Abstract
Background
There is robust evidence that creatine monohydrate supplementation can enhance short-term high-intensity exercise in athletes. However, the effect of creatine monohydrate supplementation on aerobic performance and its role during aerobic activities is still controversial.
Objective
The purpose of this systematic review and meta-analysis was to evaluate the supplementation effects of creatine monohydrate on endurance performance in a trained population.
Methods
The search strategy in this systematic review and meta-analysis was designed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and PubMed/MEDLINE, Web of Science, and Scopus databases were explored from inception until 19 May, 2022. Only human experimental trials, controlled with a placebo group, evaluating the effects of creatine monohydrate supplementation on endurance performance in a trained population were analyzed in this systematic review and meta-analysis. The methodological quality of included studies was evaluated using the Physiotherapy Evidence Database (PEDro) scale.
Results
A total of 13 studies satisfied all the eligibility criteria and were included in this systematic review and meta-analysis. The results for the pooled meta-analysis showed a non-significant change in endurance performance after creatine monohydrate supplementation in a trained population (p = 0.47), with a trivial negative effect (pooled standardized mean difference = − 0.07 [95% confidence interval − 0.32 to 0.18]; I2 = 34.75%). Further, after excluding the studies not evenly distributed around the base of the funnel plot, the results were similar (pooled standardized mean difference = − 0.07 [95% confidence interval − 0.27 to 0.13]; I2 = 0%; p = 0.49).
Conclusions
Creatine monohydrate supplementation was shown to be ineffective on endurance performance in a trained population.
Clinical Trial Registration
The study protocol was registered in the Prospective Register of Systematic Review (PROSPERO) with the following registration number: CRD42022327368.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Creatine monohydrate seems ineffective when the primary purpose is to improve endurance performance. |
Creatine monohydrate inefficacy to improve running endurance performance could be associated with its capability to increase body mass. |
Creatine monohydrate could improve aerobic performance in sports modalities where the increment in body mass does not increase the energy cost of exercise, and strength is an essential factor for the sport. |
1 Introduction
Creatine (Cr) is a non-protein amino acid endogenously synthesized primarily in the liver and kidneys through several enzyme processes from arginine, glycine, and methionine [1, 2]. Creatine is predominantly stored in skeletal muscle (~ 95%), with ~ 66% of intramuscular Cr stored as phosphocreatine (PCr), and the remaining as free Cr [3]. However, only 60–80% of muscle Cr and PCr stores are saturated in a regular diet [3]. Hence, dietary supplementation of creatine monohydrate (CrM) could help to increase muscle Cr and PCr by 20–40% [4, 5].
There are two main strategies to increase muscle Cr and PCr concentration following CrM ingestion: rapid or slow loading. The rapid loading consists of four daily dosages of 5 g of CrM (or 0.3 g/kg body mass) for 5–7 days [4, 5]. After reaching the maximum saturation of muscle Cr, a maintenance dose of CrM (3 g/day or 0.03 g/kg body mass) is recommended in order to sustain a high Cr concentration [3]. In contrast, the slow loading protocol consists of ingesting the maintenance dose (3 g/day or 0.03 g/kg body mass) for at least 28 days [4].
Creatine monohydrate is an ergogenic aid with considerable evidence concerning sports performance improvement [6,7,8,9]. Specifically, large effectiveness in optimizing power and strength performance has been shown in athletes after the ingestion of this supplement [10,11,12]. Nevertheless, the effectiveness of CrM on endurance performance in a trained population is still unclear. Previously, it has been hypothesized that CrM could improve endurance performance via greater shuttling of adenosine triphosphate (ATP) from mitochondria [3]. The Cr/PCr system could improve aerobic capacity by maintaining ATP availability during aerobic exercise [13]. Therefore, additional energy availability could be provided by resynthesizing PCr from Cr in the muscle cell’s mitochondria [13,14,15]. Furthermore, hydrogen cations are utilized in this process to produce ATP through adenosine diphosphate rephosphorylation. Hence, CrM may act as a proton buffer, helping to delay fatigue [16]. In addition, this supplement could enhance endurance performance by increasing glycogen storage [17]. Furthermore, it is well known that supplementation with CrM could increase body mass [18], and the increase in body mass could negatively influence endurance performance [19, 20]. In light of these relevant physiological pathways influencing endurance performance, athletes need to be aware of the effectiveness of this ergogenic aid in improving or impairing endurance performance.
A recent meta-analysis showed a negative effect of Cr supplementation on the maximum rate of oxygen consumption (VO2max) [21]. However, in that review, the analysis was conducted in a trained and untrained population and the only endurance outcome measured was VO2max. To the best of the authors’ knowledge, none of the previous systematic reviews and meta-analyses (SRMAs) analyzed CrM effectiveness on endurance performance, specifically in a trained population. Therefore, this SRMA aims to evaluate CrM supplementation’s influence on endurance performance in a trained population.
2 Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22] was followed in order to evaluate the effects of CrM supplementation on endurance performance in a trained population. Before starting the search strategy, the study protocol was registered in the Prospective Register of Systematic Review (PROSPERO) with the following registration number: CRD42022327368.
2.1 Literature Search
The search was independently conducted by two authors (JFL and NT), and disagreements were solved by third-party adjudication (ASG). Studies were identified by searching PubMed/MEDLINE, Web of Science, and Scopus databases from inception until 19 May, 2022. Records were identified using the following Boolean search: ((“creatine monohydrate” OR “oral creatine” OR “creatine supplementation” OR “Cr supplementation”) AND (endurance AND aerobic) AND (athlete OR trained OR elite OR competitive)). Moreover, so as to detect any missed study in the literature search, the snowball strategy [23] was used.
2.2 Inclusion and Exclusion Criteria
The a priori inclusion criteria to select the articles for this SRMA were: (i) CrM supplementation; (ii) trained population (trained/developmental, highly trained/national level, and/or elite/international level [24]); and (iii) endurance performance measurements (VO2max, peak oxygen consumption (VO2peak), individual anaerobic/lactate threshold, stages, time trial and time to exhaustion) involving the following tests: 6-km terrain run, continuous treadmill test, Leger shuttle run test, incremental test (rowing ergometer or cycle ergometer), maximal discontinuous incremental running test, 1000-m rowing test, 2000-m rowing test, 2500-m rowing test, and 400-m swimming test; (iv) human experimental trial; (v) controlled with a placebo group; (vi) original and peer-reviewed studies written in the English language.
Studies were excluded when: (i) CrM was combined with other supplements (except when the data for each supplement were given separately); (ii) volunteers in the studies were not considered as a trained population; (iii) there was no placebo group for the comparison of the results; and (iv) studies had no pre- and post-exercise data.
2.3 Text Screening
Two authors (JFL and NT) conducted the process independently, and potential discrepancies between reviewers were resolved by consensus with a third author (ASG). The first step of the process was to screen abstracts and titles in order to efficiently reduce the number of studies not meeting the inclusion and exclusion criteria. Subsequently, the same researchers screened the full texts to determine which experimental trials were relevant to be included in the SRMA.
2.4 Data Extraction and Study Coding
The following data from all studies satisfying inclusion and exclusion criteria were extracted: study authors and publication year, study design, participant’s sex, participant’s age, supplementation dose, duration of supplementation protocol, supplementation form, body mass (pre- and post-data), and endurance test outcomes (pre- and post-data). When there were no numerical data available, and the data were expressed in images (e.g., graphs), Image J software® (National Institutes of Health, Bethesda, MD, USA) was used in order to calculate mean and standard deviation values by measuring the pixel length of each magnitude. Finally, all the information was carefully reviewed and added to a spreadsheet (Microsoft Excel; Microsoft Corporation, Washington, DC, USA).
Most of the investigations included in the SRMA showed more than one relevant outcome measuring endurance performance. When more than one outcome per study was included in a meta-analysis, the final results could be affected because one effect size was given for each outcome [25]. Therefore, with the aim of reducing possible bias, the “MAd” package in R software (R Foundation for Statistical Computing, Vienna, Austria) was utilized to obtain a unique effect size estimate for each study [26]. This package needs a within-study correlation to give an accurate effect size to each study; hence, the within-study correlation was 0.70, the same Trexler et al. previously used [27].
2.5 Quality Assessment of Included Studies
Two independent researchers (JFL and ASG) conducted the process, and potential discrepancies between reviewers were resolved through discussion. The methodological quality of included studies was evaluated using the Physiotherapy Evidence Database (PEDro) scale [28]. This scale consists of 11 items, but only items from 2 to 11 can be rated. When an item receives a positive answer, it is rated with 1 point, whereas with a negative answer it is rated 0 points. Therefore, the maximum possible score on this scale is 10 points. A high PEDro score means that there is a low risk of bias, while a low PEDro score means a high risk of bias. The PEDro scale was assessed as excellent quality (a score of 9 or 10 points), good quality (a score between 6 and 8 points), fair quality (a score between 4 and 5 points), or poor quality (a score of 3 points or lower) [29].
2.6 Statistical Analysis
The statistical analyses were performed using R software’s “metafor” package (R Foundation for Statistical Computing, Vienna, Austria). Every study included in the SRMA received a weighted estimation of a standardized mean difference (SMD) and variance calculated as Hedges’ G [30], using the inverse variance random-effects model by the DerSimonian and Laird method [31]. In order to obtain the variance, the correlation coefficient used was 0.70, following Rosenthal’s recommendation [32]. The calculation of the effects of CrM supplementation versus placebo on endurance performance was measured using the SMD with a 95% confidence interval (95% CI [lower bound to–upper bound]), and the significance was set at p < 0.05. The SMD was classified as trivial (when the SMD was < 0.2); small (when the SMD was between 0.2 and 0.3); moderate (when the SMD was between 0.4 and 0.8); and large (when the SMD was > 0.8), following the Cohen criteria [33].
The heterogeneity among studies was evaluated using the I2 statistic, and ranked as low (when I2 < 25%), moderate (when I2 = 25–75%), or considerable (when I 2 > 75%) risk of heterogeneity [34]. The I2 statistic was calculated based upon the restricted maximum likelihood estimation of tau-square.
In order to determine the potential publication bias of the pooled data from each study, funnel plot asymmetry was visually evaluated. Moreover, funnel plot asymmetry was evaluated through Egger’s regression test [35] and with Duval and Tweedie’s trim and rill method [36]. The meta-analysis was repeated after excluding studies not evenly distributed around the base of the funnel plot in order to reduce publication bias.
3 Results
3.1 Literature Search
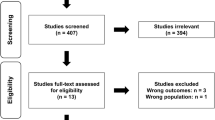
A total of 201 records were found through the database search and two [37, 38] were identified through the snowball strategy. Subsequently, duplicates were removed, including 146 unique records in the SRMA. Titles and abstracts were screened and 105 unrelated studies were eliminated. Consequently, 41 eligible studies were included for the full-text screening. Finally, 13 articles were considered to be included in this SRMA, involving 277 participants [37,38,39,40,41,42,43,44,45,46,47,48,49]. Figure 1 displays the information concerning the PRISMA flow diagram.
All relevant information regarding studies meeting the inclusion criteria is summarized in Table 1. Nine studies reported a loading supplementation protocol [37,38,39, 41, 44,45,46,47,48], while six studies reported a maintenance supplementation protocol [40, 42, 43, 45, 48, 49]. Two studies started with a loading protocol and continued with the maintenance protocol [45, 48]. In the studies that completed the loading supplementation protocol, ingested dosages ranged from 5 g [39] to 30 g per day [47]. In the maintenance supplementation protocol, the dose intake varied from 2 g [49] to 10 g per day [45]. Both supplementation duration protocols ranged from 5 days [37, 38, 41, 44] to 70 days [42].
Concerning the body mass change, five studies showed a significant body mass increase after CrM supplementation [39, 40, 43,44,45], while three studies observed no change in body mass after a period of CrM ingestion [37, 42, 48]. Five studies included in this SRMA did not provide data regarding body mass change [38, 41, 46, 47, 49].
Endurance performance was assessed through the following tests: 6-km terrain run [39], continuous treadmill test [39], Leger shuttle run test [40, 47], incremental exercise test in a rowing ergometer [41, 42], incremental exercise test in a cycle ergometer [43, 45, 46], maximal discontinuous incremental running test [44], maximal 2500-m rowing ergometer test [37], 1000-m time trial in a rowing ergometer [38], 2000-m rowing test [48], and 400-m swimming time trial [49].
Four studies reported significant improvements in endurance test outcomes after CrM supplementation. One study noticed an increase in the individual threshold [41]. In addition, the study conducted by Fernández-Landa et al. [42] reported an improvement in the 8-mml/L lactate threshold. The remaining two studies observed greater results in time trials [37, 38]. Otherwise, only one study showed negative effects on endurance performance outcomes. Balsom et al. study participants had impaired time trial results after CrM ingestion [39]. Finally, VO2max/peak [37, 39, 43, 45, 46, 48], individual anaerobic/lactate threshold [41, 42], time trial [48, 49], stages [40], and time to exhaustion [44, 45, 47] remained unchanged after the supplementation protocol.
3.2 Study Quality
The PEDro scale mean score for the included studies was 7.69, considered as good quality. Four studies [40,41,42, 44] were classified as excellent quality, eight investigations [37, 39, 43, 45,46,47,48]) were categorized as good quality, and one study [38] was classified as fair quality. The PEDro scale is shown in Table 2.
3.3 Pooled Effect Estimate
The I2 test found no significant heterogeneity between studies (p = 0.19). Nevertheless, the I2 statistic observed a moderate risk of heterogeneity (I2 = 34.75%). The visual analysis of the funnel plot indicated asymmetry showing publication bias (Fig. 2); however, no significant results were found in the Egger’s regression test for funnel plot asymmetry (df = 11; p = 0.70), and Duval and Tweedie’s trim and fill method did not identify missing studies on either side of the plot. After excluding the studies not evenly distributed around the base of the funnel plot, the heterogeneity between studies was drastically reduced, showing a low risk of heterogeneity (I2 = 0%; p = 0.89). Egger’s regression test showed no funnel plot asymmetry (df = 9; p = 0.90) and Duval and Tweedie’s trim and fill method did not identify missing studies on either side of the plot after the bias correction. Funnel plots are displayed in Fig. 2.
The results for the pooled meta-analysis showed a non-significant change in endurance performance after CrM supplementation in a trained population (p = 0.47), with a trivial negative effect (pooled SMD = − 0.07 [95% CI − 0.32 to 0.18]). Following the exclusion of the studies not evenly distributed around the base of the funnel plot, the results were similar (pooled SMD = − 0.07 [95% CI − 0.27 to 0.13]; p = 0.49). Forest plots are shown in Fig. 3.
4 Discussion
Thirteen studies satisfied the inclusion criteria to assess the effect of CrM supplementation on endurance performance. All of the participants (n = 277) in the analyzed studies followed a slow or rapid CrM supplementation protocol in order to assure fully saturated muscle PCr and Cr storage. The primary aim of this SRMA was to examine and summarize the current scientific literature regarding the effectiveness of CrM supplementation on endurance performance in a trained population. The main finding was that CrM supplementation had no effects on endurance performance in a trained population. In addition, the meta-analysis results revealed no significant change (p = 0.57) with a trivial negative effect (pooled SMD = − 0.07 [95% CI − 0.32 to 0.18]; I2 = 34.75%) in endurance performance compared with placebo. In addition, so as to reduce publication bias, the same analysis was carried out after excluding two studies [41, 43] not evenly distributed around the base of the funnel plot. After excluding those studies, the result of the meta-analysis was similar (pooled SMD = − 0.07 [95% CI − 0.27 to 0.13]; p = 0.49; I2 = 0%).
The results found in the current SRMA differ from those in the Gras et al. meta-analysis [21], where a negative influence of Cr on endurance capacity (measured as VO2max) was found. In contrast, the results of this SMRA showed no effect of CrM on endurance performance (measured as VO2max/VO2peak, individual anaerobic/lactate threshold, time trial, and time to exhaustion). Another considerable difference between reviews was the inclusion criteria applied to the population. In the Gras et al. meta-analysis [21], all young and healthy participants were included, while in this meta-analysis, only trained populations met the inclusion criteria. The results of both meta-analyses showed that this supplement could differently influence trained and untrained populations.
In this systematic review, some endurance outcomes of analyzed studies were improved after CrM ingestion. All studies with significant improvements in the measured outcomes (individual threshold [41], 8-mmol/L lactate threshold [42], time trial [37, 38], and time to exhaustion [41]) were found when a rowing ergometer test was carried out. The enhancement of performance was only found in rowers. Rowing is considered an endurance sport because of its high demand for aerobic energy, which ranges from 70 to 86% of the total energy demands [50]. However, upper and lower body strength also plays a key role in achieving the maximum performance and has been established as one of the most important rowing performance predictors [51]. In this context, CrM has shown effectiveness in improving upper and lower body strength [6, 52]. That might be the main reason for the improvement in the tests carried out in rowing ergometers by these athletes compared with the remaining trained population included in the SRMA. Moreover, the increase in the Cr/PCr system after CrM ingestion shuttling additional ATP from mitochondria [3, 13] and the capability of this supplement to increase muscle glycogen storage [17] may also have had a positive influence on endurance performance. However, one study showed a significant impairment in an endurance performance outcome [39]. In order to find an explanation for these findings, Balsom et al. [39] suggested that the impairment could have been related to the increase in body mass in the group that ingested CrM.
Although four studies found positive effects on endurance performance and only one found adverse effects, the meta-analysis showed a non-significant negative effect after the CrM supplementation (pooled SMD = − 0.07; p = 0.57). Specifically, the studies with a larger negative effect size (SMD > − 0.20) in the meta-analysis [37, 39, 40, 43, 47] were generally associated with a body mass increase after CrM ingestion. In three studies [39, 40, 43], participants increased their body mass, while one [47] did not report body mass change data, and only one study [37] did not find changes in that parameter. More precisely, results from Balsom et al. [39] and Chilibeck et al. [40], both carried out on running performance tests (6-km terrain run [39] and Leger shuttle run test [40]), could have been more affected by a body mass increase. In these studies, the impairment of endurance performance might be explained by an increase in body weight, which could augment energy costs during running [53]. Otherwise, the three studies showing higher positive effects on endurance performance (effect size > 0.20) did not report changes in body mass [41, 42], or the body mass was not measured [49]. This shows that the results of this SRMA could be considered a negative interaction between endurance performance and the CrM supplementation-induced increase in body mass.
Even though it previously has been hypothesized that CrM could improve endurance performance by shuttling additional ATP from mitochondria through the Cr/PCr system [3, 13], the ingestion of this ergogenic aid should not be the most appropriate to enhance aerobic capacity. One explanation for this result might be the action of CrM at the peripheral muscle level. This supplement is well known for its effectiveness in enhancing muscle hypertrophy and increasing the recruitment of fast-twitch muscle fibers [54]. Therefore, these changes in skeletal muscle could negatively affect endurance performance. Another reason explaining why this supplement is ineffective in enhancing endurance performance could be associated with the capacity for CrM to augment body mass. The increase in body mass could negatively influence endurance performance raising the energy cost during exercise, mainly during running [53]. However, in events where the effect of body mass does not increase the energy cost of exercise (e.g., rowing in a rowing ergometer) and strength is an essential factor for the sport (e.g., rowing), it might be a good option to ingest CrM so as to enhance endurance performance.
5 Conclusions
The result obtained in this SRMA showed that CrM supplementation was ineffective, regardless of the supplementation protocol, at improving endurance performance in a trained population. Considering that this supplement is one of the most popular ergogenic aids in the sports field, athletes, coaches, nutritionists, dietitians, and sports scientists should be aware of the finding of the current SRMA when the primary purpose is to improve endurance performance.
References
Kreider RB, Jung YP. Creatine supplementation in exercise, sport, and medicine. J Exerc Nutr Biochem. 2011;15:53–69.
Ostojic SM. Creatine synthesis in the skeletal muscle: the times they are a-changin’. Am J Physiol Endocrinol Metab. 2021;320:E390–1.
Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. J Int Soc Sports Nutr. 2017;14:18.
Hultman E, Söderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. J Appl Physiol. 1996;81:232–7.
Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci. 1992;83:367–74.
Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage F-X, Dutheil F. Creatine supplementation and upper limb strength performance: a systematic review and meta-analysis. Sports Med. 2017;47:163–73.
Fernández-Landa J, Calleja-González J, León-Guereño P, Caballero-García A, Córdova A, Mielgo-Ayuso J. Effect of the combination of creatine monohydrate plus HMB supplementation on sports performance, body composition, markers of muscle damage and hormone status: a systematic review. Nutrients. 2019;11:2528.
Cooper R, Naclerio F, Allgrove J, Jimenez A. Creatine supplementation with specific view to exercise/sports performance: an update. J Int Soc Sports Nutr. 2012;9:33.
Zuniga JM, Housh TJ, Camic CL, Hendrix CR, Mielke M, Johnson GO, et al. The effects of creatine monohydrate loading on anaerobic performance and one-repetition maximum strength. J Strength Cond Res. 2012;26:1651–6.
Claudino JG, Mezêncio B, Amaral S, Zanetti V, Benatti F, Roschel H, et al. Creatine monohydrate supplementation on lower-limb muscle power in Brazilian elite soccer players. J Int Soc Sports Nutr. 2014;11:32.
Kaviani M, Abassi A, Chilibeck PD. Creatine monohydrate supplementation during eight weeks of progressive resistance training increases strength in as little as two weeks without reducing markers of muscle damage. J Sports Med Phys Fitness. 2019;59:608–12.
Lamontagne-Lacasse M, Nadon R, Goulet ED. Effect of creatine supplementation on jumping performance in elite volleyball players. Int J Sports Physiol Perform. 2011;6:525–33.
Saks VA, Kongas O, Vendelin M, Kay L. Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta Physiol Scand. 2000;168:635–41.
Hespel P, Eijnde BO, Derave W, Richter EA. Creatine supplementation: exploring the role of the creatine kinase/phosphocreatine system in human muscle. Can J Appl Physiol. 2001;26 Suppl.:S79-102.
Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–96.
Demant TW, Rhodes EC. Effects of creatine supplementation on exercise performance. Sport Med. 1999;28:49–60.
Eijnde BO, Ursø B, Richter EA, Greenhaff PL, Hespel P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes. 2001;50:18–23.
Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Exerc Metab. 2003;13:198–226.
Swain DP. The influence of body mass in endurance bicycling. Med Sci Sport Exerc. 1994;26:58–63.
Rüst CA, Knechtle B, Knechtle P, Wirth A, Rosemann T. Body mass change and ultraendurance performance: a decrease in body mass is associated with an increased running speed in male 100-km ultramarathoners. J Strength Cond Res. 2012;26:1505–16.
Gras D, Lanhers C, Bagheri R, Ugbolue UC, Coudeyre E, Pereira B, et al. Creatine supplementation and VO2max: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021. https://doi.org/10.1080/10408398.2021.2008864 (Epub ahead of print).
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331:1064–5.
McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17:317–31.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. 2nd ed. Hoboken: Wiley; 2021.
Cooper H, Hedges LV, Valentine JC. The handbook of research synthesis and meta analysis. 2nd ed. New York: Russell Sage Foundation; 2009.
Trexler ET, Persky AM, Ryan ED, Schwartz TA, Stoner L, Smith-Ryan AE. Acute effects of citrulline supplementation on high-intensity strength and power performance: a systematic review and meta-analysis. Sports Med. 2019;49:707–18.
De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–33.
Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) scale. J Physiother. 2020;66:59.
Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Behav Stat. 1981;6:107–28.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Rosenthal R. Meta-analytic procedures for social research. 1st ed. Newbury Park: SAGE Publications; 1991.
Cohen J. A power primer. Psychol Bull. 1992;112:155–9.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Lawrence SR, Preen DB, Dawson BT, Beilby J, Goodman C, Cable NT. The effect of oral creatine supplementation on maximal exercise performance in competitive rowers. Sport Med Train Rehabil. 1997;7:243–53.
Rossiter HB, Cannell ER, Jakeman PM. The effect of oral creatine supplementation on the 1000-m performance of competitive rowers. J Sports Sci. 1996;14:175–9.
Balsom PD, Harridge SD, Söderlund K, Sjödin B, Ekblom B. Creatine supplementation per se does not enhance endurance exercise performance. Acta Physiol Scand. 1993;149:521–3.
Chilibeck PD, Magnus C, Anderson M. Effect of in-season creatine supplementation on body composition and performance in rugby union football players. Appl Physiol Nutr Metab. 2007;32:1052–7.
Chwalbinska-Moneta J. Effect of creatine supplementation on aerobic performance and anaerobic capacity in elite rowers in the course of endurance training. Int J Sport Exerc Metab. 2003;13:173–83.
Fernández-Landa J, Fernández-Lázaro D, Calleja-González J, Caballero-García A, Córdova A, León-Guereño P, et al. Effect of ten weeks of creatine monohydrate plus HMB supplementation on athletic performance tests in elite male endurance athletes. Nutrients. 2020;12:193.
Hickner RC, Dyck DJ, Sklar J, Hatley H, Byrd P. Effect of 28 days of creatine ingestion on muscle metabolism and performance of a simulated cycling road race. J Int Soc Sports Nutr. 2010;7:1–13.
Izquierdo M, Ibañez J, González-Badillo JJ, Gorostiaga EM. Effects of creatine supplementation on muscle power, endurance, and sprint performance. Med Sci Sport Exerc. 2002;34:332–43.
Murphy JA, Watsford ML, Coutts AJ, Richards AB. Effects of creatine supplementation on aerobic power and cardiovascular structure and function. J Sci Med Sport. 2005;8:305–13.
Nelson AG, Day R, Glickman-Weiss EL, Hegsted M, Kokkonen J, Sampson B. Creatine supplementation alters the response to a graded cycle ergometer test. Eur J Appl Physiol. 2000;83:89–94.
Ostojic SM. Creatine supplementation in young soccer players. Int J Sport Nutr Exerc Metab. 2004;14:95–103.
Syrotuik DG, Game AB, Gillies EM, Bell GJ. Effects of creatine monohydrate supplementation during combined strength and high intensity rowing training on performance. Can J Appl Physiol. 2001;26:527–42.
Thompson CH, Kemp GJ, Sanderson AL, Dixon RM, Styles P, Taylor DJ, et al. Effect of creatine on aerobic and anaerobic metabolism in skeletal muscle in swimmers. Br J Sports Med. 1996;30:222–5.
Russell AP, Le Rossignol PF, Sparrow WA. Prediction of elite schoolboy 2000m rowing ergometer performance from metabolic, anthropometric and strength variables. J Sports Sci. 1998;16:749–54.
Izquierdo-Gabarren M, Expósito RG, de Villarreal ES, Izquierdo M. Physiological factors to predict on traditional rowing performance. Eur J Appl Physiol. 2010;108:83–92.
Lanhers C, Pereira B, Naughton G, Trousselard M, Lesage F-X, Dutheil F. Creatine supplementation and lower limb strength performance: a systematic review and meta-analyses. Sports Med. 2015;45:1285–94.
Bourdin M, Belli A, Arsac LM, Bosco C, Lacour JR. Effect of vertical loading on energy cost and kinematics of running in trained male subjects. J Appl Physiol. 1995;79:2078–85.
Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol. 1996;271:31–7.
Acknowledgements
The authors thank all the authors for providing their data.
Author information
Authors and Affiliations
Contributions
JFL designed and conceived the systematic review and meta-analysis. JFL wrote the first draft of the manuscript. JFL, NT, and ASG conducted the literature search. JFL, NT, and ASG selected the articles for inclusion in the review. JFL and ASG assessed the quality of the included studies. JFL conducted the statistical analysis. JFL, ASG, NT, VS, and SMO revised the original manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests
Julen Fernández-Landa, Asier Santibañez-Gutierrez, Nikola Todorovic, Valdemar Stajer, and Sergej M. Ostojic have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
This project was performed in accordance with PRISMA guidelines.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data for the current analysis are available upon request and can be obtained by contacting the corresponding author.
Code Availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández-Landa, J., Santibañez-Gutierrez, A., Todorovic, N. et al. Effects of Creatine Monohydrate on Endurance Performance in a Trained Population: A Systematic Review and Meta-analysis. Sports Med 53, 1017–1027 (2023). https://doi.org/10.1007/s40279-023-01823-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-023-01823-2