduous Forests enclaves. The abiotic factors commonly related to the structure and dynamics of tropical forests are solar radiation and availability of water and mineral nutrients in soils (Hugget 1995). The importance of each one differs significantly among the terrestrial biomass or even within a similar physiognomy, especially when this is inserted in form of enclaves in different vegetation matrices, as is the case of the FED. The Dry Forest occurs on fertile soils where the rainfall is less than 1,800 mm per year (Dryflor et al. 2016). Here we found that except for the solar radiation that we did not evaluate, the fertility, texture, acidity and relief were the main responsible for floristic-structural differentiation in the enclaves of Deciduous Forests.
When we consider that the particle size distribution, expressed by soil texture, is the characteristic that best describes the water retention capacity of the soil (Beutler et al. 2002; Fernandes and Corá 2004), we assumed that ZTMA showed large ability to water storage, with higher levels of clay. Moreover, ZTCA 1 has shown the highest values of sand, thus the lower water holding capacity. Relationship between the texture and water retention capacity of the soil occurs as a result of the relationship between macro and micropores, respectively, soils with higher levels of sand and clay (Paiva et al. 2000). Thus, besides the availability of nutrients in the soil, which naturally tends to be high in the FED, the availability of water in the soil was also important in the distribution of vegetation.
Just as the physical properties of soils, the decrease in rainfall toward ZTMA > CE > ZTCA 1 > ZTCA 2, also exerted influence on soil water availability. Similar pattern was also observed in the Brazilian semiarid region (Rodal et al. 2008), where they found that physiognomic changes are common along the rainfall gradient and that the seasonality is as important as the total precipitation and the number of dry months per year. Thus, our results corroborate the assertion that Deciduous Seasonal Forests are associated with a strong seasonal rainfall regime (Murphy and Lugo 1986; Gentry 1995), where it is assumed that the water storage capacity of each locality presents a preponderance in the distribution of species (Rizzini 1977).
In addition, we observed the action of the full set of rainfall and soil properties also influenced the species distribution and structure of woody vegetation in the seasonal Dry Forests. After all, it is expected that, the more it is pronounced and the less their capacity to resist the water stress is, the more it decreases (Andrade et al. 2009). In this case, the environmental characteristics, whether of the soil or climate nature, can be the difference that allow or restrict the arrival and the establishment and development of species in each locality.
Although the studied fragments are located on the same type of geological origin material derived from limestone “Grupo Bambuí” (Iglesias and Uhlein 2009), the differences in the physical properties of the soils also may have influenced the chemical characteristics. The texture of the soil also acts in nutritional availability, since clay particles are derived from weathering of larger fractions, and although they are more resistant, they have lower nutrient reserves in its constitution than the material from which they came from.
Regarding this, the relationship of texture and nutritional availability soil can be established once ZTMA, area of higher concentration of clay, had the lowest fertility. However, the remnants with higher nutritional availability (CE and ZTCA 2) showed lower levels of clay. Thus, it was noticed that, as well as for precipitation, there is also an increasing gradient of fertility in the following sequence: ZTMA < ZTCA1 < CE < ZTCA 2. Nevertheless, one should notice that all the areas had levels of basis saturation (V) greater than 50%, which indicates the existence of eutrophic soils (Embrapa 1999), that is, with high levels of nutrients, moderate to high pH and low aluminum content, which are characteristic of the environments with occurrence of FED (Pennington et al. 2000; Prado 2000).
The differences between structural vegetations parameters probably reflected the relationship with the environmental conditions imposed on her. Factors such as climate, physical composition and chemical properties of soil and relief are important in the distribution and structure of forest formations (Oliveira Filho et al. 1994, 1998; Oliveira Filho and Fontes 2000), reflecting mainly in the horizontal and vertical communities’ stratification (Fagundes et al. 2007). Works performed in Brazilian Deciduous Forests find relationship between vegetation and soil characteristics, most of them related to soil nutrient availability (Fagundes et al. 2007; Santos et al. 2012). This relationship directly influences the height, density and biomass of these communities (Sampaio et al. 1981) which reinforces our hypothesis that abiotic factors govern the vegetation structural patterns of the Deciduous Forest enclaves.
Limitations associated with fertility recorded in the ZTMA, as well as the history of disturbances, may also have influenced the patterns described in the vegetation. Several studies indicate that previous events may affect vegetation development (Oliveira Filho et al. 1997; Kennard et al. 2002; Fagundes et al. 2007). Signals of degrading events were observed inside the fragment in ZTMA, and that may have influenced the vegetation structure due to, for example, competition for exotic species that would limit both the germination and the development of the tree individuals. This would explain the low density and basal area recorded in this fragment. In general, Dry Forest is in a critical conservation state, because so little of it is no disturbed and of the remnant areas little is protected (Miles et al. 2006). Thus, we believe that both the environmental conditions, especially those related to nutrient availability and acidity of the soil, and the history of disturbance in the area resulted in lower density and height of vegetation sampled in ZTMA.
We assume that the texture and acidity of soils were responsible for the floristic separation of ZTMA from the other enclaves and this possessed soils with higher clay content and higher pH and potential acidity (H + Al), and other areas more neutral soils pH having low-to-intermediate clay contents. Variations in clay and soil pH were also responsible for the distinction of areas Deciduous Forests of southeastern Brazil (Oliveira Filho et al. 2001). Thus, we found that differences between environmental variables besides influencing the vegetation structure also influenced the separation of FED in floristic terms.
We attribute the floristic separation between CE and other fragments due to the influence of relief, rockiness and organic matter, since CE presented planner surface, soils with higher organic matter content and lower concentration of rocks, than the other areas, similar to that found by others studies (Fagundes et al. 2007). While the separation of ZTCA 1 and ZTCA 2 we associate to the differences in the availability of nutrients and once more the texture and rockiness. ZTCA 2 presented more fertile soils, with higher percentage of sand and high concentration of rocks. Others authors (Oliveira Filho et al. 1994, 1998) also observed that fertility variables and the rocky concentration exerted a strong influence on the distinction of seasonal environments. In fact, the floristic variation of dry forest species is also explained by environmental conditions (Neves et al. 2015).
When we take as a basis the model of succession for Seasonal Forests in the Atlantic Forest (Machado and Oliveira Filho 2010), we can infer from the large number of individuals and low basal area that ZTCA 1 would be the initial phase of construction with degradation. Thus, in forests where degradation evidences remain, even in restructure processes are in progress, it is possible to observe the characteristics of environments that are in the stage of degradation, such as reduced basal area (Machado and Oliveira Filho 2010). Actually, in general, in mature forests, a major number of trees with higher basal areas are observed, while those at early stages of regeneration form large densification of thin trees (Parthasarathy 1999).
The size structure of the tree individuals, represented by a greater number of individuals in the smaller classes indicated that communities are self-regenerative, the J-Reverse-type distributions, can characterize community stock, that is, presenting considerable abundance in the natural regeneration of individuals available for the establishment of individuals. This is typical of stable tropical forests, which do not exhibit large disturbances and composition in different species that create permanent seedling bank. However, most forests known as native forests with inverted exponential distribution do not present a balanced distribution, but tend to converge to that standard (Harper 1977).
The differences in vertical stratification observed allowed us to infer that ZTCA 2, CE and ZTCA 1 are more compartmentalized due to the larger amplitudes between the Brazilian minimum and maximum values shown for these areas, which resulted in greater diversification of strata. Well-stratified forests have high diversity and differentiation of ecological niches and comprise most flora and fauna diversity in different vertical strata, in the same plant community (Hunter Júnior 1990). Thus, the most plausible explanation for the observed differences in the vertical stratification is its relationship with the environmental and historical factors. For example, the fertility of soils which limits the development of the individuals, or even the selective cutting larger species that may have influenced both the structure of the studied remnants and their floral compositions (Emmerich 1990).
We conclude that the floristic, structural and environmental variations observed allowed us to assume our initial hypothesis that the chemical and textural properties of the soil rockiness, declivity and altitude have influenced in the composition, and structure of the woody vegetation in the Deciduous Forests enclaves as true, which has reflected in the identification of environmental-floral-structural gradient in the studied fragments.
References
Andrade WM, Araújo EL, Rodal MJN, Encarnação CRF, Pimentel RMM (2009) Influência da precipitação na abundância de populações de plantas da caatinga. Rev de Geogr 26:161–184
Apgaua DMG, Coelho PA, Santos RM, Santos PF, Oliveira Filho AT (2014) Structure of tree community in a remnant of seasonally dry tropical forest, Brazil. Cerne 20:173–182. doi:10.1590/01047760.201420021540
Ayoade JO (2010) Introdução à climatologia para os trópicos, 14th edn. Bertrand Brasil, Rio de Janeiro
Ayres M, Ayres Júnior M, Ayres DL, Santos AA (2007) BIOESTAT: aplicações estatísticas nas áreas das ciências bio-médicas. Ong. Mamiraua, Belém
Beutler AN, Centurion JF, Souza ZM, Andrioli I, Roque CG (2002) Retenção de água em dois tipos de latossolos sob diferentes usos. Rev Bras Ciênc Solo 26:829–834
Braun Blanquet J (1979) Fitosociologia. Bases para el estudio de las comunidades vegetales. Blume Ediciones, Madrid
Brower JE, Zar JH (1984) Field and laboratory methods for general ecology. Brown Publishers, Boston
Chown SL, van Rensburg BJ, Gaston KJ, Rodrigues ASL, van Jaarsveld AS (2003) Energy, species richness, and human population size: conservation implications at a national scale. Ecol Appl 13:1233–1241
Denslow JS (1980) Gap partitioning among tropical rainforest trees. Biotropica 12:47–55
DRYFLOR et al (2016) Plant diversity patters in neotropical dry Forest and their conservations implications. Science 353:1383–1387. doi:10.1126/science.aaf5080
EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária) (1997) Manual de métodos de análise de solo. EMBRAPA, Rio de Janeiro
EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária) (1999) Sistema brasileiro de classificação de solos. EMBRAPA, Rio de Janeiro
Emmerich KH (1990) Influence of landform, landscape development and soil moisture balance on forest and savanna ecosystem patterns in Brazil. Pédologie 40:5–17
Espírito Santo FDB, Oliveira Filho AT, Machado ELM, Souza JS, Fontes MAL, Marques JJGSM (2002) Variáveis ambientais e a distribuição de espécies arbóreas em um remanescente de Floresta Estacional Semidecídua Montana no campus da Universidade Federal de Lavras, MG. Acta Bot Bras 16:331–356
Fagundes LM, Carvalho DA, van den Berg E, Melo Marques JJGS, Machado ELM (2007) Florística e estrutura do estrato arbóreo de dois fragmentos de florestas deciduais às margens do rio Grande, em Alpinópolis e Passos, MG, Brasil. Acta Bot Bras 21:65–78
Felfili, JM, Carvalho FA, Libano AM, Venturoli F, Pereira BAS, Machado ELM (2011) Análise multivariada: princípios e métodos em estudos de vegetação. In: Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira-Neto JAA (eds) Fitossociologia no Brasil: Métodos e estudos de casos. Editora UFV, Viçosa, pp 122–155
Fernandes C, Corá JE (2004) Bulk density and relationship air/water of horticultural substrate. Sci Agric 61:446–450
Gentry AH (1995) Diversity and floristic composition of neotropical dry forests. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 146–194
Gonzaga APD, Oliveira Filho AT, Machado ELM, Hargreaves P, Machado JNM (2008) Diagnóstico florístico-estrutural do componente arbóreo da floresta da serra de São José, em Tiradentes, Minas Gerais, baseado na comparação com 23 remanescentes florestais da região. Acta Bot Bras 22:501–516
Gonzaga APD, Pinto JRR, Machado ELM, Felfili JM (2013) Similaridade florística entre estratos da vegetação em quatro Florestas Estacionais Deciduais na bacia do Rio São Francisco. Rodriguésia 64:11–19
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Harper JL (1977) Population biology of plants. Academic Press, New York
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–473
Hugget RJ (1995) Geoecology: an evolutionary approach. Routledg, London
Hunter Júnior ML (1990) Wildlife forests, and forestry: principles of managing forests for biological diversity. Prentice-Hall, New Jersey
Iglesias M, Uhlein A (2009) Estratigrafia do Grupo Bambuí e coberturas fanerozóicas no vale do rio São Francisco, norte de Minas Gerais. RBG 39:256–266
Kellman M, Tackaberry R (1997) Tropical environments: the functioning and management of tropical ecosystems. Routledge, London
Kennard DK, Gould K, Putz FE, Fredericksen TS, Morales F (2002) Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. For Ecol Manag 162:197–208
Kent M, Coker P (1992) Vegetation description analyses. Behaven Press, London
Kolb A, Diekmann M (2004) Effects of environment, habitat configuration and forest continuity on the distribution of forest plant species. J Veg Sci 15:199–208
Machado ELM, Oliveira Filho AT (2010) Spatial patterns of tree community dynamics are detectable in a small (4 ha) and disturbed fragment of the Brazilian Atlantic forest. Acta Bot Bras 24:250–261. doi:10.1590/S0102-33062010000100027
Machado ELM, Oliveira Filho AT, van den Berg E, Carvalho WAC, Souza JS, Marques JJGSM, Calegário N (2008) Efeitos da proximidade espacial, substrato e bordas na estrutura da comunidade arbórea de um fragmento florestal em Lavras, MG. Rev B Bot 31:287–302
McCune B, Mefford MJ (2011) PC-ORD. Multivariate analysis of ecological data. Version 6. MjM Software, Gleneden Beach
Melo AS (2008) O que ganhamos “confundindo” riqueza de espécies e equabilidade em um índice de diversidade? Biota Neotrop 8:21–27
Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505. doi:10.1111/j.1365-2699.2005.01424.x
Moro MF, Martins FR (2011) Métodos de levantamentos do componente arbóreo-arbustivo. In: Felfili JM, Eisenlohr PV, Melo MMRF, Andrade LA, Meira Neto JAA (eds) Fitossociologia no Brasil: métodos e estudos de caso. Editora UFV, Viçosa, pp 174–212
Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Annu Rev Ecol Syst 17:67–88
Neves DRM, Dexter KG, Pennington RT, Bueno ML, Oliveira Filho AT (2015) Environmental and historical controls on floristic composition across the South American Dry Diagonal. J of Biogeogr 42:1365–2699. doi:10.1111/jbi.12529
Oliveira Filho AT, Fontes MAL (2000) Patterns of floristic differentiation among Atlantic forests in south-eastern Brazil, and the influence of climate. Biotropica 32:793–810. doi:10.1111/j.1744-7429.2000.tb00619.x
Oliveira Filho AT, Vilela EA, Carvalho DA, Gavilanes ML (1994) Effects of soils and topography on the distribution of tree species in a tropical riverine forest in south-eastern Brazil. J Trop Ecol 10:483–508. doi:10.1017/S0266467400008178
Oliveira Filho AT, Mello JM, Scolforo JRS (1997) Effects of past disturbance and edges on tree community structure and dynamics within a fragment of tropical semideciduous forest in south-eastern Brazil over a five-year period (1987–1992). Plant Ecol 131:45–66. doi:10.1023/A:1009744207641
Oliveira Filho AT, Curi N, Vilela EA, Carvalho DA (1998) Effects of canopy gaps, topography and soils on the distribution of woody species in a central Brazilian deciduous dry forest. Biotropica 30:362–375. doi:10.1111/j.1744-7429.1998.tb00071.x
Oliveira Filho AT, Curi N, Vilela EA, Carvalho DA (2001) Variation in tree community composition and structure with changes in soil properties within a fragment of semideciduous forest in south-eastern Brazil. Edinb J Bot 58:139–158. doi:10.1017/S0960428601000506
Paiva AQ, Souza LS, Ribeiro AC, Costa LM (2000) Propriedades físico-hídricas de solos de uma toposseqüência de tabuleiro do estado da Bahia. Pesqui Agropecu Bras 35:2295–2302. doi:10.1590/S0100-204X2000001100023
Parthasarathy N (1999) Tree diversity and distribution in undisturbed and human-impacted sites of tropical wet evergreen forest in southern Western Ghats, India. Biodivers Conserv 8:1365–1381. doi:10.1023/A:1008949407385
Pennington RT, Prado DA, Pendry C (2000) Neotropical seasonally dry forests and Pleistocene vegetation changes. J Biogeogr 27:261–273
Prado DE (2000) Seasonally dry forests of tropical South America: from forgotten ecosystems to a new phytogeographic unit. Edinb J Bot 57:437–461
Rabinowitz D (1981) Seven forms of rarity. In: Synge H (ed) The biological aspects of rare plant conservation. Wiley, Chichester, pp 205–217
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do bioma Cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora, 2nd edn. Embrapa, Brasília, pp 151–199
Ricklefs RE (1977) Environmental heterogeneity and plant species diversity: a hypothesis. Am Nat 111:376–381
Rizzini CT (1977) Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. Âmbito Cult, Rio de Janeiro
Rodal MJN, Barbosa MRV, Thomas WW (2008) Do the seasonal forests in northeastern Brazil represent a single floristic unit? Braz J Biol 68:467–475. doi:10.1590/S1519-69842008000300003
Rodrigues LA, Carvalho DA, Oliveira Filho AT, Curi N (2007) Efeitos de solos e topografia sobre a distribuição de espécies arbóreas em um fragmento de floresta estacional semidecidual, em Luminárias, MG. R Árvore 31:25–35
Sampaio EVSB, Andrade-Lima D, Gomes MAF (1981) O gradiente vegetacional das caatingas e áreas anexas. Rev Bras Bot 4:27–30
Santos RM, Vieira FA, Fagundes M, Nunes YRF, Gusmão E (2007) Riqueza e similaridade florística de oito remanescentes florestais no norte de Minas Gerais, Brasil. R Árvore 31:135–144
Santos RM, Oliveira Filho AT, Eisenlohr PV, Queiroz LP, Cardoso DBOS, Rodal MJN (2012) Identity and relationships of the Arboreal Caatinga among other floristic units of seasonally dry tropical forests (SDTFs) of North-eastern and Central Brazil. Ecol Evol 2:409–428. doi:10.1002/ece3.91
Shmida A, Wilson MV (1985) Biological determinants of species diversity. J Biogeogr 12:1–20
Waide RB, Willig MR, Steiner CF, Mittelbach G, Gough L, Dodson SI, Juday GP, Parmenter R (1999) The relationship between productivity and species richness. Ann Rev Ecol Syst 30:257–300
Whitmore TC (1990) An introduction to tropical rain forests, 2nd edn. Oxford University Press, Oxford
Wilson SD (2000) Heterogeneity, diversity, and scale in plant communities. In: Hutchings MJ, John E, Stewart AJA (eds) The ecological consequences of environmental heterogeneity. Cambridge University Press, Cambridge, pp 53–69
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, New Jersey
Acknowledgements
We acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), by the concession of Doctorate Scholarship to the first author and the research productivity scholarship—PQ to the second and fourth authors. The ones who assisted during the botanical collecting identifications, especially Ary Teixeira de Oliveira Filho, Manoel Cláudio da Silva Junior, Hisaias de Souza Almeida, Rubens Manoel dos Santos and Newton Rodrigues, and the contributors that helped during the data collecting.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jeanine Maria Felfili: deceased.
Rights and permissions
About this article
Cite this article
Gonzaga, A.P.D., Machado, E.L.M., Maria Felfili, J. et al. Brazilian Decidual Tropical Forest enclaves: floristic, structural and environmental variations. Braz. J. Bot 40, 417–426 (2017). https://doi.org/10.1007/s40415-016-0346-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0346-z