Abstract
Purpose
High-grade gliomas are aggressive primitive brain tumors presenting aberrant vasculature, regional necrosis, and areas of hypoxia. Tumor hypoxia is associated with resistance to conventional treatment and worse prognosis. [18F]-fluoromisonidazole (18F-FMISO) is the most extensively investigated radiotracer for the evaluation of hypoxia. However, the use of 18F-FMISO in clinical practice has been hampered mainly due to the slow clearance of the unbound tracer from normoxic tissue and its low tumor-to-background ratio (TBR). The research community has therefore investigated other radiotracers to overcome the drawbacks of 18F-FMISO. This mini-review aims to present an update on the most relevant PET studies published in the last 15 years evaluating the utility of radiotracers for hypoxia imaging other than 18F-FMISO in high-grade glioma (HGG) patients.
Methods
A comprehensive computer literature search of studies was carried out in PubMed/MEDLINE database to identify the most relevant studies published in the last 15 years which investigated the utility of hypoxia PET tracers other than 18F-FMISO in the assessment of tumor hypoxia in patients with HGG.
Results
18F-flouroazomycin arabinoside (18F-FAZA) has been proposed as a valid alternative to 18F-FMISO for the assessment of hypoxia, due to its improved biodistribution and enhanced tumor-to-background ratio. Also 1-(2-[18F]fluoro-1[hydroxymethyl]ethoxy)methyl-2-nitroimidazole(18F-FRP170) seems a valuable hypoxia tracer in patients with brain tumor. The value of copper-diacetyl-bis(N4-methylthiosemicarbazone)(Cu-ATSM) seems controversial. Few evidences still exist regarding the utility of 18F-2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide (18F-EF5).
Conclusion
Hypoxia PET imaging has the potential to provide useful information for the clinicians and to guide hypoxia tailored treatments. According to the present literature, the most promising hypoxic tracer seems to be 18F-FAZA, but well-designed and wide trials to validate hypoxia radiotracers and evaluate their clinical utility in daily practice are still lacking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-grade glioma (HGG) are aggressive primitive brain tumors arising from the glial tissue [1]. HGG, and particularly grade IV glioma (formerly known as glioblastoma multiforme—GBM), are characterized by aberrant vasculature (a phenomenon known as angiogenesis), regional necrosis, and viable areas of hypoxia [2]. Hypoxia is a pathophysiological status characterized by an oxygen concentration in a tissue lower than normal [3]. In brain tumors, the median pO2 has been reported to be around 13 mmHg in contrast to the median pO2 (35 mmHg) documented in normal brain tissue [4, 5].
A well-documented link between hypoxia, prognosis, and resistance to conventional treatments exists in hypoxic tumors [3]. The clinical relevance of mapping and measuring tumor hypoxia in HGG using non-invasive imaging techniques, such as positron emission tomography (PET) or magnetic resonance imaging (MRI), relies on the possibility of delivering hypoxia-modifying treatments, leading theoretically to an improvement of patient outcome [3, 6,7,8].
Quantification of hypoxia in HGG using PET has been extensively documented [9, 10]. Hypoxia PET imaging agents can be divided into nitroimidazole-based radiotracers and other compounds and further divided in fluorinated and non-fluorinated agents [11]. In the assessment of tissue hypoxia in brain tumors, further important aspects to consider for a tracer are the lipophilicity and the ability to cross the blood–brain barrier (BBB) [12].
[18F]-fluoromisonidazole (18F-FMISO) is the most extensively used and validated radiotracer so far for the evaluation of hypoxia in patients with brain tumors [10, 13,14,15,16,17,17]. However, other radiotracers have been investigated to overcome the main drawbacks of 18F-FMISO which have limited its translation from the research setting to the clinical practice: the slow clearance of the unbound tracer from normoxic tissue, the slow plasma clearance, and the low tumor-to-background ratio (TBR) [11].
This review aimed to discuss the most relevant PET clinical studies, published in the last 15 years, evaluating the utility of PET radiopharmaceuticals for imaging tumor hypoxia, other than 18F-FMISO, in patients with HGG, offering also an insight, when available, on the complementarity between PET and advanced MRI techniques in this setting.
Methods
We searched on PubMed and Scopus databases using combinations of the following search terms: ‘tumor hypoxia', ‘brain tumor’, ‘glioma’ ‘PET’, ‘positron emission tomography’, ‘nitroimidazoles’, ‘fluoromisonidazole’, ‘FMISO’, ‘FAZA’, EF5′, ‘FRP170′ ‘Cu-ATSM’, ‘DiFA’. The search results were screened for relevance and the reference lists of relevant publications were also crosschecked. Only papers published in English were considered. The final reference list was compiled by considering papers published in the last 15 years through December 2019.
18F-FAZA
Several hypoxia-specific PET tracers have been proposed for tumor hypoxia imaging after 18F-FMISO. One of the most promising second-generation 2-nitroimidazole radiolabeled compounds is 18F-flouroazomycin arabinoside (18F-FAZA) (Tables 1 and 2, Fig. 1) [11]. Compared to 18F-FMISO, 18F-FAZA has an improved biodistribution, related to the addition of a sugar moiety, making it less lipophilic [14]. 18F-FAZA has faster diffusion and more rapid clearance from normal tissue compared to 18F-FMISO, providing an enhanced tumor-to-background ratio [19].
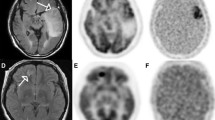
(Adapted with the permission of Mapelli et al. [26]. A 77-year-old patient with a suspected brain tumor. a MRI gadolinium-enhanced T1 image showing an enhancing, oedematous lesion in the right basal ganglia and "corona radiata" with mass effect on the right ventricle. The lesion also showed high values of relative cerebral blood volume, b (rCBV; yellow arrow), transfer constant (Ktrans), c red arrow and fractional plasma volume (Vp)(d)—white arrow. e) 18F-FAZA PET image showed uptake in correspondence of the brain lesion with a central photopenic area possibly due to necrotic tissue. f MRI and 18F-FAZA PET/CT images were co-registered and fused and an area of both high perfusion MRI markers and high 18F-FAZA uptake was selected for biopsy. The histological analysis confirmed the presence of a grade IV glioblastoma
A higher contrast with non-target tissues for 18F-FAZA compared to 18F-FMISO, has been reported by Souvatzoglou et al., with an average tumor-to-muscle ratio of 2.0 ± 0.3 at 2 h post-injection acquisition [20]. The same group also reported that 18F-FAZA has overall superior pharmacokinetics and that the use of dynamic analysis offers further potential improvement [21]. The different pharmacokinetics of 18F-FAZA can be inferred evaluating its octanol/water partition coefficient (P). This is a parameter reflecting the hydrophilic/lipophilic nature of a tracer, with lower values indicating lower lipophilicity. Whereas 18F-FMISO has a P of 0.4, the corresponding value is 0.027 for 18F-FAZA. The lower P, as demonstrated in preclinical experiments, results in a faster plasma clearance and lower uptake in organs (especially liver, kidneys and small intestine) 3 h post-injection compared to 18F-FMISO [21, 22].
First-in-human biodistribution and dosimetry data for 18F-FAZA have been recently presented by Savi et al. The authors reported an effective dose equivalent (EDE) similar to that of 18F-FMISO (0.015 mSv/MBq vs. 0.013 mSv/MBq, respectively) with the highest absorbed dose measured in the urinary bladder wall [23].
Few studies investigated the application and relevance of 18F-FAZA in the specific clinical setting of HGG and there is a lack of studies evaluating the utility of the combined use of 18F-FAZA and MRI in the assessment of hypoxia. Postema et al. were the first group reporting clinical experience on 18F-FAZA in HGG [24]. Their study included 50 oncological patients, 7/50 of whom were affected by GBM. A high tumor-to-blood ratio of the radiotracer was reported, mainly related to the lack of uptake of 18F-FAZA in the normal brain tissue. Additionally, 18F-FAZA provided good quality images at 2–3 h post-injection, thus avoiding later scanning. This study confirmed that 18F-FAZA was highly representative of tumor hypoxia within glioblastoma. Although a disrupted BBB certainly plays a role in 18F-FAZA uptake in gliomas, the radiotracer retention is strictly dependent from the reduction process, which happens only under hypoxic conditions, and therefore 18F-FAZA uptake is absent in normal brain tissue.
In the setting of treatment planning, Mapelli et al. reported the potentiality of 18F-FAZA PET/CT in guiding tailored radiotherapy treatment in HGG. The group described the possibility to provide an additional boost on more hypoxic regions using 18F-FAZA PET images in a simulated radiotherapy scheme. Additionally, this molecular imaging technique has been also used to assess tumor response after radiotherapy with promising implication for clinical practice [25]. The same group reported for the first time the ability of 18F-FAZA to identify the more hypoxic regions within the tumor and consequently its ability to guide stereotactic biopsy on the tumor areas with the highest aggressive potential. To achieve this goal, a co-registration of MRI and 18F-FAZA PET/CT images has been performed to identify the areas showing either high perfusion MRI markers expression and high 18F-FAZA uptake, therefore indicating the most representative hypoxic tumor region [26].
Other hypoxia PET tracers
Research is continuously addressing the development of new radiotracers, which can evaluate the heterogeneous nature of glial tumors, with particular regards to the extent of hypoxia within the tumor tissue, overcoming the limitations of “traditional” hypoxia PET tracers [3]. Currently, there is a lack of studies that systematically correlate these tracers with MRI in patients with HGG. Therefore, in this paragraph, we discuss the most promising hypoxic tracers considering both clinical and preclinical evidence.
2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)-acetamide (EF5) is a lipophilic compound that binds to hypoxic tumor cells [27, 28]. Koch et al. evaluated the clinical usefulness of 18F-EF5 PET by comparing 18F-EF5 uptake with EF5 bindings, assessed by immunohistochemistry, in 3 patients with glioblastoma (Fig. 2). The authors found that the uptake of 18F-EF5 corresponded to the regions characterized by high EF5 [29]. The same group proved that the 18F-EF5 distribution is positively correlated with intertumoral variation of radiation response in twenty-two 9Lgliosarcoma tumors grown in Fischer rats [30]. The main limit for the widespread use of such compound relies on the requirement for electrophilic fluorination using 18F-F2, at the time of the radiolabeling process. Another limitation of 18F-EF5 could be its low tumor-to-normal tissue ratio due to the slow clearance of the unbound tracer [15].
Adapted with the permission from Koch et al. [29]. CE MRI image of tumor (upper left) and the correspondent PET image (lower left). The anterior area of the tumor (red arrow) displays little 18F-EF5 uptake and tissue obtained from this region has no EF5 binding, determined by IHC (upper right). The inferior area (green arrow) is characterized by high 18F-EF5 uptake and tissue obtained from this area has high EF5 binding, determined by IHC (lower right)
Another novel hydrophilic tracer for hypoxia assessment is 1-(2-[18F]fluoro-1[hydroxymethyl]ethoxy)methyl-2-nitroimidazole (18F-FRP170; Table 2). In preliminary evaluations, low distribution has been reported in normal brain tissue [31]. Shibahara et al. assessed hypoxia in glial tumors of different grades in a group of eight patients. The authors found that high 18F-FRP170 uptake was associated with a remarkable hypoxia-inducible-factor (HIF-1a) expression in case of GBM, whereas the uptake was moderate for lower-grade gliomas [32]. In four out of eight patients, an 18F-FDG PET/CT scan was also performed to compare the distribution of the two radiotracers. The uptake of FDG did not match well with the FRP170 uptake, presumably due to their different pharmacodynamics. The same was true also for two of the three patients who underwent to 11Cmethionine PET/CT together with the 18F-FRP170 PET; the only concordant case was a recurrent anaplastic astrocytoma, possibly because cell proliferation was high enough to induce hypoxia in the tumor mass, or the vascular supply was adequate for hypoxic tumor cells to continue proliferating. Also, Beppu et al. reported interesting findings on the potential usefulness of 18F-FRP170 for the detection of hypoxic areas within glial tumors. In a preliminary study involving twelve patients with GBM undergoing 18F-FRP170 PET 1 h after injection, the authors reported that areas with high 18F-FRP170 uptake, calculated as the SUVmean within the 10 mm-ROI drawn in the areas with the highest uptake, corresponded to areas with low pO2, measured with microelectrodes during the tumor excision [33]. More recently, the same group compared low and high 18F-FRP170 uptake areas with immunohistochemical staining for Ki-67 within tumor specimens and hypoxia-inducible factor (HIF)-1 expression in 13 patients with GBM. A good match was found between high 18F-FRP170 uptake and high HIF-1a levels and also between high Ki-67 expressions and high 18F-FRP170 uptake areas [34]. According to these findings, it has therefore been postulated that 18F-FRP170 uptake may be considered as a marker of proliferation rather than hypoxia.
Among novel hypoxic radiotracers, also copper-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) has shown some promising results (Table 3). In hypoxic cells, Cu-ATSM is trapped by the reaction with thiol groups or redox-active proteins. Conversely, Cu-ATSM is less retained in tissues with an inadequate amount of oxygen, owing to a less reducing environment, with its subsequent wash-out from the cells [16, 35, 36]. Four different Copper-isotopes were bound with ATSM compound: 60Cu-, 61Cu-, 62Cu-, 64Cu [37]. Among them, 64Cu- seems to be the most promising for clinical applications, owing to its feasible half-life (12.7 h) and a more remarkable TBR, which, once assessed 1-hour post-injection, was shown to be higher than the TBR provided by 2-nitroimidazole based tracers. Moreover, it demonstrated the fastest clearance, with an easier radiolabeling procedure and synthesis, compared to other radiotracers targeting hypoxia [19]. A pioneering preclinical study evaluated the relationship between copper-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) uptake with pO2 and its changes obtained by the administration of hydralazine, in a 9Lgliosarcoma rat model [38] and found an inverse correlation between pO2 and 61Cu-ATSM uptake.
62Cu-ATSM uptake has proved also to be an independent prognostic factor in glioma patients (in terms of PFS and OS; p < 0.05) and is a more promising imaging method to predict prognosis compared to 18F-FDG PET/CT, as shown in a study including 56 patients with grade 2–4 glioma [39]. More recently, Tateishi et al. evaluated the feasibility of 62Cu-ATSM PET to discriminate patients with different grade of glioma, even in association with 11CMET PET and/or 18F-FDG PET. The authors found higher SUVmax and TBR in both 62Cu-ATSM PET and 11CMET PET scans in patients with GBM than in patients with grade II or III gliomas. By establishing a TBR cut-off of 1.9, the authors confirmed that 62Cu-ATSM PET could be a useful tool for distinguishing patients with GBM from patients with lower-grade glioma [40].
Intratumoral distribution of Cu-ATSM has also been compared with the distribution of 18F-FMISO and 18F-FDG [41,42,43]. The rationale of this comparison relies on the fact that 18F-FMISO and 64Cu-ATSM have different retention mechanisms and different half-lives, t1/2 = 110 min and 12.7 h, respectively. Furthermore, in a 9Lgliosarcoma rat model, Dence and coworkers found a strong positive correlation between intra-tumoral 18F-FMISO uptake, assessed 2 h post injections, with 64Cu-ATSM uptake either at 10 min and 24 h post-injection [44].
However, further evidence warns to consider Cu-ATSM as a marker for hypoxia tout court since the validity of 64Cu-ATSM uptake as a marker of hypoxia is dependent on tumor type [45]. Indeed, it is still a matter of debate whether Cu-ATSM could be able to detect a pure hypoxic phenomenon rather than cell-redox potential and vascular delivery [46, 47].
More recently, another PET tracer, 1-(2,2-dihydroxymethyl-3-[18F]-fluoropropyl)-2-nitroimidazole (18F-DiFA) has been proposed for measuring hypoxia [48]. This tracer accumulates in hypoxic tissue via a glutathione conjugation reaction and displays a more hydrophilic nature than 18F-FMISO [8]. In vitro experiments in Fadu head and neck cancer cells, preincubated under hypoxia (O2 = 1%), documented higher radioactivity uptake at 2 h after incubation with for 18F-DiFA compared to 18F-FMISO (0.53 ± 0.02 vs. 0.34 ± 0.07; p < 0.01) [49]. A subsequent preclinical study, conducted in EMT6 mammary carcinoma cell-bearing mice, showed higher accumulation in tumor than 18F-FMISO at 1 h and 2 h post-injection [50]. Preliminary clinical data in seven patients with different types of tumors undergoing both 18F-FMISO and 18F-DiFA PET scan showed at a visual analysis similar diagnostic ability for tumor hypoxia for 18F-DiFA (evaluated at 1 h and 2 h) compared to 18F-FMISO (assessed 4 h post-injection). Unfortunately, no data are still available for brain tumors [48].
Conclusion
Well-designed trials are required to validate hypoxia radiotracers and evaluate their clinical utility in daily clinical practice. Hypoxia PET imaging has the potential to provide useful information for clinicians to guide hypoxia-tailored treatments. Based on current literature, the most promising second-generation hypoxic tracer seems to be 18F-FAZA, although additional research trials should be performed before introducing this tracer into the clinical scenario. The ideal tracer for quantifying hypoxia has not yet been found in brain tumor imaging. Although hydrophilic compounds present undeniable advantages compared to lipophilic tracers in terms of pharmacokinetics, there is still an open debate between supporters of lipophilic tracers and supporters of hydrophilic tracers. Despite the undeniable advantages of hydrophilic profile in terms of pharmacokinetics, the impact of BBB disruption on tracer delivery should be further investigated.
Another problem to consider is that hypoxia PET imaging requires fully equipped facilities with trained staff for tracer production and accurate image analysis. A consensus in the quantification methodology of hypoxia in PET imaging is still missing. Another point that needs to be addressed is the comparison of the hypoxic volume (defined in PET imaging) and the volume of the radio-resistant portion of the tumor. Last but not least, trials evaluating the utility of hypoxia PET imaging in the assessment of response to hypoxia-targeted treatments are warranted to define the potential impact in patient outcome.
References
Ostrom QT et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(4):1–63
Brahimi-Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia and cancer. J Mol Med (Berl) 85(12):1301–1307
Walsh JC et al (2014) The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal 21(10):1516–1554
McKeown SR (2014) Defining normoxia, physoxia and hypoxia in tumours—implications for treatment response. Br J Radiol 87(1035):20130676
Carreau A et al (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15(6):1239–1253
Huang W-J, Chen W-W, Zhang X (2016) Glioblastoma multiforme: effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol Lett 12(4):2283–2288
Rockne RC et al (2015) A patient-specific computational model of hypoxia-modulated radiation resistance in glioblastoma using 18F-FMISO-PET. J R Soc Interface 12(103):20141174
Hirata K et al (2019) The roles of hypoxia imaging using (18)F-fluoromisonidazole positron emission tomography in glioma treatment. J Clin Med 8(8):1088
Bell C et al (2015) Hypoxia imaging in gliomas with 18F-fluoromisonidazole PET: toward clinical translation. Semin Nucl Med 45(2):136–150
Bonnitcha P, Grieve S, Figtree G (2018) Clinical imaging of hypoxia: current status and future directions. Free Radic Biol Med 126:296–312
Quartuccio N, Asselin MC (2018) The validation path of hypoxia PET imaging: focus on brain tumours. Curr Med Chem 25(26):3074–3095
Rajendran JG, Krohn KA (2015) F-18 fluoromisonidazole for imaging tumor hypoxia: imaging the microenvironment for personalized cancer therapy. Semin Nucl Med 45(2):151–162
Bekaert L et al (2017) [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 44(8):1383–1392
Busk M et al (2013) PET hypoxia imaging with FAZA: reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur J Nucl Med Mol Imaging 40(2):186–197
Chitneni SK et al (2011) Molecular imaging of hypoxia. J Nucl Med 52(2):165–168
Fujibayashi Y et al (1997) Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 38(7):1155–1160
Gerstner ER et al (2016) ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma using 18F-FMISO PET and MRI. Clin Cancer Res 22(20):5079–5086
Fleming IN et al (2014) Imaging tumour hypoxia with positron emission tomography. Br J Cancer 112(2):238–250
Lopci E et al (2014) PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am J Nucl Med Mol Imaging 4(4):365–384
Souvatzoglou M et al (2007) Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: a pilot study. Eur J Nucl Med Mol Imaging 34(10):1566–1575
Piert M et al (2005) Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med 46(1):106–113
Reischl G et al (2007) Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA–first small animal PET results. J Pharm Pharm Sci 10(2):203–211
Savi A et al (2017) First evaluation of PET-based human biodistribution and dosimetry of (18)F-FAZA, a tracer for imaging tumor hypoxia. J Nucl Med 58(8):1224–1229
Postema EJ et al (2009) Initial results of hypoxia imaging using 1-alpha-D: -(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA). Eur J Nucl Med Mol Imaging 36(10):1565–1573
Mapelli P et al (2017) 18F-FAZA PET/CT hypoxia imaging of high-grade glioma before and after radiotherapy. Clin Nucl Med 42(12):e525–e526
Mapelli P et al (2017) Hypoxia 18F-FAZA PET/CT imaging in lung cancer and high-grade glioma: open issues in clinical application. Clin Transl Imaging 5(4):389–397
Evans SM et al (2004) Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 10(24):8177–8184
Evans SM et al (2004) Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res 64(5):1886–1892
Koch CJ et al (2010) Biodistribution and dosimetry of (18)F-EF5 in cancer patients with preliminary comparison of (18)F-EF5 uptake versus EF5 binding in human glioblastoma. Eur J Nucl Med Mol Imaging 37(11):2048–2059
Koch CJ et al (2009) The radiation response of cells from 9L gliosarcoma tumours is correlated with [F18]-EF5 uptake. Int J Radiat Biol 85(12):1137–1147
Kaneta T et al (2007) Initial evaluation of dynamic human imaging using 18F-FRP170 as a new PET tracer for imaging hypoxia. Ann Nucl Med 21(2):101–107
Shibahara I et al (2010) Imaging of hypoxic lesions in patients with gliomas by using positron emission tomography with 1-(2-[18F] fluoro-1-[hydroxymethyl]ethoxy)methyl-2-nitroimidazole, a new 18F-labeled 2-nitroimidazole analog. J Neurosurg 113(2):358–368
Beppu T et al (2014) Standardized uptake value in high uptake area on positron emission tomography with 18F-FRP170 as a hypoxic cell tracer correlates with intratumoral oxygen pressure in glioblastoma. Mol Imaging Biol 16(1):127–135
Beppu T et al (2015) High-uptake areas on positron emission tomography with the hypoxic radiotracer (18)F-FRP170 in glioblastomas include regions retaining proliferative activity under hypoxia. Ann Nucl Med 29(4):336–341
Padhani AR et al (2007) Imaging oxygenation of human tumours. Eur Radiol 17(4):861–872
Vavere AL, Lewis JS (2007) Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans 43:4893–4902
Niccoli Asabella A et al (2014) The copper radioisotopes: a systematic review with special interest to 64Cu. Biomed Res Int 2014:786463
Lewis JS et al (2001) Tumor uptake of copper-diacetyl-bis(N(4)-methylthiosemicarbazone): effect of changes in tissue oxygenation. J Nucl Med 42(4):655–661
Toriihara A et al (2018) Prognostic implications of (62)Cu-diacetyl-bis (N(4)-methylthiosemicarbazone) PET/CT in patients with glioma. Ann Nucl Med 32(4):264–271
Tateishi K et al (2014) (62)Cu-diacetyl-bis (N(4)-methylthiosemicarbazone) PET in human gliomas: comparative study with [(18)F]fluorodeoxyglucose and L-methyl-[(11)C]methionine PET. AJNR Am J Neuroradiol 35(2):278–284
Valk PE et al (1992) Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med 33(12):2133–2137
Rajendran JG et al (2004) Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 10(7):2245–2252
Rajendran JG et al (2003) [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging 30(5):695–704
Dence CS et al (2008) Autoradiographic and small-animal PET comparisons between (18)F-FMISO, (18)F-FDG, (18)F-FLT and the hypoxic selective (64)Cu-ATSM in a rodent model of cancer. Nucl Med Biol 35(6):713–720
Yuan H et al (2006) Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med 47(6):989–998
Bowen SR et al (2011) Characterization of positron emission tomography hypoxia tracer uptake and tissue oxygenation via electrochemical modeling. Nucl Med Biol 38(6):771–780
Carlin S et al (2014) A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J Nucl Med 55(3):515–521
Watanabe S et al (2019) Biodistribution and radiation dosimetry of the novel hypoxia PET probe [(18)F]DiFA and comparison with [(18)F]FMISO. EJNMMI Res 9(1):60–60
Shimizu Y et al (2019) A novel PET probe "[(18)F]DiFA" accumulates in hypoxic region via glutathione conjugation following reductive metabolism. Mol Imaging Biol 21(1):122–129
Nakata N et al (2019) Comparative evaluation of [(18)F]DiFA and its analogs as novel hypoxia positron emission tomography and [(18)F]FMISO as the standard. Nucl Med Biol 70:39–45
Acknowledgements
Italian Association for Cancer Research (Grant IG 2014 Id.1524; EudraCT: 2015–000679-28).
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Consortia
Contributions
NQ, RL: literature search, literature review, manuscript writing, manuscript editing, content planning; PM, PG, DAP: literature search, literature review, manuscript writing; MB, GA, MP: manuscript editing, content planning.
Corresponding author
Ethics declarations
Conflict of interest
Quartuccio N, Laudicella R, Mapelli P, Guglielmo P, Pizzuto DA, Boero M, Arnone G, Picchio M declare no conflict of interest related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quartuccio, N., Laudicella, R., Mapelli, P. et al. Hypoxia PET imaging beyond 18F-FMISO in patients with high-grade glioma: 18F-FAZA and other hypoxia radiotracers. Clin Transl Imaging 8, 11–20 (2020). https://doi.org/10.1007/s40336-020-00358-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-020-00358-0