Abstract
This study aimed to document variation in the composition and structure of regenerating woody vegetation along an altitudinal gradient of a rupestrian grassland and to describe the relationship between species and characteristics of the soil. All woody plants with a diameter at ground height of ≤1 cm were collected in seven sampling sites at altitudes between 800 and 1400 m in Serra do Cipó (Minas Gerais State, Brazil). Phytosociological parameters and the Shannon diversity index were calculated and compared using Hutcheson’s t test. Floristic composition was evaluated by nonmetric multidimensional scaling (NMDS) ordination with analysis of variance as a confirmatory analysis, in which the response variables were the axis of the NMDS. Correlation between soil characteristics and vegetation patterns was evaluated with multiple regression models and canonical redundancy analysis. For all analyses, we performed analysis of variation partitioning to decompose the explanation of spatial, soil, and altitudinal variation. A total of 809 individuals of 127 species belonging to 26 families were sampled. Species richness and structure varied among altitudes. Species diversity followed an altitudinal pattern and was higher at lower altitudes. Some soil variables, such as remaining phosphorus, base saturation, and structural components, were more strongly correlated with species distribution than the others. Although variation in altitude leads to changes in the composition of the regenerating woody species, variation in plant communities was determined by a combination of environmental factors, of which soil properties played an important role.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Altitude is a geographic factor that is able to generate patterns of biological diversity due to the climate, soil, and vegetation changes that occur along its gradients (Whittaker 1960; Rahbek 1995; Lomolino 2001; Fritzsons et al. 2008; Rana et al. 2011). Environmental conditions that vary with altitude influence plant species distributions (Moreno et al. 2003), such that one of the most well-documented biogeographic patterns on Earth is the increase in plant diversity with decreasing altitude (e.g., Whittaker 1956; Hamilton 1975; Fernandes and Price 1988; Gentry 1988; Kitayama 1992; Huston 1994; Aiba and Kitayama 1999; Hussain and Malik 2012). However, several other studies have shown non-unimodal patterns for plant richness along altitudinal gradients (e.g., Fernandes 1992; Liberman et al. 1996; Wang et al. 2002; Joly et al. 2012), while yet others report peaks of diversity at intermediate elevations (e.g., Lomolino 2001; Sang 2009). Hence, a clear and accurate description of the distributional pattern of plant richness in relation to altitude is yet to be achieved (Wang et al. 2002). Mountains provide excellent opportunities to develop and test theories regarding the relationship between biodiversity and altitude, and empirical works are the most convincing tests when there are covariables that are not directly related to altitude (Korner 2007). Therefore, the study of the distribution of plant species along environmental gradients is important, particularly in several mountainous tropical regions where such knowledge is still needed.
Multiple environmental factors vary along altitudinal gradients, but few studies have attempted to interpret vegetation patterns associated with these factors (Wang et al. 2002). An altitudinal gradient basically corresponds to a temperature gradient (Kitayama 1992) and variation in other atmospheric variables, with implications for the distribution and survival of most organisms (Korner 2007). However, differences between distinct areas can also be attributed to soil characteristics (Moreno et al. 2008), and an altitudinal difference of a few hundred meters can cause significant changes in soil, climate, and vegetation (Fritzsons et al. 2008). For example, the decrease of nutrients, especially nitrogen, with increasing altitude was suggested as a cause for the decline of plant diversity and forest stature on tropical mountains (Grubb 1977; Aiba and Kitayama 1999). On the other hand, the effects of area and isolation likely contribute to the observed decreases in species richness with altitude (Korner 2007). However, the environmental processes responsible for structuring communities may also be spatially organized, so that spatial structure can corroborate false correlations between species and environment (Legendre et al. 2002; Peres Neto and Legendre 2010). Therefore, it is necessary to consider spatial structure in studies of correlations between components of vegetation and environmental variables.
The tropical flora of the southeastern Brazilian mountains represents an excellent opportunity for ecological studies. The main formation in these mountains is low-stature sclerophyllous vegetation called rupestrian grasslands, locally known as “campo rupestre”. This vegetation comprises a complex mosaic of plant communities with high species richness and many endemic species (Giulietti et al. 1987; Filgueiras 2002; Medina and Fernandes 2007). It occurs on a mosaic of soils of quartzite and igneous origin (Semir 1991; Benites et al. 2007) and is found mainly along the Espinhaço Mountain Range, generally above 900 m a.s.l., on shallow, Al-rich and water- and nutrient-deficient soils (Giulietti et al. 1987; Benites et al. 2007; Negreiros et al. 2009). These environments are highly diverse (Giulietti et al. 1987; Conceição and Pirani 2007; Carvalho et al. 2012; Gastauer et al. 2012) and represent a large proportion of the flora of the "Cerrado" (savanna) biome, as well as all tropical flora (Rapini et al. 2008; see Fernandes 2016).
Environmental heterogeneity and soil-climatic factors influence plant species composition in this environment and are partly responsible for the species diversity present in the rupestrian grassland complex (Ribeiro and Walter 2008; Carvalho et al. 2012). Floristic composition in this ecosystem may change within meters, and plant species density was argued to be directly associated with the soil substrate (Ribeiro and Fernandes 1999, 2000; Benites et al. 2001; Medina and Fernandes 2007; Carvalho et al. 2012). Therefore, more detailed studies on floristic diversity in this vegetation type are needed for understanding the relationship between species density and environmental conditions and for estimating the overall species richness of the region (Torres et al. 1997).
Although many studies have been conducted on the flora of rupestrian grassland vegetation in the Espinhaço Mountain Range (e.g., Giulietti et al. 1987; Conceição and Pirani 2007; Rapini et al. 2008; Nunes et al. 2008) making it the most studied plant typology in the state of Minas Gerais (Costa et al. 1998), only a few studies have assessed changes in its flora along altitudinal gradients (e.g., Nunes et al. 2008; Borges et al. 2011). Likewise, although essential for conservation and understanding the processes that determine the resilience of this ecosystem, studies on the natural regeneration of rupestrian grasslands are virtually nonexistent (see Medina and Fernandes 2007). Thus, focusing on natural regeneration is crucial to understand the behavior of different native species that compose this singular plant community. The presence of species in the early stages of regeneration indicates the flux of new species to the community, while a small number of these species indicate an interruption in the regeneration process (e.g., Menino et al. 2012). Due to a lack of research, the processes and patterns involved in the natural regeneration of rupestrian grasslands, and the factors that determine which species will colonize a given environment, are largely unknown. Furthermore, this knowledge has acquired legal significance, as a Brazilian law erroneously placed this vegetation under the domain of the Atlantic Forest (Law n. 11,428; Brasil 2006), and so knowledge regarding the colonization and regeneration of its species is necessary for the required environmental assessment strategies and plans (GWF, pers. inf.).
The present study aims to provide the very first information on the regeneration of woody species of the rupestrian grassland by attempting to answer the following questions: (1) Do floristic composition and structure of regenerating woody communities of the rupestrian grassland complex vary along an altitudinal gradient? (2) Is the floristic similarity among areas influenced by soil characteristics? (3) Is there an interaction between soil and altitude that promotes a pattern of plant diversity and distribution in the rupestrian grassland? Although we expect the diversity of the regenerating plant species to decrease with increasing altitude (e.g., Whittaker 1956; 1960; Hamilton 1975; Gentry 1988; Kitayama 1992), this diversity may also be influenced by soil characteristics independent of altitude (see Grubb 1977; Aiba and Kitayama 1999).
Materials and methods
Study area
This study was conducted in the rupestrian grasslands of Serra do Cipó from December 2010 to March 2011. Serra do Cipó is located in the southern portion of the Espinhaço Mountain Range between the latitudes of 19°12′–19°34′S and longitudes of 43°27′–43°38′W in the central region of the state of Minas Gerais of southeastern Brazil, (Melo Júnior et al. 2001). It encompasses the municipalities of Santana do Riacho and Jaboticatubas, and, according to the Köppen classification, the regional climate is tropical highland (Cw) with hot summers and pronounced dry seasons (Madeira and Fernandes 1999). The region experiences relatively cold winters and a narrow annual temperature range; the average annual temperature and precipitation are 21.2 °C and 1622 mm, respectively (Madeira and Fernandes 1999).
Serra do Cipó includes small and large patches of Atlantic Forest above 1300 m a.s.l., “cerrado” stricto sensu (“cerrado” s. s.; shrub-savanna) from 700 throughout 1400 m a.s.l., patches of seasonally dry forests at 800–900 m a.s.l. and riparian forest in drainages, while the rupestrian grasslands are distributed primarily between 900 and 1400 m a.s.l. (Giulietti et al. 1987). Although the soils in the region contain moderate levels of organic matter, they are highly acidic and macronutrient deficient, and have high aluminum saturation and a predominantly sandy texture (Negreiros et al. 2008).
Sampling of regenerating woody vegetation
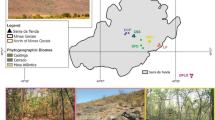
The sampling area was established along an altitudinal gradient with sites stratified at approximately 100-m intervals from 800 to 1400 m for a total of seven altitudinal sites (Fig. 1–8; Table 1). At each altitudinal site, a 250-m north-to-south transect with 13 plots of 1 m2 (1 m × 1 m) placed 20 m apart was established. In each plot, all regenerating woody plants (including seedlings, shrubs, and subshrubs, but excluding grasses and lianas) with a diameter at ground height (DGH) of ≤1 cm were inventoried, measured with a digital caliper, and labeled with a numbered aluminum plate that was affixed with nylon thread. Plants sampled in this category (DGH ≤ 1) were considered juveniles or regenerating woody individuals (regeneration stratum). Even though they are non-woody, species that form rosettes were also included in the sampling because their young individuals are characteristic of the regeneration stratum and are an important component of this plant community (pers. obs.).
Regenerating woody component of a rupestrian grassland complex sampled in 1-m2 (1 m × 1 m) plots (1) in Serra do Cipó (Minas Gerais, southeastern Brazil) at different altitudes (2. 1400 m; 3. 1300 m; 4. 1200 m; 5. 1100 m; 6. 1000 m; 7. 900 m; 8. 800 m), between 800 and 1400 m. Photographers: Graciene da Silva Mota and Nayara Mesquita Mota
Species were identified through consultations with experts and/or the botanical literature. Biological material from all species was sampled, treated according to conventional herborization techniques, and stored at Montes Claros Herbarium (MCMG), located at the Universidade Estadual de Montes Claros (UNIMONTES), as part of a reference collection.
Soil samples were collected at the same 13 plots of each 250-m transect for evaluation of the effect of soil variables on species composition. We collected composite soil samples within the 100-m2 plots in which adult woody plants were sampled (see Mota 2012 for details), where the 1 m × 1 m plots of regenerating woody plants were located. Following the protocol of EMBRAPA (1999), physical and chemical analyses of each composite sample were performed at the Laboratory for Soil Analysis at the Institute of Agricultural Sciences (Universidade Federal de Minas Gerais). The following soil variables were measured: water pH (pH), potassium (K), phosphorus (P-Mehlic), remaining phosphorus (P-rem), calcium (Ca), magnesium (Mg), aluminum (Al), hydrogen and aluminum (H + Al), sum of bases (SB), base saturation (V), effective cation exchange capacity (t), aluminum saturation (m), cation exchange capacity at pH 7.0 (T), organic matter (OM), and proportions of coarse sand (CS), fine sand (FS), silt, and clay.
Data analyses
The efficiency of sampling was evaluated from rarefaction curves constructed for each site using EstimateS software (Colwell 2013). Traditional quantitative parameters (Mueller-Dombois and Ellenberg 1974), including density, frequency, absolute and relative dominance, and importance value (IV), were calculated for each species at each altitude using the software FITOPAC 2.1 (Shepherd 2010). The alpha Shannon diversity index (H′) was used to estimate the diversity within each altitude (Krebs 1989). A pairwise Hutcheson’s t test with a significance level of 5 % was used to compare the variation in diversity between altitudes (Zar 1996). The seven sampled altitudes were coupled for comparisons (21 combinations) and Bonferroni correction was used to correct for multiple comparisons.
Variation in richness, abundance, basal area, and Shannon diversity index with respect to altitude was assessed using simple linear regression (Zar 1996). In this model, we used seven samples, one for each altitude. Variation in average richness, Shannon index, abundance, basal area, relative density, and absolute dominance was evaluated using multivariate analysis of variance with 1000 permutations (PERMANOVA) and a post hoc test with 5 % significance level and sequential Bonferroni correction using the software PAST 3.01 (Hammer 2013). For this purpose, a block design with the same number of plots and altitudes as the sampled treatments was established, in which we used the thirteen samples of each altitude.
To investigate variation in species composition and structure across the altitudinal gradient without direct interference from environmental and spatial variables, we applied NMDS (nonmetric multidimensional scaling; McCune and Grace 2002) coupled with the Sørensen coefficient as the similarity measure using the program PC-ORD 6.0 (McCune and Mefford 2011). The similarity of the floristic composition of the woody regeneration stratum among altitudes was determined using ANOVA (analysis of variance) in which the response variable was the first axis of the NMDS. With respect to the SAC (spatial autocorrelation), we added spatial eigenvectors (filters) as additional predictors (Diniz Filho and Bini 2005) using the ‘spacemakeR’ package of R (Dray 2013). We progressively selected the MEMs (Moran’s eigenvector maps) according to the method of Blanchet et al. (2008) using the ‘packfor’ package in R (Dray et al. 2013), and partitioning variance based on vegetation type using the ‘varpart’ function of the ‘vegan’ package (Oksanen et al. 2015) following Eisenlohr’s (2014) script. We performed a Tukey’s HSD test (Smith 1971) to determine the differences between pairs that were separated on this axis using the above MEMs as covariables. We confirmed the assumptions of the models following Eisenlohr (2013) and Eisenlohr (2014). To test normality of residuals we used Shapiro–Wilk test.
We constructed multiple linear models in order to determine the relationship between altitude, soil variables, and species composition. Three matrices were constructed that included the seven study areas. The first matrix contained the response variables (species composition), the second matrix the environmental variables (altitude and soil components), and the third matrix contained coordinate data. Prior to constructing models, we removed collinearities by performing a principal components analysis (PCA) to detect any redundant variables. For each set of redundant variables, we kept the variables that were most strongly correlated with the axis. We then performed a forward selection of environmental and spatial (MEMs) variables. To construct a linear model, we obtained the standardized coefficients for each selected variable. Finally, we partitioned the variance by separating the environmental effect from that exerted by space and the shared fraction between them, and tested each fraction with permutation-based ANOVA (Peres Neto et al. 2006), following the script of Eisenlohr (2014). We used the ‘vegan,’ ‘spacemakeR,’ and ‘packfor’ packages of R (Oksanen et al. 2015; Dray 2013; Dray et al. 2013).
A canonical redundancy analysis (RDA) was performed to confirm correlations between vegetation and soil variables (ter Braak 1987) using the software PC-ORD 6.0 (McCune and Mefford 2011). The primary matrix was composed of species abundance data for each site, while the secondary matrix included the soil variables with the variables selected by the PCA. To perform RDA, the environmental matrix should not contain correlated variables and must have a strong relationship with the response patterns of the variable (Peck 2010). We considered variables that represent a cutoff value >0.3 as significant.
Results
Floristic composition and structure of regenerating woody vegetation
Altogether, 809 individuals of 127 species belonging to 26 families and 13 unidentified morphospecies were recorded (Table 2). The family Fabaceae was the most represented (149 individuals), followed by Eriocaulaceae (102), Asteraceae (98), Melastomataceae (89), Lythraceae (61), and Velloziaceae (57), altogether accounting for nearly 70 % of all sampled individuals. Eriocaulaceae had the highest IV, followed by Velloziaceae and Melastomataceae. The most diverse genera were Vellozia (six species) and Chamaecrista and Miconia (five species each). The species with the highest IV were Paepalanthus sp. 3, Leiothrix crassifolia (Bong.) Ruhland, Calliandra dysantha Benth., Vellozia epidendroides Mart. ex Schult. & Schult., and Diplusodom orbicularis Koehne. The species with the highest IV at each site were Acosmium dasycarpum (Vog) Yakovlev. (800 m), Myrcia guianensis (Aubl.) Kuntze (900 m), Erytroxylum suberosum St. Hil (1000 m), Lessingianthus linearifolius (Less.) H.Rob. (1100 m), Paepalanthus sp. 3 (1200 m), Calliandra dysantha Benth. (1300 m), and Actinocephalus polyanthus (Bong.) Sano. (1400 m).
The rarefaction curve for the total area exhibited a tendency toward stabilization (Fig. 9). At higher altitudes (800 and 900 m), the rarefaction curves did not stabilize, while at lower altitudes there were fewer species. A clear pattern of variation in species richness with altitude is apparent from the rarefaction curves.
Of the sampled species, 35 were found at two or more altitudes, while no species occurred at all altitudes (Table 2). The most common species was the restricted native and threatened species Diplusodom orbicularis Koehne (Medina and Fernandes 2007), which was found at all except at the highest (1400 m) and lowest (800 m) altitudes. More than 92 (70 %) of the species were found only at one altitude: 24 at 800 m, 15 at 900 m, 16 at 1000 m, 8 at 1100 m, 16 at 1200 m, 8 at 1300 m, and 5 at 1400 m. The altitudes sharing the greatest number of species were 800 and 1000 m with four species, whereas the altitude pairs of 1000 and 1200, 1000 and 1100, 900 and 1100, and 800 and 900 each shared three species, and 900 and 1000 m shared two. The highest species richness (37 species) and abundance (154 individuals) of regenerating woody species were observed at the altitudes of 800 m and 1200 m, respectively (Table 3). The highest species density occurred at 1300 m, while the highest dominance was observed at 1200 m. According to linear regression analysis, abundance and basal area did not vary across altitudes (P = 0.357 and P = 0.845, respectively), but species richness decreased with altitude (n = 7, r 2 = 0.816, P < 0.01, y = −0.0821x + 135.79, Fig. 10). More than 80 % of the variation in species richness was explained by altitude.
Species richness also varied significantly among altitudes (PERMANOVA: df = 90; F = 2.99; P < 0.001, Table 3). Plant species richness was significantly higher at 800 and 900 m, and lowest at 1400, 1300, and 1100 m (Table 3). Abundance (df = 90; F = 2.28; P < 0.001, Table 3) and absolute dominance (df = 90; F = 3.21; P < 0.001, Table 3) varied among altitudes. The 800- and 1200-m sites had the highest dominance values, while the sites at 1100, 1300, and 1400 m had the lowest.
The diversity (H′) for the entire sampled area (0.091 ha) was 4.14 (Table 3) and decreased with increasing altitude (n = 7, r 2 = 0.821, P < 0.05; y = −0.236x + 3.61; Fig. 11). According to PERMANOVA, Shannon index varied significantly among sites (df = 90; F = 4.25; P < 0.001). Sites can be grouped into three classes according to the significance of the Hutcheson’s t test followed by Bonferroni correction: high altitude (1300- and 1400-m sites), intermediate altitude (1100-m and 1200-m sites), and low altitude (800- to 1100-m sites) (Table 3). The 1100-m site was placed into two groups because its diversity value did not differ from that of the sites at 800, 900, 1000, and 1200 m, while H′ at the 1200-m site was significantly different from the H′ at all other sites except 1100-m site (Table 3).
Floristic similarity
The NMDS ordination (Fig. 12) indicated a significant correlation between altitude and species composition. The ordination axis explained 87.7 % of the variation, with 52.8 % explained by the first axis and 35.0 % by the second (35 %). The first axis separated the sites at the lower altitudes (800 and 900 m) from those at the higher altitudes (1300 and 1400). Plots of the sites at 1200, 1100, and 1000 m were dispersed. It was not possible to identify variation in altitude with the second axis; however, it was able to separate the plots at 1100 m. The first axis revealed a significant correlation with altitude composition (df = 81, F = 103.4, P < 0.0001), after considering the effects of spatial autocorrelation. Tukey’s HSD test showed that the highest three sites were significantly different from the lowest four sites.
Soil attributes
The collected soils were acidic, and especially so at higher altitudes (1100 through 1400 m) which exhibited lower average pH values (pH < 5). The altitude of 1400 m had the highest average levels of Al, Mg, H + Al, Ca, effective cation exchange capacity, and cation exchange capacity. The highest average levels of organic matter were found at the altitudes of 1200 and 1400 m. On the other hand, the altitudes of 800 and 1200 m had higher average levels of K, whereas the altitudes of 1100 and 1300 m had the lowest. The greatest average proportion of fine sand was found in the soils at 1100 and 1400 m, and the highest proportion of coarse sand was at 800, 1100, and 1300 m, silt at 800 and 900 m, and clay at 800 and 1100 m.
Relationship between environmental data and vegetation
For the correlation between community data and environmental variables (soils and altitude), altitude, V, Al, P-rem, and silt were significant and could be used to predict the variation in community data (F = 1.266, P < 0.002), as well as the spatial predictors (F = 1.617, P < 0.001). The variation explained by the spatial factors (12 %) was highest among fractions [a], [b], and [c] in the variance partitioning. The environment explained 2 %, and the spatially structured environment explained 4 % of the variation in species distribution. The residual fraction of variation was 82.4 %. In the RDA graph (Fig. 13), the first axis was positively correlated with the proportion of silt levels (0.67), base saturation (V; 0.52), and K (0.47) and negatively correlated with fine sand (FS; −0.61) (Fig. 13).
Ordination diagram of the canonical redundancy analysis (RDA) of significant soil variables and sampling altitudes according to the distribution of the 127 species sampled in 90 1-m2 plots at different altitudes of a rupestrian grassland complex of Serra do Cipó, and the correlation between the following variables: FS fine sand proportion, P-rem remaining phosphorus, V base saturation, Silt silt proportion, K potassium level
Discussion
Even in tropical mountains and within a relatively short altitudinal gradient, altitude causes changes in plant species composition and diversity. Such variation is also related to soil components at different altitudes. This study highlights the high species diversity and rarity of the vegetation of the rupestrian grassland complex at Serra do Cipó and the influence of environmental variation on plant diversity.
The families (Asteraceae, Eriocaulaceae, Lythraceae, Fabaceae, and Melastomataceae) and genera (Lychnophora, Eremanthus, Paepalanthus, Miconia, and Vellozia) of the regenerating strata found in this study have been considered characteristic of the rupestrian grassland (Romero 2002). Not surprisingly, elements of other vegetation types, especially “cerrado” s. s., are common in the rupestrian grassland ecosystem (Rapini et al. 2008), including some of the most abundant species detected in this study: Guapira venosa Lundell (typical of semideciduous, deciduous, and gallery forests), Erytroxylum suberosum (cerrado s.s.), and Acosmium dasycarpum (Vog) Yakovlev. (semideciduous, deciduous, gallery forests and “cerrado” s.s.) (Oliveira Filho 2005). Pinto et al. (2009) found that the woody vegetation in a rupestrian “cerrado” was composed mostly of species typical of cerrado s.s. vegetation, with smaller contributions from species characteristic of “cerrado” forest formations and endemic rupestrian grassland species. However, species of Eriocaulaceae had the highest IV, as these species have a large basal area and are common in the study area, especially at higher altitudes. Species of Eriocaulaceae are characterized by spiral leaves in rosettes or along the stems (Miranda and Giulietti 2001). They are extremely common in rupestrian grasslands (Costa et al. 2008) and contributed a significant basal area in the present study. In this study, floristic composition differed markedly among altitudes, as has been shown in altitudinal zones in the Atlantic Forest (Moreno et al. 2003). Vegetation patches vary in structure at different altitudes; as altitude increases both plant size and species richness are reduced (e.g., Whittaker 1956; 1960; Hamilton 1975; Gentry 1988; Kitayama 1992; Rocha and Amorim 2012). Structural aspects varied significantly between altitudes; however, again no altitudinal pattern was observed and, in other words, basal area, density, and dominance had no clear relationship with altitude. Species and habitat characteristics at each altitude may be confounding dominance values. The altitudes with higher dominance were 800 and 1200 m, and vegetation structure at 800 m had woody plant species with greater height and basal area. At this altitude, higher temperatures, lower wind velocity, and deeper soil (authors’ pers. inf.) ensure better conditions for colonization by woody species and seedling development. In the same manner, this elevation presents a dominance of “cerrado” physiognomy (Table 1). At an altitude of 1200 m, the influence of Paepalanthus sp. 3 was determinant for basal area and, consequently, for the dominance values. In contrast, at 1100, 1300, and 1400 m dominance values were low. These differences may have been the result of fewer individuals being sampled at 1100 and 1400 m altitudes, or by the presence of individuals with lower basal area at the 1300-m site (high abundance of Caliandra dysantha). Medina and Fernandes (2007) argued that low abundance of emergent seedlings at this altitude could be related to high seed dormancy. Only detailed studies on seed dormancy in the rupestrian grassland can address this hypothesis.
The diversity found in the present study for the total sampling area (H′ = 4.14) may be considered high, even for rupestrian grasslands (Conceição and Giulietti 2002; Conceição et al. 2005, 2007; Conceição and Pirani 2007; Gastauer et al. 2012; Mota 2012). However, it should be recognized that the different altitudes sampled encompass heterogeneous areas with different environmental factors that may contribute to the high diversity observed (Medina and Fernandes 2007; Carvalho et al. 2012). Sampling in the present study incorporated several habitat types commonly found in the rupestrian grasslands of Serra do Cipó (see Carvalho et al. 2012; Table 1). Therefore, these environmental variations likely contributed to the increased diversity of species in the area. The geomorphological features of each particular area, such as elevation, depression, and the general shapes of mountains, may also contribute to high local and regional diversity. In addition, the high species richness of the rupestrian grassland has a strong relationship with different substrates (Conceição and Giulietti 2002), while the relevance of the surrounding vegetation matrix cannot be neglected (e.g., Jacobi et al. 2007).
The highest species richness and diversity were found at lower altitudes, hence providing support for the altitudinal gradient hypothesis (Whittaker 1960; Lomolino 2001). Bhatt and Purohit (2011) also reported a decline of species richness with increasing altitude in a study conducted on a 1600–2200 m altitudinal gradient in the Himalayan region, while Sharma et al. (2009) showed the same trend for temperate forests (see also Huston 1994). On the other hand, highest plant diversity at mid-altitude has also been reported (Wang et al. 2002; Sang 2009; Joly et al. 2012). However, our study focused on a type of vegetation (rupestrian grasslands) that is naturally restricted to high altitudes (above 900 m). In this scenario, the regenerating woody species had a strong negative response to an increase in altitude. The regenerating community at the lower altitude had more species from the “cerrado” s.s., which contribute to the local diversity. The 1000-m site is a good example of an altitudinal transition zone, as it was the most diverse and contained species typical of high altitudes as well as of neighboring vegetation. Therefore, regional peculiarities and the relationship between sampled altitudes and neighboring phytophysiognomies seem to affect regenerating species composition, presumably through dispersion and colonization events.
In another sense, the presence of rocky outcrops in different proportions among the altitudes is another environmental characteristic that may influence variation in species composition and floristic similarity among sites. Some species prevail in areas with shallow soils using the fractures of the rocks for establishment (Conceição and Giulietti 2002; Negreiros et al. 2008). For example, the 1400-m elevation presents a more homogeneous area, with no rocky outcrops, and comprises a rupestrian grassland landscape (Table 1), with the most stressful physical conditions, which limit colonization by woody species resulting in low species diversity. Besides, rupestrian grassland vegetation occurs mostly at altitudes above 1100 m, and in this altitude, regenerating species of Eriocaulaceae and Velloziaceae prevail (Romero 2002). Korner (2007) attributes the high diversity at low altitudes to the effects of regional peculiarities and general altitude phenomena. Thus, in the study region, this pattern is probably a result of climatic trends and altitude, with strong roles played by light, UV radiation, and other factors such as wind velocity. These factors, unmeasured in our models, appear to have a significant contribution since the residual fraction was high in the partitioning of the variance of our study.
Typically, rupestrian grassland soils have low pH, phosphorus, and base saturation values (Vincent and Meguro 2008) and little organic matter; they are generally porous and friable (Reatto et al. 1998) as well as nutrient poor, with yellowish hues, sandy textures, and high levels of exchangeable aluminum (Benites et al. 2003). According to the RDA results, the soil variables distinguished some plots, although there was only slight variation in soil characteristics between altitudes in relation to fertility. For all chemical variables measured, remaining phosphorus, potassium, and base saturation were related to plots, but this relationship was not sufficient to separate the plots according to altitude. Communities that include species that are more demanding with respect to soil nutritional status occur where the potassium availability is higher (Moreno et al. 2008). The plant species of rupestrian grasslands differ in their responses to variation in soil fertility (Negreiros et al. 2009), so that soil nutritional deficiency favors only species adapted to nutritional stress (Grime 2006). According to Grime (2006), certain common features of vegetation types are immediately apparent among sites with severely nutrient-deficient soil, such as rosette species and a high proportion of dicotyledons being of creeping habit. Therefore, availability of nutrients appears to be one of the factors that allow the establishment of tree species (with higher nutritional requirements) at lower altitudes and perhaps in specific locations at higher altitudes that act as islands of resources.
In rock outcrops, physical characteristics of the soil, such as the influence of particle size on soil penetration by roots, water retention capacity and permeability, and low root/shoot ratios (Negreiros et al. 2009; Messias 2011), can determine features of the vegetation and affect plant colonization. The physical characteristics of the soil, specifically fine sand and silt, differed among the study plots. Fine sand showed a strong relationship with the distribution and composition of regenerating species at 1100 and 1400 m, while proportions of silt were higher at lower altitudes (1000, 900, and 800 m), and likely affected species richness. Thus, structural characteristics of soil are generally responsible for the heterogeneity of this habitat because variation in these characteristics affects species composition. This conclusion corroborates the results of Messias (2011), who found a strong relationship between species composition and variables associated with soil particle size, as well as the results of Negreiros et al. (2008) who found that soils differ significantly in texture in the rupestrian grasslands at Serra do Cipó. Carvalho et al. (2012) attributed the high diversity of the rupestrian grasslands of Serra do Cipó to heterogeneity in the physical attributes of soil among habitats.
In turn, it is likely that the proportion of fine sand and silt in the soil influences the similarity between locations, as this factor distinguished some plots. Furthermore, there were significant differences in the soils of some endemic legumes in the rupestrian grasslands of Serra do Cipó with respect to sand, silt, and clay (Negreiros et al. 2008). On the other hand, features of microtopography, such as the presence of rock outcrops, can have a crucial influence on rupestrian grassland flora (Benites et al. 2007), and may explain the low response of the environmental variables in the variance partitioning. The dispersion of the 1200-m plots, for example, may be due to the presence of large, steep outcrops at the site, which may limit the number of taxa (see also Conceição and Giulietti 2002). Thus, the lithology and geomorphology of the sampling area (Messias et al. 2012) and substrate (Conceição and Giulietti 2002) could have an important role in plant distribution in rupestrian grasslands.
Our study highlighted the relevance of altitude, physical soil characteristics, base saturation, potassium, and remaining phosphorus levels on the composition, structure, and richness of regenerating woody species along an altitudinal gradient in Brazil. Studies elsewhere have also shown strong plant response to similar environmental factors (e.g., Odland 2009; Sang 2009; Eisenlohr et al. 2013; Gentili et al. 2013; Graham et al. 2014). Other important contributors to variation in species diversity and composition were immediacy altitudes and habitat microtopography, which may influence the abrupt changes in richness and composition in a given altitudinal gradient. Restricting factors interfere with colonization by establishing limits to plant recruitment and establishment. These factors act as strong environmental filters mainly at higher altitudes where they restrict colonization by woody species and favor stress-tolerant ones (Grime 2006; Graham et al. 2014). It is likely that this has resulted in communities with phylogenetically closely related species at higher altitudes (Machac et al. 2011), such as mostly monocots, many of which are endemic. Additional studies are needed to investigate these possibilities.
References
Aiba S, Kitayama K (1999) Structure, composition and species diversity in an altitude-substrate matrix of rain forest tree communities on Mount Kinabalu, Borneo. Plant Ecol 140:139–157
Benites VM, Schaefer CEGR, Mendonça ES, Martin Neto L (2001) Caracterização da matéria orgânica e micromorfologia de solos sob campos de altitude no Parque Estadual da Serra do Brigadeiro. Rev Bras Cienc Solo 25:661–674
Benites VM, Caiafa AN, Mendonça ES, Schaefer CE, Ker JC (2003) Solos e vegetação nos complexos rupestres de altitude da Mantiqueira e do Espinhaço. Floresta Ambient 10:76–87
Benites VM, Schaefer CEGR, Simas FNB, Santos HG (2007) Soils associated with rocky outcrops in the Brazilian montain range Mantiqueira and Espinhaço. Rev Bras Bot 30:569–577
Bhatt P, Purohit VK (2011) Floristic structure and phytodiversity along an elevational gradient in Peepalkoti–Joshimath area of Garhwal Himalaya, India. Nat Sci 9:57–67
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Borges RAX, Carneiro MAA, Viana PL (2011) Altitudinal distribution and species richness of herbaceous plants in campos rupestres of Southern Espinhaço Range, Minas Gerais, Brazil. Rodriguésia 62:139–152
Brasil (2006) Lei no 11.428, de 22 de dezembro de 2006. Dispõe sobre a utilização e proteção da vegetação nativa do Bioma Mata Atlântica, e dá outras providências
Carvalho F, Souza FA, Carrenho R, Moreira FMS, Jesus EC, Fernandes GW (2012) The mosaic of habitats in the high-altitude Brazilian rupestrian fields is a hotspot for arbuscular mycorrhizal fungi. Appl Soil Ecol 52:9–19
Colwell RK (2013) EstimateS: statistical estimation of taxa richness and shared taxa from samples, version 9. User’s Guide and application published. http://purl.oclc.org/estimates. Acessed 25 Nov 2015
Conceição AA, Giulietti AM (2002) Composição florística e aspectos estruturais de campo rupestre em dois platôs do Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Hoehnea 29:37–48
Conceição AA, Pirani JR (2007) Diversidade em quatro áreas de campos rupestres na Chapada Diamantina, Bahia, Brasil: espécies distintas, mas riquezas similares. Rodriguésia 58:193–206
Conceição AA, Rapini A, Pirani JR, Giulietti AM, Harley RM, Silva TRS et al (2005) Campos rupestres. In: Juncá FA et al (eds) Biodiversidade e conservação da Chapada Diamantina. Ministério do Meio Ambiente, Brasília, pp 153–180
Conceição AA, Giulietti AM, Meirelles ST (2007) Ilhas de vegetação em afloramentos de quartizito-arenito no Morro do Pai Inácio, Chapada Diamantina, Bahia, Brasil. Acta Bot Bras 21:335–347
Costa CMR, Hermann GCS, Martins C, Lins LV, Lamas IR (1998) Biodiversidade em Minas Gerais: um atlas para sua conservação. Fundação Biodiversitas
Costa FN, Trovó M, Sano PT (2008) Eriocaulaceae na Cadeia do Espinhaço: riqueza, endemismo e ameaças. Megadiversidade 4(1–2):89–97
Diniz Filho JAF, Bini LM (2005) Modelling geographical patterns in species richness using eigenvector-based spatial filters. Glob Ecol Biogeogr 14:177–185
Dray S (2013) SpacemakeR: Spatial modelling. R package version 0.0-5/r113. http://R-Forge.R-project.org/projects/sedar. Accessed 10 Jan 2015
Dray S, Legendre P, Blanchet G (2013). Packfor: forward selection with permutation (Canoco p.46). R package version 0.0-8/r109. http://R-Forge.R-project.org/projects/sedar. Accessed 20 Jan 2015
Eisenlohr PV (2013) Challenges in data analysis: pitfalls and suggestions for a statistical routine in vegetation ecology. Braz J Bot 3:83–87
Eisenlohr PV (2014) Persisting challenges in multiple models: a note on commonly unnoticed issues regarding collinearity and spatial structure of ecological data. Braz J Bot 37:365–371
Eisenlohr PV, Alves LF, Bernacci LC, Padgurschi MCG, Torres RB, Prata EMB et al (2013) Disturbances, elevation, topography and spatial proximity drive vegetation patterns along an altitudinal gradient of a top biodiversity hotspot. Biodivers Conserv 22:2767–2783
Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) (1999) Centro Nacional de Pesquisa de Solos Sistema brasileiro de classificação de solos. EMBRAPA, Rio de Janeiro
Fernandes GW (1992) A gradient analysis of plant forms from Northern Arizona. J Ariz Nev Acad Sci 24–25:21–30
Fernandes GW (2016) The megadiverse rupestrian grassland. In: Fernandes GW (ed) Ecology and conservation of mountaintop grasslands in Brazil. Springer, New York, pp 3–14
Fernandes GW, Price PW (1988) Biogeographical gradients in galling species richness. Oecologia 76:161–167
Filgueiras TS (2002) Herbaceous plant communities. In: Oliveira PS, Marquis RJ (eds) The cerrados of Brazil: ecology and natural history of a neotropical savanna. Columbia University Press, New York, pp 121–139
Fritzsons E, Mantovani LE, Aguiar AV (2008) Relação entre altitude e temperatura: uma contribuição ao zoneamento climático no Estado do Paraná. Rev Estud Amb 10:49–64
Gastauer M, Messias MCTB, Meira Neto JAA (2012) Floristic composition, species richness and diversity of campo rupestre vegetation from the Itacolomi State Park, Minas Gerais, Brasil. Environ Nat Resour Res 2:115–130
Gentili R, Armiraglio S, Sgorbati S, Baroni C (2013) Geomorphological disturbance affects ecological driving forces and plants turnover along an altitudinal stress gradient on alpine slopes. Plant Ecol 214:571–586
Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard 75:1–34
Giulietti AM, Menezes NL, Pirani JR, Meguro M, Wanderley MGL (1987) Flora da Serra do Cipó, Minas Gerais: caracterização e lista de espécies. Bol Bot USP 9:1–151
Graham CH, Carnaval AC, Cadena CD, Zamudio KR, Roberts TE, Parra JL et al (2014) The origin and maintenance of montane biodiversity: integrating evolutionary and ecological processes. Ecography 37:711–719
Grime JF (2006) Plant strategies, vegetation processes, and ecosystem properties, 2nd edn. Wiley, London
Grubb PJ (1977) Control forest growth and distribution on wet tropical mountains: with special references to mineral nutrition. Ann Rev Ecol Syst 8:83–107
Hamilton AC (1975) A quantitative analysis of altitudinal zonation in Uganda forests. Vegetatio 30:99–106
Hammer O (2013) PAST—paleontological statistics version 3.01. http://folk.uio.no/ohammer/past. Accessed 20 Aug 2014
Hussain MZ, Malik NZ (2012) High altitude forest composition diversity and its component in a part of Ganga Chotti and Bedori Hills District Bagh. Azad Jammu and Kashmir, Pakistan. AGD Lands Environ 6:31–40
Huston MA (1994) Biological diversity. Cambridge University Press, Cambridge
Jacobi CM, Carmo FF, Vincent RC, Stehmann JR (2007) Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodivers Conserv 16:2185–2200
Joly CA, Assis MA, Bernacci LC, Tamashiro JY, Campos MCR, Gomes JAMA et al (2012) Florística e fitossociologia em parcelas permanentes da Mata Atlântica do sudeste do Brasil ao longo de um gradiente altitudinal. Biota Neotrop 12:125–145
Kitayama K (1992) An altitudinal transect study of the vegetation on Mount Kinabalu, Borneo. Vegetatio 102:149–171
Korner C (2007) The use of ‘‘altitude’’ in ecological research. Trends Ecol Evol 22:569–574
Krebs CJ (1989) Ecological methodology. Harper and Row, New York
Legendre P, Dale MRT, Fortin MJ, Gurevitch J, Hohn M, Myers D (2002) The consequences of spatial structure for the design and analysis of ecological field surveys. Ecography 25:601–615
Liberman D, Lieberman M, Peralta R, Hartshorn G (1996) Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J Ecol 84:137–152
Lomolino VM (2001) Elevation gradients of species-density: historical and prospective view. Glob Ecol Biogeogr 10:3–13
Machac A, Janda M, Dunn R, Sanders NJ (2011) Elevational gradients in phylogenetic structure of ant communities reveal the interplay of biotic and abiotic constraints on diversity. Ecography 34:364–371
Madeira JA, Fernandes GW (1999) Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil. J Trop Ecol 15:463–479
McCune B, Grace JB (2002) Analysis of ecological communities, 2nd edn. MjM Software Design, Gleneden Beach, p 300
McCune B, Mefford MJ (2011) PC-ORD: multivariate analysis of ecological data, version 6.0. MjM Software Design, Gleneden Beach, p 28
Medina BMO, Fernandes GW (2007) The potential of natural regeneration of rocky outcrop vegetation on rupestrian fields soils in “Serra do Cipó”, Brazil. Rev Bras Bot 30:665–678
Melo Júnior TA, Vasconcelos SMF, Fernandes GW, Marini MA (2001) Bird species distribution and conservation in Serra do Cipó, Minas Gerais, Brazil. Bird Conserv Int 11:189–204
Menino GCO, Nunes YRF, Santos RM, Fernandes GW, Fernandes LA (2012) Environmental heterogeneity and natural regeneration in riparian vegetation of Brazilian semi-arid region. Edinb J Bot 69:29–51
Messias MCTB (2011) Fatores Ambientais Condicionantes da Diversidade Florística em Campos Rupestres Quartzíticos e Ferruginosos no Quadrilátero Ferrífero, Minas Gerais. Thesis, Universidade Federal de Ouro Preto
Messias MCTB, Leite MGP, Meira-Neto JAA, Kosovits AR (2012) Fitossociologia de campos rupestres quartzíticos e ferruginosos no Quadrilátero Ferrífero, Minas Gerais. Acta Bot Bras 26:230–242
Miranda EB, Giulietti AM (2001) Eriocaulaceae no Morro do Pai Inácio (Palmeiras) e Serra da Chapadinha (Lençóis), Chapada Diamantina, Bahia, Brasil. Sitientibus Ser Cienc Biol 1:15–32
Moreno MR, Nascimento MT, Kurtz BC (2003) Estrutura e composição florística do estrato arbóreo em duas zonas altitudinais na Mata Atlântica de encosta na região do Imbé, RJ. Acta Bot Bras 17:371–386
Moreno MIC, Schiavini I, Haridasan M (2008) Fatores edáficos influenciando na estrutura de fitofisionomias do Cerrado. Cam Geogr 9:173–194
Mota GS (2012) Variação na estrutura, na composição florística e nas formas de vida ao longo de um gradiente altitudinal na Cadeia do Espinhaço. Dissertation, Universidade Estadual de Montes Claros
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York, p 547
Negreiros D, Moraes MLM, Fernandes GW (2008) Caracterização da fertilidade dos solos de quatro leguminosas de campos rupestres, Serra do Cipó, MG, Brasil. Rev Cienc Suelo Nutr 8:30–39
Negreiros D, Fernandes GW, Silveira FAO, Chalub C (2009) Seedling growth and biomass allocation of endemic and threatened shrubs of rupestrian fields. Acta Oecol 35:301–310
Nunes YRF, Landau EC, Veloso MDM (2008) Diversidade de Melastomataceae em diferentes altitudes de campos rupestres na Serra do Cipó, MG. Unimontes Cient 10:34–45
Odland A (2009) Interpretation of altitudinal gradients in South Central Norway based on vascular plants as environmental indicators. Ecol Indic 9:409–421
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. (2015) Vegan: community ecology package. R package version 2.2-1. http://CRAN.R-project.org/package=vegan. Accessed 20 Jan 2015
Oliveira Filho AT (2005) Catálogo das Árvores Nativas de Minas Gerais: mapeamento e inventário da flora nativa e dos reflorestamentos de Minas Gerais. Universidade Federal de Lavras, Lavras
Peck JE (2010) Multivariate analysis for community ecologists: step-by-step using PC-ORD. MjM Software Design, Gleneden Beach
Peres Neto PR, Legendre P (2010) Estimating and controlling for spatial autocorrelation in the study of ecological communities. Glob Ecol Biogeogr 19:174–184
Peres Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Pinto JRR, Lenza E, Pinto AS (2009) Composição florística e estrutura da vegetação arbustivo-arbórea em um cerrado rupestre, Cocalzinho de Goiás, Goiás. Rev Bras Bot 32:1–10
Rahbek C (1995) The elevation gradient of species richness: a uniform pattern? Ecography 18:200–205
Rana MS, Samant SS, Rawat YS (2011) Plant communities and factors responsible for vegetation pattern in an alpine area of northwestern Himalaya. J Mount Sci 8:817–826
Rapini A, Ribeiro PL, Lambert S, Pirani JR (2008) A flora dos campos rupestres da Cadeia do Espinhaço. Megadiversidade 4:16–24
Reatto A, Correia JR, Spera ST (1998) Solos do Bioma Cerrado: aspectos pedológicos. In: Sano SM, Almeida SP (eds) Cerrado: ambiente e flora. Embrapa-CPAC, Planaltina, pp 47–83
Ribeiro KT, Fernandes GW (1999) Geographic distribution of Coccoloba cereifera Schw. (Polygonaceae), a narrow endemic plant from Serra do Cipó, Brazil. Bios Cad Biol 7:7–12
Ribeiro KT, Fernandes GW (2000) Patterns of abundance of a narrow endemic species in a tropical and infertile montane habitat. Plant Ecol 147:205–218
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do Bioma Cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Embrapa, Planaltina, pp 151–212
Rocha DSB, Amorim AMA (2012) Heterogeneidade altitudinal na Floresta Atlântica setentrional: um estudo de caso no sul da Bahia, Brasil. Acta Bot Bras 26:309–327
Romero R (2002) Diversidade da flora dos campos rupestres de Goiás, Sudoeste e Sul de Minas Gerais. In: Araújo EL, Moura NA, Sampaio EVSB et al (eds) Biodiversidade, conservação e uso sustentável da flora do Brasil. Universidade Federal Rural de Pernambuco, Sociedade Botânica do Brasil, Recife, pp 81–86
Sang W (2009) Plant diversity patterns and their relationships with soil and climatic factors along an altitudinal gradient in the middle Tianshan Mountain area, Xinjiang, China. Ecol Res 24:303–314
Semir J (1991) Revisão taxonômica de Lychnophora Mart. (Vernonieae: Compositae). Thesis, Universidade de Campinas
Sharma CM, Suyal S, Gairola S, Ghildiyal SK (2009) Species richness and diversity along in altitudinal gradient in moist temperate forest of Garhwal Himalaya. J Am Sci 5:119–128
Shepherd GJ (2010) FITOPAC 21: manual do usuário. Universidade Estadual de Campinas, Campinas
Smith RA (1971) The effect of unequal group size on Tukey’s HSD procedure. Psychometrika 36:31–34
ter Braak CFJ (1987) The analysis of vegetation-environment relationships bay canonical correspondence analysis. Vegetatio 69:69–77
Torres RB, Martins FR, Inoshita LS (1997) Climate, soil and tree flora relationships in forests in the state of São Paulo, southeastern Brazil. Rev Bras Bot 20:41–49
Vincent RC, Meguro M (2008) Influence of soil properties on the abundance of plant species in ferruginous rocky soils vegetation, southeastern Brazil. Rev Bras Bot 31:377–388
Wang G, Zhou G, Yang L, Li Z (2002) Distribution species diversity and life-form spectra of plant communities along an altitudinal gradient in the northern slopes of Qilianshan Mountains, Gansu, China. Plant Ecol 165:169–181
Whittaker RH (1956) Vegetation of the great smoky mountains. Ecol Monogr 26:1–80
Whittaker RH (1960) Vegetation of the Siskiyou mountains. Ecol Monogr 30:279–338
Zar JH (1996) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico; 558250/2009-2; 307039/2013-7), CNPq/Peld-Site 17, and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais; APQ-04105-10) for grants funding this research. We also thank the field assistance provided by ES Coutinho, SR Souza, MH Oliveira, FM Costa, DL Silva, RCV Araújo, PL Rodrigues, LL Braga, TM Veloso, and LP Lima. GR Luz and TO Bahia provided suggestions to the manuscript, RM Santos, R Romero, MC Mamede, M Sobral, and D Negreiros helped with identifying botanical specimens, and UNIMONTES (Universidade Estadual de Montes Claros), Parque Nacional da Serra do Cipó, and Reserva Vellozia provided logistical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mota, N.M., Rezende, V.L., da Silva Mota, G. et al. Forces driving the regeneration component of a rupestrian grassland complex along an altitudinal gradient. Braz. J. Bot 39, 845–860 (2016). https://doi.org/10.1007/s40415-016-0287-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0287-6