Abstract
Introduction
Evidence on myocardial deformation, detected by speckle tracking echocardiography (STE), in patients with acromegaly is scanty.
Aim
The aim of the present meta-analysis was to provide an updated information on left ventricular (LV) systolic function assessed by global longitudinal strain (GLS) in patients with acromegaly and preserved LVEF.
Methods
Following the PRISMA guidelines, systematic searches were conducted across bibliographic databases (Pub-Med, OVID, EMBASE and Cochrane library) to identify eligible studies from inception up to June 30-2024. Clinical studies published in English reporting data on LV mechanics in patients with acromegaly and controls were included. The statistical difference of the echocardiographic variables of interest between groups such as LVEF and global longitudinal strain (GLS) was calculated by standardized mean difference (SMD) with 95% confidence interval (CI) by using random-effects models.
Results
Seven studies including 288 patients with acromegaly and 294 healthy individuals were considered for the analysis. Pooled average LVEF values were 64.6 ± 1.5% in the healthy control group and 64.0 ± 1.3% in the acromegaly group (SMD: − 0.21 ± 0.22, CI -0.62/0.22, p = 0.34); the corresponding values of GLS were − 19.1.1 ± 1.2% and − 17.5 ± 1.2% (SMD: -0.52 ± 0.27, CI − 1.05/0.01, p = 0.05). No difference was found between the two groups for both global circumferential strain (GCS) and global radial strain (GRS).
Conclusions
Our findings suggest that patients with acromegaly in which LVEF is completely comparable to healthy controls show an impairment in GLS of borderline statistical significance. Whether GLS assessment can actually unmask early alterations of systolic function in patients with acromegaly better than LVEF will need to be investigated by future studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Acromegaly is a neuroendocrine disease, due to hypersecretion of growth hormone (GH), most commonly from a pituitary adenoma [1]. Recent epidemiological data support the view that acromegaly is a rare syndrome with an estimated prevalence of between 30 and 13.5 cases/million inhabitants and an annual incidence of between 2 and 11 cases/million per year, depending on the diagnostic criteria, clinical setting and ethnicity [2, 3].
Chronic excess secretion of GH and insulin-like growth factor 1 (IGF1) induces structural and functional alterations in many organs and tissues such as osteo-muscular and respiratory system, brain, kidneys, liver, pancreas, thyroid, and, last but not least, heart and blood vessels [4]. It should be further noted that acromegaly is linked to several cardiovascular (CV) risk factors such as hypertension, dyslipidaemia, sleep apnoea, glucose intolerance and diabetes. CV disease, together with cancer, is the leading cause of mortality in patients with acromegaly [5]. Until a few decades ago life expectancy was reported to be reduced by approximately 10 years compared to the general population with a double standardized mortality rate for CV diseases. More recently, thanks to an early diagnosis and the modern, multimodal therapy mortality rates have declined [6], but this favourable trend has been denied by other authors [7].
CV complications remain an important public health burden in this setting in relation to the high risk of cardiomyopathy, coronary artery disease, valve disease, heart failure, and arrhythmias (i.e. atrial fibrillation) [8,9,10]. Increased GH and IGF-I secretion affects cardiac function and morphology resulting in biventricular concentric remodelling due to myocyte hypertrophy and accumulation of fibrous tissue within the cardiac interstitium [11, 12]. These alterations in myocardial texture lead to progressive deterioration of diastolic and systolic cardiac performance [13, 14]. Many echocardiographic studies and, more recently, magnetic resonance imaging (MRI) studies have investigated the prevalence of abnormal cardiac phenotypes in patients with acromegaly, showing a high prevalence of LVH and LV diastolic dysfunction [15, 16]. On the contrary, impairment of systolic function, assessed by LVEF, has been reported to be much less frequent than diastolic dysfunction. Whether this feature is linked to the pathophysiology of acromegaly or to the inherent limitations of LVEF in assessing LV systolic performance remains currently undefined. It should be remarked that a large body of evidence supports the view that early changes in LV systolic function can hardly be revealed by LVEF [17]. The growing implementation of two (2D) and three-dimensional (3D) speckle tracking echocardiography (STE) both in research and clinical practice has shown that global longitudinal strain (GLS) is a more reliable and sensitive index of LV systolic function than LVEF [18]. To date, information on LV mechanics in acromegaly patients is scanty. Therefore, the aim of the present meta-analysis was to provide a comprehensive, updated findings on LV systolic function assessed by GLS in patients with acromegaly and preserved LVEF.
2 Methods
The review was performed according to the key recommendations provided by the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 [19], and prospectively registered with the International Prospective Register of Systematic Reviews (unique identifier: CRD42024539686). Medical literature was reviewed in order to identify all articles evaluating LV mechanics by STE in patients with acromegaly. To this purpose, a systemic search was performed using four electronic databases (Pub-Med, OVID, EMBASE and Cochrane library) from inception up to June 30th 2024. Searches were limited to clinical investigations published in English. Studies were identified by using MeSH terms and crossing the following search items: “acromegaly”, “heart”, “cardiac disease”, “myocardial strain” “left ventricular mechanics”, “longitudinal global strain”, “speckle tracking echocardiography”, “systolic dysfunction”, “left ventricular ejection fraction”.
Checks of the reference lists of original papers and pertinent review articles were also searched for additional relevant literature. Data were examined and extracted by three independent investigators (EG, AF and CS). In case of no agreement on a specific record, the full text of the study was analyzed by all reviewers in order to establish its eligibility according to the inclusion criteria mentioned below.
Main inclusion criteria were: (I) English articles published in peer-reviewed journals; (II) studies providing data on GLS by STE in patients with acromegaly and preserved LVEF compared to healthy individuals; (III) minimum set of clinical/demographic data (i.e. sex and age)
Two independent investigators based on the Newcastle-Ottawa Scale (http://www.ohrica/programs/clinical_epidemiologyoxford.html) assessed the methodological quality of each study (CC and CS). The Newcastle-Ottawa Scale of seven or more was considered as a good quality.
2.1 Statistical Analysis
The primary aim of the meta-analysis was to compare LV systolic function assessed by GLS in patients with acromegaly and preserved LVEF with that of their healthy counterparts. Additional conventional parameters were also considered in the analysis (see results below).
A pooled analysis of demographic and clinical variables was performed using fixed or random effects meta-analysis by Comprehensive Meta-Analysis Version 2, Biostat, Englewood, NJ. Standardized mean difference (SMD) with 95% confidence interval (CI) was calculated to test the statistical difference of continuous echocardiographic variables between healthy controls and patients with acromegaly.
Demographic, clinical and echocardiographic data provided by selected studies were expressed as absolute numbers, percentages, mean ± SD, mean ± SE or mean with CI.
The random effect model was applied due to the high heterogeneity across studies (I2 > 75). To assess the effect of individual studies on the pooled result, we conducted a sensitivity analysis by excluding each study one by one and recalculating the combined estimates on the remaining studies. Publication bias was assessed by using the funnel plot method (Trim and fill test) Statistical significance was set at p < 0.05.
3 Results
3.1 Search Results
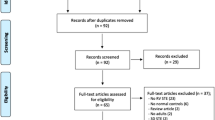
The PRISMA flowchart as presented in Fig. 1 describes the full selection process. After removing duplicates, the first literature search identified 683 papers. After the initial screening of titles and abstracts, 581 studies were excluded as they were not related to the topic. Therefore 102 studies were reviewed; of these, 70 did not report data on echocardiographic speckle tracking parameters, 23 were review, commentary, editorial articles, case reports and double publications and 2 did not include healthy controls. Thus, a total of 7 studies focusing on LV mechanics (i.e. GLS) were included in the analysis (Fig. 1). The Newcastle-Ottawa Score, used for assessing the quality of the studies, ranged from 7 to 9, the mean score being 7.5. Therefore, no study was excluded based on its limited quality.
3.2 Main Study Features
On the whole 288 patients with acromegaly, defined according to clinical symptoms as well as results of testing (standard hormonal criteria) and medical imaging (magnetic resonance imaging) and 294 healthy controls were included in 7 studies (acromegaly sample size ranging from 25 to 81 participants) performed in four countries (Brazil = 2; Poland = 2, Turkey = 2; Hungary = 1) [19,20,21,22,23,24,25,26].
Table 1 shows demographic, clinical and echocardiographic characteristics of participants from selected studies such as setting (i.e. active or controlled acromegaly), sample size, GH levels, mean age, LVMI, GLS and LVEF and LAVI.
Mean age ranged from 45 to 57 years in acromegaly and from 44 to 52 years in controls. LVEF varied from 58 to 67% in acromegaly and from 59 to 67% in controls; the corresponding average GLS values ranged from − 11 to − 20% and − 14 to − 23% in controls, respectively.
Table 2 summarizes the results of the meta-analysis comparing the demographic and clinical characteristics of the pooled acromegaly and control group (48% and 46% female, respectively). Mean age, systolic, diastolic BP values and sex distribution were similar between groups; whereas, body surface area (BSA) (SMD: 0.13 ± 0.02, CI 0.11/0.62, p = 0.005) and body mass index (BMI) (SMD: 0.27 ± 0.11, CI 0.06/0.48, p = 0.01) were significantly higher in acromegaly than in their counterparts without it.
3.3 Echocardiographic Methods
In all studies, the conventional analysis of cardiac structure and function was performed according to the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging published in 2015. LV myocardial deformation was measured offline from 2D or 3D echocardiographic images using commercial dedicated softwares. R-R gating was used for LV strain assessment. In all studies, LV endocardium was manually traced and corrected, if necessary, and average longitudinal strain curve was automatically provided by the software.
3.4 Echocardiographic Findings
The following echocardiographic parameters of LV structure, geometry and function were considered in the present review: LV wall thicknesses (i. e. interventricular septum and posterior wall, 4 studies), relative wall thickness (RWT, 3 studies), LVMI (6 studies), LVEF (7 studies), GLS (7 studies), GCS (3 studies), GRS (3 studies), LAVI (4 studies), E/A ratio (4 studies), E/e’ ratio (3 studies).
3.5 LV Function
Pooled average LVEF values were 64.6 ± 1.3% in the healthy control group and 64.0 ± 1.5% in the acromegaly group. As depicted by the forest plot in Fig. 2 the meta-analysis of selected studies revealed a non-significant difference between groups (SMD: − 0.21 ± 0.22, CI − 0.64/0.22, p = 0.34).
Pooled mean GLS values were 19.1 ± 1.2 % in the control and 17.5 ± 1.2% in the acromegaly group. Figure 3 shows the results of the meta-analysis of seven studies where SMD indicated that this index of systolic function tended to be lower in the acromegaly group, reaching borderline statistical significance (− 0.52 ± 0.27, CI − 1.06/0.01, p = 0.05). In a further meta-analysis restricted to 3 studies reporting data on GCS, the SMD revealed that also this parameter was not different between groups (− 0.09 ± 0.14, CI − 0.38/0.19, p = 0.52). This was also the case for GRS [data from 3 studies, SMD: − 0.44 ± 0.58, CI − 1.58/0.70, p = 0.45). As for LV diastolic function, as assessed by the E/A ratio (data from 4 studies), the average value was lower in acromegaly patients (0.97 ± 0.06) than in controls (1.18 ± 0.07) with a significant SMD of − 0.37 ± 0.11, CI − 0.59/− 0.15, p = 0.001. The value of the E/e′ ratio was greater in the pooled acromegaly group (data from 3 studies) than in the control group (8.27 ± 1.02 vs 7.28 ± 0.34) without however reaching statistical significance (SMD: 0.18 ± 0.13, CI − 0.06/0.43, p = 0.14).
3.6 LV Structure
Pooled mean absolute LVM index values (data from 6 studies) were 85.2 ± 6.3 g/m2 in controls and 109.2 ± 7.3 g/m2 in acromegaly patients (SMD: 0.84 ± 0.09, CI 0.65/1.02, p < 0.0001) (Figure 4). The corresponding values of interventricular septum thickness (IVST, 9.7 ± 0.2 mm versus 11.5 ± 0.6 mm, SMD: 0.77 ± 0.27, CI: 0.24/1.29, p = 0.004), and posterior wall thickness (PWT, 9.7 ± 0.1 mm vs 11.3 ± 0.4 mm, SMD: 0.84 ± 0.37, CI 0.11/1.56, p = 0.02) were significantly lower in the control than in the acromegaly group.
Pooled RWT, an established index of LV geometry, was higher in acromegalic patients than in controls (0.39 ± 0.01 versus 0.37 ± 0.021) suggesting a more concentric geometry, without however reaching statistical significance.
3.7 LA Size
Average LAVI (4 studies) was significantly higher in acromegaly patients than in their normal counterparts (34.9 ± 5.0 versus 25.5 ± 1.9 ml/m2) with a SMD of 0.72 ± 0.26, CI 0.22/1.23; p = 0.005) (Fig. 5).
3.8 Publication Bias
No publication bias was observed for all the analysis performed in the study. No single study effect was observed for the analysis of LVEF, GLS (Supplementary Fig. 1).
4 Discussion
The results of our meta-analysis add new information on cardiac structural and functional changes in patients with acromegaly that can be summarized as follows: (I) both LVMI and LAVI were increased compared to healthy controls matched for sex, age and BP; (II) these alterations were associated with impaired diastolic function; (III) no difference between the two pooled groups was evident for LV systolic function, assessed with LVEF; (IV) conversely, GLS was lower in patients with acromegaly, with a difference of borderline statistical significance.
Increased secretion of GH has been shown to directly affect heart resulting in a specific cardiomyopathy whose main phenotype is LVH. In line with this view, the present meta-analysis showed that LVMI was markedly higher in patients with acromegaly than in controls (+ 24 g/m2) and this marker of LVH was associated with greater LV wall thicknesses, and LA dilatation. Data on cardiac involvement in acromegaly are based on a variety methods such as electrocardiogram, echocardiogram and MRI.
An early MRI study showed that 72% of patients with untreated active acromegaly had LVH, whereas it was detected in only 36% of patients by echocardiography emphasizing how echocardiographic assessment may underestimate LVH [10]. However, subsequent larger MRI studies have provided lower prevalence rates, reporting that LVH may occur in approximately a quarter of patients with active acromegaly and a cluster of biventricular hypertrophy due to increased intracellular and myocardial mass and functional impairment has been described in these patients. [15, 27]. Chronic excess of GH and IGF-I secretion increases cardiac myocyte size, and, at the same time, extracellular collagen deposition, resulting in myofibrillar derangement, and areas of monocyte necrosis and lymphomononuclear infiltration, all of which gradually impair the whole cardiac texture. GH affects water balance and therefore acromegaly potentially can determine interstitial myocardial oedema. Some authors reported increased MRI-derived myocardial T2 relaxation time, which implies the existence of myocardial oedema that was normalized after effective treatment and significantly correlated with reduction of GH and IGF-1 levels [28]. GH-induced insulin resistance, comorbidities such hypertension and obstructive apnea syndrome can be likely contributing factors to acromegalic heart disease.
Increased LAVI might be the consequence of increased LV stiffness, increased LV filling pressure and overall diastolic dysfunction [29]. In addition, plasma volume expansion in patients with acromegaly stretches the myocardial fibers, inducing the cardiac muscle to contract more effectively and, through this mechanism, improving mechanical performance. Atrial fiber shortening and contractility cannot follow progressive LA dilation in acromegaly patients, which means that further enlargement will only result in LA function reduction [30]. Irrespective of the mechanisms that induce LA enlargement in acromegaly, it results in elevated risk of arrhythmias, particularly atrial fibrillation, atrio-ventricular valve insufficiency, stroke, and heart failure.
Moving from structural to functional modifications, our findings did not reveal significant differences in LVEF between acromegaly patients and controls. This is in agreement with previous findings [20, 22, 24, 25]. Although sex-related differences have been reported by some authors who have highlighted that acromegalic women have a higher LVEF than men [31].
Data about LV diastolic function is not that straight forward and current data on this topic remain controversial [21, 22, 26, 31, 32]. Their inconsistency, however, must be interpreted in relation to the different characteristics of the studies, different from each other. regarding age, comorbidities (diabetes and hypertension), concomitant therapy and other confounding factors that were not possible to control (or avoid) in small populations of patients in these studies.
As LVEF remains preserved in acromegaly patients for a very long time, until cardiomyopathy develops, early identification of systolic dysfunction regardless of LVEF may be a step forward in preventing heart failure in this setting. Timely detection is nowadays feasible with STE, which enables revealing subclinical myocardial dysfunction. GLS is the leading strain parameter which indicates subtle cardiac changes before any other conventional echocardiographic parameter. Our meta-analysis showed that GLS was lower in acromegaly patients with borderline statistical significance, whereas GCS and GRS did not show any difference between acromegaly and control subjects. Data from single clinical studies are sparse: some of them found some deterioration in GLS [21, 25, 31], but other reports did not [20, 22, 33]. A significant difference in 3D multidirectional strain [longitudinal, circumferential, radial and area) between acromegaly patients and controls has been also described [26]. Koca et al. documented that GLS was decreased in 48% of patients with active acromegaly being systolic BP, IGF-1 and LVMI were associated with global GLS [25].
The impairment of GLS in acromegaly is essentially explained by two mechanisms. The first is linked to concomitant diseases such as diabetes and hypertension, which are frequently seen in acromegaly patients. The second is related with LVH and myocardial fibrosis due to increased IGF-1 regardless of comorbidities [34, 35].
5 Limitations
There are some limitations of the current meta-analysis. The number of patients in individual studies is limited and there is a significant proportion of patients with comorbidities such as hypertension, obesity and diabetes. Therefore, it is difficult to distinguish the effect of acromegaly per se from concomitant effects of comorbidities. The selected studies used various echocardiographic machines and software for strain evaluation, which might be the source of variation and heterogeneous results among researches.
6 Conclusions
The present meta-analysis suggest that in patients with acromegaly subclinical cardiac damage, phenotyped by increased LVMI, LAVI volume and diastolic dysfunction, is associated with a reduction in GLS of borderline statistical significance, this is not the case for LVEF completely superimposable to that of healthy controls. Whether GLS can provide valuable information about LV functional impairment which precedes changes in LVEF and may be an early predictor of worse clinical outcome in the setting of acromegaly needs to be investigated in future studies.
References
Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552–61. https://doi.org/10.1038/s41574-018-0058-5.
Rosendal C, Arlien-Søborg MC, Nielsen EH, Andersen MS, Feltoft CL, Kistorp C, Dekkers OM, Jørgensen JOL, Dal J. The changing landscape of acromegaly—an epidemiological perspective. Rev Endocr Metab Disord. 2024;25(4):691–705. https://doi.org/10.1007/s11154-024-09875-z.
Rosendal C, Arlien-Søborg MC, Nielsen EH, Andersen MS, Feltoft CL, Klose M, Andreassen M, Bruun NH, Jørgensen JOL, Dal J. Changes in acromegaly comorbidities, treatment, and outcome over three decades: a nationwide cohort study. Front Endocrinol (Lausanne). 2024;4(15):1380436. https://doi.org/10.3389/fendo.2024.1380436.
Sharma MD, Nguyen AV, Brown S, Robbins RJ. Cardiovascular disease in acromegaly. Methodist Debakey Cardiovasc J. 2017;13:64–6. https://doi.org/10.14797/mdcj-13-2-64.
Bolfi F, Neves AF, Boguszewski CL, Nunes-Nogueira VS. Mortality in acromegaly decreased in the last decade: a systematic review and meta-analysis. Eur J Endocrinol. 2018;179:59–71. https://doi.org/10.1530/EJE-18-0255.
Arosio M, Sciannameo V, Contarino A, Berchialla P, Puglisi S, Pesatori AC, Ferrante E, Filopanti M, Pivonello R, Dassie F, Rochira V, Cannavò S, De Menis E, Pigliaru F, Grottoli S, Cambria V, Faustini-Fustini M, Montini M, Peri A, Ceccato F, Puxeddu E, Borretta G, Bondanelli M, Ferone D, Colao A, Terzolo M, Reimondo G. Disease control of acromegaly does not prevent excess mortality in the long term: results of a nationwide survey in Italy. J Endocrinol Invest. 2024;47:1457–65. https://doi.org/10.1007/s40618-023-02257-3.
Wolf P, Maione L, Kamenický P, Chanson P. Acromegalic cardiomyopathy: an entity on its own? The effects of GH and IGF-I excess and treatment on cardiovascular risk factors. Arch Med Res. 2023;54(8): 102921. https://doi.org/10.1016/j.arcmed.2023.102921.
Kamenický P, Maione L, Chanson P. Cardiovascular complications of acromegaly. Ann Endocrinol (Paris). 2021;82:206–9. https://doi.org/10.1016/j.ando.2020.03.010.
Hong S, Kim KS, Han K, Park CY. Acromegaly and cardiovascular outcomes: a cohort study. Eur Heart J. 2022;43:1491–9. https://doi.org/10.1093/eurheartj/ehab822.
Wolf P, Bouazizi K, Kachenoura N, Piedvache C, Gallo A, Salenave S, Maione L, Young J, Prigent M, Lecoq AL, Kuhn E, Agostini H, Trabado S, Redheuil A, Chanson P, Kamenický P. Increase in intracellular and extracellular myocardial mass in patients with acromegaly: a cardiac magnetic resonance imaging study. Eur J Endocrinol. 2023;189:199–207. https://doi.org/10.1093/ejendo/lvad105.
Hinojosa-Amaya JM, Varlamov EV, Yedinak CG, Cetas JS, McCartney S, Banskota S, Fleseriu M. Echocardiographic findings in acromegaly: prevalence of concentric left ventricular remodeling in a large single-center cohort. J Endocrinol Invest. 2021;44:2665–74. https://doi.org/10.1007/s40618-021-01579-4.
Guo X, Fu H, Pang H, Xing B. Risk of left ventricular hypertrophy and diastolic and systolic dysfunction in acromegaly: a meta-analysis. J Clin Neurosci. 2018;48:28–33. https://doi.org/10.1016/j.jocn.2017.10.067.
Colao A, Grasso LFS, Di Somma C, Pivonello R. Acromegaly and heart failure. Heart Fail Clin. 2019;15:399–408. https://doi.org/10.1016/j.hfc.2019.03.001.
Guo X, Cao J, Liu P, Cao Y, Li X, Gao L, Wang Z, Fang L, Jin Z, Wang Y, Xing B. Cardiac abnormalities in acromegaly patients: a cardiac magnetic resonance study. Int J Endocrinol. 2020;14(2020):2018464. https://doi.org/10.1155/2020/2018464.
De Alcubierre D, Feola T, Cozzolino A, Pofi R, Galea N, Catalano C, Auriemma RS, Pirchio R, Pivonello R, Isidori AM, Giannetta E. The spectrum of cardiac abnormalities in patients with acromegaly: results from a case-control cardiac magnetic resonance study. Pituitary. 2024;27(4):416–27. https://doi.org/10.1007/s11102-024-01403-1.
Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–74. https://doi.org/10.1016/j.jcmg.2017.11.017.
Kuznetsova T, Cauwenberghs T, Knez J, Yang WY, Herbots L, D’hooge J, Haddad F, Thijs L, Voigt JU, Staessen JA. Additive prognostic value of left ventricular systolic dysfunction in a population-based cohort. Circ Cardiovasc Imaging. 2016;9: e004661. https://doi.org/10.1161/CIRCIMAGING.116.004661.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. https://doi.org/10.1136/bmj.b2535.
Volschan ICM, Kasuki L, Silva CMS, Alcantara ML, Saraiva RM, Xavier SS, Gadelha MR. Two-dimensional speckle tracking echocardiography demonstrates no effect of active acromegaly on left ventricular strain. Pituitary. 2017;20:349–57. https://doi.org/10.1007/s11102-017-0795-9.
Popielarz-Grygalewicz A, Gąsior JS, Konwicka A, Grygalewicz P, Stelmachowska-Banaś M, Zgliczyński W, Dąbrowski M. Heart in acromegaly: the echocardiographic characteristics of patients diagnosed with acromegaly in various stages of the disease. Int J Endocrinol. 2018;2018:1–7. https://doi.org/10.1155/2018/6935054.
Kormányos Á, Domsik P, Kalapos A, Gyenes N, Valkusz Z, Lengyel C, Forster T, Nemes A. Active acromegaly is associated with enhanced left ventricular contractility: results from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path Study. Rev Port Cardiol. 2020;39:189–96. https://doi.org/10.1016/j.repc.2019.08.010.
Uziȩbło-Życzkowska B, Jurek A, Witek P, Zieliński G, Gielerak G, Krzesiński P. Left heart dysfunction in acromegaly revealed by novel echocardiographic methods. Front Endocrinol. 2020;11:418. https://doi.org/10.3389/fendo.2020.00418.
Gadelha P, Santos ECL, Castillo J, Vilar L. Subclinical ventricular dysfunction in long-term acromegaly assessed by speckle-tracking echocardiography. Front Endocrinol. 2022;13: 812964. https://doi.org/10.3389/fendo.2022.812964.
Koca H, Koc M, Sumbul HE, Icen YK, Gulumsek E, Koca F, Ozturk HA, Baykan AO, Kaypakli O. Subclinical left atrial and ventricular dysfunction in acromegaly patients: a speckle tracking echocardiography study. Arq Bras Cardiol. 2022;118:634–45. https://doi.org/10.36660/abc.20201174.
Firlatan B, Karakulak UN, Hekimsoy V, Iremli BG, Lay I, Yuce D, Dagdelen S, Kabakci G, Erbas T. Evaluation of the relation between subclinical systolic dysfunction defined by four-dimensional speckle-tracking echocardiography and growth differentiation factor-15 levels in patients with acromegaly. Hormones. 2024. https://doi.org/10.1007/s42000-024-00558-7.
Bogazzi F, Lombardi M, Strata E, Aquaro G, Di Bello V, Cosci C, Sardella C, Talini E, Martino E. High prevalence of cardiac hypertrophy without detectable signs of fibrosis in patients with untreated active acromegaly: an in vivo study using magnetic resonance imaging. Clin Endocrinol (Oxf). 2008;68:361–8. https://doi.org/10.1111/j.1365-2265.2007.03047.x.
Gouya H, Vignaux O, Le Roux P, Chanson P, Bertherat J, Bertagna X, Legmann P. Rapidly reversible myocardial edema in patients with acromegaly: assessment with ultrafast T2 mapping in a single-breath-hold MRI sequence. AJR Am J Roentgenol. 2008;190:1576–82. https://doi.org/10.2214/AJR.07.2031.
Cansu GB, Yılmaz N, Yanıkoğlu A, Özdem S, Yıldırım AB, Süleymanlar G, Altunbaş HA. Assessment of diastolic dysfunction, arterial stiffness, and carotid intima-media thickness in patients with acromegaly. Endocr Pract. 2017;23:536–45. https://doi.org/10.4158/EP161637.
Mehrzad R, Rajab M, Spodick DH. The three integrated phases of left atrial macrophysiology and their interactions. Int J Mol Sci. 2014;15(9):15146–60. https://doi.org/10.3390/ijms150915146.
Popielarz-Grygalewicz A, Stelmachowska-Banaś M, Raczkiewicz D, Czajka-Oraniec I, Zieliński G, Kochman W, Dąbrowski M, Zgliczyński W. Effects of acromegaly treatment on left ventricular systolic function assessed by speckle tracking echocardiography in relation to sex differences: results from a prospective single center study. Front Endocrinol (Lausanne). 2023;14:1154615. https://doi.org/10.3389/fendo.2023.1154615.
Popielarz-Grygalewicz A, Stelmachowska-Banaś M, Gąsior JS, Grygalewicz P, Czubalska M, Zgliczyński W, Dąbrowski M, Kochman W. Subclinical left ventricular systolic dysfunction in patients with naive acromegaly—assessment with two-dimensional speckle-tracking echocardiography: retrospective study. Endokrynol Pol. 2020;71:227–34. https://doi.org/10.5603/EP.a2020.0021.
Akdeniz B, Gedik A, Turan O, Ozpelit E, Ikiz AO, Itil O, Badak O, Baris N, Cömlekçi A. Evaluation of left ventricular diastolic function according to new criteria and determinants in acromegaly. Int Heart J. 2012;53:299–305. https://doi.org/10.1536/ihj.53.299.
Nemes A, Kormányos Á, Domsik P, Kalapos A, Gyenes N, Lengyel C, Valkusz Z. Diabetes mellitus deteriorates left ventricular deformation in acromegaly-analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Path study. Quant Imaging Med Surg. 2021;11:410–4. https://doi.org/10.21037/qims-20-159.
Lie JT. Pathology of the heart in acromegaly: anatomic findings in 27 autopsied patients. Am Heart J. 1980;100:41–52. https://doi.org/10.1016/0002-8703(80)90277-x.
Clayton RN. Cardiovascular function in acromegaly. Endocr Rev. 2003;24:272–7. https://doi.org/10.1210/er.2003-0009.
Acknowledgements
This study is dedicated to the memory of Prof. Fabio Magrini MD, recently deceased, mentor and friend of many of us.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
Data to verify study outcomes are available on request to the corresponding author from qualified clinical researchers with approval from an Institutional Review Board.
Conflict of interest
The authors report no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
40292_2024_667_MOESM1_ESM.tif
Supplementary file1 Figure S1 Funnel plot assessing publication bias for standardized means difference (SMD) of left ventricular global longitudinal strain (GLS) in patients with acromegaly and controls (TIF 30 KB)
Rights and permissions
About this article
Cite this article
Gherbesi, E., Faggiano, A., Sala, C. et al. Myocardial Mechanics in Acromegaly: A Meta-Analysis of Echocardiographic Studies. High Blood Press Cardiovasc Prev (2024). https://doi.org/10.1007/s40292-024-00667-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40292-024-00667-9