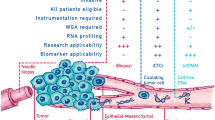
Abstract
Background
Cancer-derived material circulating in the bloodstream and other bodily fluids, referred to as liquid biopsies (LBs), has become an appealing adjunct or alternative to tissue biopsies, showing vital promise in several clinical applications.
Purpose
A systematic literature review was conducted to (1) summarize the current health economic evidence for LB assays and (2) identify and analyze the studies addressed or reported on the challenges of health economic modeling in precision medicine.
Methods
Relevant studies were identified in the EMBASE, MEDLINE, Cochrane Library, EconLit, and the University of Melbourne Full Text Journal databases from 1 January 2013 to 16 September 2022. Included papers were selected if they were economic evaluations and/or budget impact analyses.
Results
A total of 24 studies were included and analyzed, with the majority being full economic evaluations (n = 19, 79.2%). Four studies (16.7%) were health and budget impact analyses, and one study (4.1%) incorporated both an economic evaluation and a budget impact analysis. Cohort-level modeling techniques were the most common approach (n = 16; 80%). LB technologies were cost-effective in 15 studies (75%) considering different biomarkers, cancer types and stages, and economic analyses. These studies evaluated LBs for screening and early detection (66.7%), treatment selection (26.7%), and monitoring treatment response (6.6%). Budget impact analysis results were varied among included studies, with the majority of studies (n = 4; 80%) reporting either cost savings, minimal, or modest budget impact, while one study (20%) reported LBs as an efficient strategy. The reviewed studies often inadequately reported or addressed modeling challenges, such as patient-level processes, the combination of tests and treatments, preferences, and uncertainty.
Conclusion
LBs could provide a cost-effective approach for treatment selection in lung cancer and aid in the screening and early detection of other cancers, including colorectal, gastric, breast, and brain cancers. This is in comparison with various alternatives, such as the standard of care (SOC) and no screening scenario. However, it is important to mention that in some comparisons, LBs were used in combination with SOC instead of replacing it. Importantly, few studies have pointed toward LBs’ cost-effectiveness for monitoring treatment response. Most health and budget impact analyses, especially those focused on lung cancer, suggest potential cost savings or a minimal-to-moderate budget impact. Nevertheless, additional research is needed to ascertain their effectiveness across various stages of lung and colorectal cancer, as well as to address potential modeling challenges.
Systematic review registration
PROSPERO CRD42022307939.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The cost-effectiveness of liquid biopsies (LBs) has been established in selecting treatments for lung cancer patients and in screening and early detection of colorectal, gastric, breast, brain, and other cancers. |
The majority of health and budget impact studies, which were primarily focused on the use of LBs in treatment selection among patients with lung cancer, reported either cost savings or a minimal-to-moderate budget impact. |
Only two studies explored the role of LB in monitoring treatment response, both suggesting cost-saving benefits. |
There seems to be a lack of health-economic evidence regarding the use of LBs in other clinical applications, such as prognostication, risk of relapse, and monitoring of disease burden. |
While current evidence suggests the potential value of LBs in these clinical applications and cancer types, further research is necessary to comprehensively evaluate associated costs and health outcomes. |
To tailor the modeling of personalized treatment using LBs, future research should consider alternative approaches such as dynamic simulation modeling utilizing real-world data to address current modeling challenges, data gaps, and the ability to analyze treatment pathways. |
1 Introduction
Cancer is a significant contributor to mortality globally, with around 10 million deaths attributed to it in 2020 (almost one-sixth of all deaths) [1]. To address the increasing burden of this disease, continuous efforts are being made to enhance cancer diagnosis and management [2]. Genomics has made a significant contribution to early disease detection and molecular tumor profiling, leading to the identification of diagnostic and prognostic biomarkers and personalized targeted therapies considering individual variability [3, 4].
Despite tumor tissue being the standard source for clinical molecular analysis, liquid biopsies (LBs) have gained popularity as a complement or alternative through analyzing cancer-derived materials circulating in the bloodstream or other body fluids. LBs have shown promise in several clinical applications, such as screening and early detection, identifying minimal residual disease, selecting treatments, and monitoring disease progression [5, 6]. Acknowledging the broad meaning of LB, this study specifically refers to cancer-derived material LBs, thereby distinguishing it from serum tumor markers such as prostate-specific antigen (PSA) for prostate cancer screening, cancer antigen 125 (CA-125) for ovarian cancer detection, and carcinoembryonic antigen (CEA) for colorectal cancer monitoring, which are also often referred to as LBs [7,8,9,10].
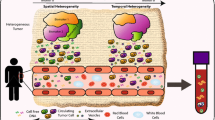
Several sources of tumor materials can be analyzed using LBs, including circulating tumor cells (CTCs); circulating cell-free DNA (cfDNA), including circulating tumor DNA (ctDNA), which makes up methylated ctDNA such as methylated Septin 9 (mSEPT9); and cell-free RNA (cfRNA) including circulating non-coding RNAs, such as small nucleolar RNAs, long non-coding RNAs, PIWI-interacting RNAs (piRNAs), and microRNAs (miRNA) [11,12,13]. Furthermore, cfDNA, which consists of brief DNA fragments that are not coupled with cells, is discharged into the bloodstream from both apoptotic and necrotic tumor cells as well as from normal cells [14]. Although LB is mainly performed using blood samples, it can also be conducted on alternative body fluids such as saliva, urine, or cerebrospinal fluid [11].
LBs confer numerous benefits over traditional solid biopsies, including their minimally invasive nature, ability to obtain multiple samples, and providing rapid turnaround times for test results. Such attributes are generally linked to diminished morbidity relative to conventional biopsy modalities. Furthermore, the substitution of solid biopsies with a series of LBs may mitigate unnecessary health risks for patients as well as reduce the risk of complications such as hypervascularized tumor rupture and consequent bleeding or hemorrhage [15, 16].
LBs may have potential utility in terms of improving clinical outcomes in several areas of oncology, such as detecting cancer (screening or early diagnosis), predicting prognosis (such as in minimal residual disease or MRD and risk of relapse), selecting treatments, and monitoring disease burden. Prognostic, molecular profiling, and monitoring translational potential of ctDNA have been demonstrated by proof-of-principle studies [17]. However, clinical evidence is required, typically obtained from well-designed, large-scale controlled trials before implementing LBs in clinical practice [18]. More than 60 trials, with an expected accrual of more than 20,000 patients, are presently addressing the challenges posed by LBs across 11 cancer types [18].
Generally, successful clinical translation and adoption of innovative health technologies in countries with a formal Health Technology Assessment (HTA) pathway requires clinical and health economic assessment, typically through an economic evaluation or budget impact analysis [19]. Moreover, in the context of healthcare technologies, conducting early model-based economic evaluations or development-focused HTAs that compare clinical benefits with related costs can also provide valuable insights for research and development (R&D) endeavors, particularly during the nascent stages of technology development. Such evaluations can aid in the design and management of new health technologies, thereby mitigating potential risks and uncertainties that may arise during the market access and reimbursement processes at later stages [20]. In addition, it is prudent to conduct a budget impact analysis to assess the financial implications of implementing an intervention. Such an analysis would consider the utilization and coverage of the intervention in a specific population, enabling an evaluation of the intervention’s budgetary impact [21, 22].
To produce health economic evidence in precision medicine (PM), it is necessary to evaluate various diagnostic tests and treatments over time. However, as treatment decisions are becoming more personalized and based on multiple sources of information, including patients’ characteristics and medical history, assessing the cost-effectiveness of health technologies is becoming more complex [23]. This complexity poses challenges not only for delivering healthcare, but also for evaluating the economic value of health technologies [24]. Therefore, in the context of PM, there is a need for methods that can consider the intricate process of making multiple treatment choices and provide valuable information to support healthcare policy decisions [25].
Annemans et al. [26], Degeling et al. [27], and Marshall et al. [25] have identified at least ten methodological challenges that must be considered when developing and implementing robust model-based economic evaluations in the setting of PM. We pose that while these challenges pertain to PM in general, they are equally relevant to the specific application of LBs. Successfully tackling these challenges necessitates the precise representation of individualized treatment decisions, accounting for diagnostic test performance, managing increased uncertainty stemming from complex analyses and data gaps, and incorporating patients’ and physicians’ preferences. Additionally, the impact of drug therapies and companion diagnostics must be taken into consideration, which adds an additional layer of complexity. Furthermore, the lack of established guidelines, criteria, and standards for evaluating new technologies in PM further complicates the field [26].
Due to the aforementioned methodological challenges, the adequacy of conventional modeling techniques, such as cohort and state-transition models, is being questioned as they may not fully capture the intricacies of the personalized treatment process. As a result, more advanced modeling methods, such as discrete event simulation, agent-based modeling, and system dynamics, have been proposed as potentially better suited to this personalized setting [25, 28, 29]. The aim of this systematic review was, firstly, to collate and synthesize the existing health economic evidence on LB assays by identifying and evaluating economic evaluation and budget impact analysis studies, and secondly, to review existing research that has explored or addressed the health economic modeling challenges previously discussed, with the aim of extracting insights that can inform the refinement of modeling methods for LBs.
2 Materials and Methods
2.1 Search Strategy
A systematic literature review of EMBASE, MEDLINE, the Cochrane Library, University of Melbourne Full Text Journals, and EconLit databases was conducted to identify the most relevant health economic studies on liquid biopsies. The full search strategy for each database is available in the Supplementary Appendix (S1). The search terms used were a combination of free-text words and subject headings used by EMBASE and MEDLINE. The search strategies were restricted to studies of humans and papers had to be written in the English language. The literature search was conducted from 1 January 2013 to 16 September 2022. The final search strategies were reviewed by a librarian to ensure the quality and all-inclusiveness of the search as well as to reduce errors. In addition to the main search strategy, the reference lists of relevant articles will also be reviewed to ensure a comprehensive search. The study protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO; registration no. CRD42022307939).
2.2 Inclusion and Exclusion Criteria
Studies were included if they met all of the following criteria:
-
Population: patients with or at risk of any solid cancer type.
-
Intervention: blood-based LB assays in solid tumors at any stage of cancer management (including screening and early detection, treatment selection, monitoring disease burden, and/or prognosis).
-
Comparator: any other diagnostic tools such as tissue biopsy, the standard of care (SOC), or “do nothing” strategy.
-
The study design includes economic evaluations such as cost-effectiveness and cost-utility analysis (CEA/CUA), cost-minimization analysis (CMA), cost-consequence analysis (CCA), and cost-benefit analysis (CBA). Budget or health impact analyses are also included. The study also considered other research designs, such as clinical trials, systematic reviews, meta-analyses, network meta-analyses (NMA), health technology assessments (HTA), and evidence-based guidelines that incorporated any of these types of analyses.
Studies were excluded if they did not report on both cost and health outcomes, lacked health economic results, or had no full-text article available.
2.3 Study Selection
After removing duplicates, two reviewers (MF and MV) first independently screened studies by title and abstract followed by full-text, using the Covidence platform against the eligibility criteria. Disagreements regarding study eligibility were resolved by consensus with a third reviewer (MIJ).
2.4 Data Extraction
Data were extracted by one reviewer (MF) using a pre-determined data extraction form developed in Microsoft Excel 2019. A second reviewer (MV) was involved in checking for inconsistencies. Data points included author, title, year of publication, country, population, biomarkers, clinical application for the LB assay, description of intervention and comparator used, method/analysis, modeling approach, time horizon, discount rate, perspective, software used, health outcomes, willingness to pay (WTP), main results, incremental cost-effectiveness ratio (ICER) reported, sensitivity analysis, and key conclusions.
2.5 Quality Assessment and Risk of Bias
The quality of the economic evaluation studies was evaluated using the latest version of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [54]. A detailed presentation of the criteria items for the CHEERS checklist is presented in Supplementary Table 2. Although this checklist was not designed to assess the methodological quality of health economic studies, it has been widely used to evaluate the reporting quality of economic evaluations of health interventions. Each publication was evaluated on the basis of the checklist’s criteria, which were categorized as reported, not reported, or not applicable. The overall reporting quality of each study was determined by calculating the proportion of rated criteria to the total number of applicable criteria. A score greater than 85% indicated excellent reporting quality, while scores ranging from 85 to 75% were classified as very good, scores between 75 and 55% as good, and scores below 55% as poor. The risk of bias was not assessed due to the absence of established tools for evaluating the risk of bias in health economic studies.
2.6 Evaluation of the Health Economic Modeling Challenges
A ten-item checklist was employed for the evaluation of the included studies, drawing on the challenges identified from the literature [25,26,27]. A full description of the context of the potential challenges for health economic modeling as identified in the literature is provided in Supplementary Table 1. This checklist aimed to highlight the modeling challenges faced within the studies. For the initial seven checklist items, a positive outcome indicated that the authors either acknowledged, managed, or addressed the challenge within the economic model, while for the remaining three checklist items, a positive outcome signified that the authors identified or reported on the specific challenge. If a challenge was acknowledged, managed, or addressed by the authors, it was recorded as ‘+,’ whereas challenges that were not otherwise were given a ‘−’ score.
3 Results
3.1 Included Studies
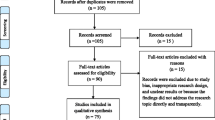
Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search. The literature search yielded a total of 7756 records, with 4 additional records identified from other sources (particularly publications’ reference lists). Of those, after removing duplicates and screening, 156 records were assessed by full text, with 132 excluded for reasons such as lack of full text and wrong intervention or study design. Ultimately, 24 studies were included in the review.
3.2 Quality Assessment and Risk of Bias
The quality of economic evaluation studies (20 studies) was assessed using the updated Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (2022). Results from the CHEERS checklist assessment are shown in Fig. 2. The majority of the studies (greater than 75% of the studies) were of excellent reporting quality when assessed against most of the CHEERS statement items. However, there were several studies that did not report on the health economic analysis plan (n = 15; 75%), characterizing heterogeneity (n = 10; 50%), and characterizing distributional effects (n = 10; 50%).
3.3 General and Clinical Characteristics
A total of 24 studies were included and analyzed, with the majority being full economic evaluations (n =19, 79.2%). Four studies (16.7%) were health and budget impact analyses, and one study (4.1%) incorporated both an economic evaluation and a budget impact analysis. The characteristics of the studies, clinical applications, evaluated strategies, and the overall judgement of the included studies are presented in Table 1.
3.3.1 Publication Year and Geographical Location
Studies were published between 2013 and 2022, with most studies (n = 15; 62.5%) published between 2021 and 2022. LBs were mostly evaluated for use in the USA (n = 8; 33.3%), followed by Canada (n = 4; 16.7%). A variety of other countries were also represented in the review, each making up 4.2% (n = 1) of the studies. These countries included Australia, China, Colombia, Germany, Greece, Japan, the Netherlands, Singapore, Spain, and the UK. One study (4.2%) reported its country of origin as both Singapore and South Korea. Moreover, another study conducted its analysis in both the USA and the UK. In one study, supplementary scenario analyses were conducted using Taiwanese data in conjunction with the base case analysis from the USA, with the results subsequently presented in the supplementary material.
3.3.2 Cancer Stream, Biomarkers, and Clinical Application
Studies analyzed the impact of LBs across various solid tumors, including lung cancer (n =8; 33.3%), colorectal cancer (n = 5; 20.8%), gastric cancer (n = 3; 12.5%), breast cancer (n = 2; 8.3%), brain cancer (n = 1; 4.2%), testicular cancer (n = 1; 4.2%), and prostate cancer (n = 1; 4.2%), while some studies investigated the role of LBs for multi-cancer detection (n = 3; 12.5%). ctDNA was the most commonly investigated biomarker (n = 10; 41.7%), followed by miRNA (n = 5; 20.8%), methylated SEPT9 DNA (n = 3; 12.5%), CTC (n = 2; 8.3%), and cfDNA using methylation signatures (n = 2; 8.3%), while the remaining study (4.2%) used Fourier-transform infrared (FTIR) spectroscopy based on spectral properties of the serum.
Most of the studies assessed the use of LB in cancer diagnosis as a screening and early detection tool (n = 12; 50%), as well as in guiding treatment selection (n = 10; 42%), whereas the remaining studies (n = 2; 8%) examined its use in surveillance and monitoring of treatment response.
3.4 Economic Evaluation
A summary of health economic analysis in conjunction with the cost-effectiveness judgement is presented in Table 2. More detailed information, including the modeling approach, perspective, time horizon, health outcomes, and primary results, are presented in Supplementary Table 3.
3.4.1 Type of Economic Evaluation and Health Outcomes
There were 20 economic evaluation studies identified in this review. Of these 20 economic evaluations, there were 19 (95%) CEA/CUA and 1 (5%) CMA. Moreover, one study (Gray et al. (2021) conducted a cost-consequence analysis (CCA) in addition to the CEA/CUA. Most of the economic evaluation studies used QALYs as health outcome (n = 17; 85%), with some studies using other outcomes such as LY gained (n = 5) and adverse events avoided (n = 1) in addition to QALYs. Of all included studies, three studies (15%) did not use QALYs and instead used other outcomes such as LYs gained, rate of treatment, and monetary loss.
3.4.2 Decision Analytic Modeling
The methodological characteristics and model-specific information of included studies are presented in Supplementary Table 3. Among all model-based economic evaluation studies (n = 20; 100%), cohort-level modeling techniques were the most common approach (n = 16; 80%). Of these studies, seven (44%) used Markov models, five (31%) used decision tree, and four (25%) combined decision tree with Markov models. Among the remaining studies, three economic evaluations used a micro-simulation approach, and one study compared two different modeling approaches [timed automata (TA) and discrete event simulation (DES)].
3.4.3 ICER and Cost-Effectiveness Judgement
Supplementary Table 3 provides a summary of detailed results for each comparison, including willingness-to-pay (WTP) thresholds and the resulting incremental cost-effectiveness ratios (ICERs). In general, out of the 20 included economic evaluations, there were 2 studies [38, 49] that demonstrated LBs were not cost-effective in all examined scenarios. Rodriguez et al. [38] investigated the use of LBs in monitoring treatment response in metastatic colorectal cancer, while Sanchez-Calderon et al. [49] examined the role of LBs in treatment selection among patients with HER2-positive breast cancer. Moreover, two studies [32, 47] did not provide any analysis of ICER or give any assessment of the cost-effectiveness of LBs. The study by Ezeife et al. [32] indicated cost savings and a gain in QALYs associated with LBs, whereas Tafazzoli et al. [47] revealed the price point at which LBs would become cost-effective.
Nonetheless, the economic analyses of 15 studies (75%) showed that LB technologies were cost-effective over varying time horizons, ranging from 2 years to a lifetime. Of these studies, ten (66.7%) evaluated the use of LBs for screening and early detection, four (26.7%) for treatment selection, and one (6.6%) for monitoring treatment response. The studies that demonstrated the cost-effectiveness of LBs differed significantly in terms of biomarkers evaluated, cancer type and stage, and the type of economic analysis performed.
Among the identified economic evaluation studies, four investigations delved into the usage of LBs in managing lung cancer [30,31,32,33,34,35,36,37]. Three of these [30,31,32] centered on the application of LBs in treatment selection, and the remaining study [33] was for screening and early detection. Out of the three studies in treatment selection, two studies [30, 32] employed CEA/CUA techniques, while Yang et al. [31] was the sole study to utilize CMA. Firstly, Ontario Health Technology Assessment [30] found that LB is cost-effective (dominant) compared with tissue biopsy when short-term costs and effects were considered, while not cost-effective (ICER > $100,000 per QALY in Canadian dollars) when considering long-term costs and effects, mainly due to the overall high cost of third-generation EGFR-TKI treatment. Secondly, Ezeife et al. [32] compared LB + tissue testing (TT) with TT alone among patients with treatment-naïve stage IV non-squamous non-small cell lung cancer, and demonstrated that LB resulted in cost savings (LB + TT strategy resulted in incremental cost savings of $3065 Canadian dollars per patient and a gain in quality-adjusted life-years of 0.02). Thirdly, Yang et al. [31], employing CMA, did not observe any cost savings from LBs. Economic analysis from Yang et al. [31] determined that liquid-based NGS (as plasma-first approach) was not a cost-saving option when compared with tissue-first and complementary approaches. However, the tissue-first approach was the best strategy for minimizing monetary loss. Lastly, the study conducted by Zhao et al. [33] was the only investigation that evaluated LBs for screening and early detection, concluding their cost-effectiveness.
In the context of colorectal cancer (CRC), five studies applied CEA/CUA methods [38,39,40,41,42]. Three of these [39, 40, 42] evaluated the cost-effectiveness of LBs for screening and early detection, consistently finding LBs to be cost-effective. To et al. [41] assessed LBs’ application for treatment selection, demonstrating their cost-effectiveness. The remaining study [38], which evaluated the cost-effectiveness of LBs versus computed tomography scans in monitoring treatment response, did not find them cost-effective.
Three studies examined the usage of LBs in gastric cancer [43,44,45], all employing CEA/CUA techniques. These universally agreed that LBs, when used for screening and early detection, are cost-effective. LBs were also studied as an MCED tool in two studies [46, 47]. In their 2022 study, Lipscomb et al. determined that the MCED test was indeed a cost-effective option.
Conversely, Tafazzoli et al. [46] calculated the value-based price of $1196 at which the MCED test would be cost-effective, set at a WTP threshold of US $100,000 per QALY. The study also assessed the clinical and economic outcomes of annual MCED testing for individuals aged between 50 and 79 years. The findings suggested that MCED tests with a high specificity could potentially improve long-term health outcomes and reduce the cost linked to cancer treatment.
In the case of breast cancer, two studies investigated the use of LBs, both adopting CEA/CUA methods [49, 50]. Van der Poort et al. [50] analyzed the use of LBs in screening and early detection, finding them to be cost-effective. In contrast, Sanchez-Calderon et al. [49] determined LBs not to be cost-effective when applied for treatment selection. This study evaluated the cost-effectiveness of ctDNA detection in HER2-positive breast cancer. It showed that including the LB test with the conventional molecular target treatment was both less effective and more costly than using the conventional molecular target treatment alone.
Additionally, two studies investigated the usage of LBs in the context of patients with testicular and prostate cancers [51, 52], specifically for treatment selection and monitoring treatment responses, respectively. Both studies agreed that LBs are cost-effective, resulting in cost savings. Lastly, only one study [53] has examined the cost-effectiveness of LBs for screening and early detection of brain cancer, successfully establishing their cost-effectiveness.
3.4.4 Modeling Challenges for Liquid Biopsies
Results from the evaluation of the included studies regarding the health economic modeling challenges are presented in Table 2. In the 20 studies analyzed, diagnostic performance (n = 17, 85%) and data gaps (n = 19, 95%) were the most commonly acknowledged, managed, or addressed challenges. Conversely, challenges such as addressing patient-level processes (n = 5, 25%), patients’ and physicians’ preferences (n = 2, 10%), identifying and reporting on greater uncertainty in complex analysis (n = 1, 5%), and the absence of guidelines (n = 2, 10%) were the least commonly acknowledged, managed, or addressed. It is essential to carefully consider these challenges, despite them not being commonly identified or managed, when conducting model-based economic evaluations in the context of PM, as they can lead to a higher degree of uncertainty in economic models and impact the interpretation of results.
3.5 Budget Impact Analysis
3.5.1 Overview and General Characteristics
Supplementary Table 4 presents a summary of the health budget impact analyses and results. Our literature search identified five publications related to health and budget impact analysis [30, 35,36,37, 48], three of which performed population health and budget impact analyses [36, 37, 48], one exclusively focused on budget impact [35], and one study that performed BIA as part of an economic evaluation [30]. Regarding clinical applications, four investigations evaluated the effects of employing LBs in the selection of cancer treatments. Meanwhile, a separate study estimated the population health impact of MCED to supplement existing cancer screening methods [48].
3.5.2 Modeling Approach
All of the health and budget impact studies (n = 5) adopted a population-based approach in their analysis, yet two studies [30, 37] employed an epidemiological approach, while one study [36] employed both epidemiological and market share approaches. The remaining two studies [35, 48] did not specify the modeling approach utilized.
3.5.3 Budget Impact Judgement
The five studies analyzed in this review revealed varying budget impacts when comparing LBs testing approaches with current testing approaches. In general, the majority of studies (n = 4; 80%) reported either cost savings, minimal, or modest budget impact. Specifically, the studies by Ontario Health Technology Assessment [30] and Cheng et al. [35] linked LBs with cost savings. Simultaneously, Johnston et al. [36] and Patel et al. [37] attested to the effectiveness of LBs, documenting minor and moderate impacts, respectively. Furrthermore, a single study (20%) by Hackshaw et al. [48] positioned LBs as an efficient strategy, endorsing MCED as an effective method to identify additional undetected cancer types by SOC, while also minimizing false-positive results.
Ontario Health Technology Assessment [30] compared the costs of using LB as a triage test followed by tissue biopsy (if patients test negative for the EGFR T790M resistance mutation) or standalone test with the current scenario (without public funding for LB). This study estimated minimal budget impact when LB was used as a triage test and cost savings when LB was used alone. Cheng et al. [35] used a budget impact model to compare the financial impact of different EGFR mutation testing approaches, including plasma test, combined testing, and reflex testing, with the current standard of tissue biopsy only. They found cost savings for plasma only and combined testing, while cost optimal for reflex testing per correctly classified patient. Johnston et al. [36] reported effective outcomes with minimal budget impact when evaluating the health and budget impacts of adopting FoundationOne® Liquid only among patients with non-small cell lung cancer (NSCLC).
In contrast, Patel et al. [37] found that incorporating LBs into the healthcare system would have an overall modest impact ($14.7 million in Canadian dollars) with 168 life-years gained to the Canadian publicly funded healthcare system in the 3-year time horizon. This study identified a difference in results compared with the study by Johnston et al. [36], which reported a lower 3-year budget of around $4.5 million. The reason for this disparity is that Johnston et al. [36] focused primarily on tissue biopsy and assumed that 5% of patients would have unavailable tissue for testing. Meanwhile, Patel et al. [37] considered all potential drug treatment costs and provided a more detailed and disaggregated breakdown of patients with tissue-limited advanced NSCLC.
Hackshaw et al. [48] assessed the population health impact of MCED in addition to the recommended screening modalities in USA and UK. The study found that an MCED test (with 25–100% uptake) detected 105,526–422,105 additional cancers of various types. The cost for each detected cancer dropped from $89,042 (with SOC) to $7060 using the MCED test in the USA. Similarly, in the UK, the cost per detected cancer fell from £10,452 (with SOC) to £2175 using the MCED test. The study concluded that including an MCED blood test in routine screening in addition to SOC could potentially be an efficient strategy.
4 Discussion
Out of the 20 economic evaluation studies reviewed, results from 15 studies [30, 33, 34, 39,40,41, 43,44,45,46, 50,51,52,53] indicate that LB is cost-effective, with two studies [51, 52] showing that LB is a cost-saving option. While it is important to interpret these findings within the context of the modeling assumptions used in each study, the current evidence suggests that LB is likely to be cost-effective for cancer management. Additionally, the majority of health and budget impact studies (4 out of 5) reported either cost-savings, minimal, or modest budget impact [30, 35,36,37]. Considering the significant diversity in the studies, the findings varied considerably. In reviewing distinct types of cancer, this evaluation noted that lung cancer (specifically NSCLC) and CRC were the most frequently studied in relation to the economic assessment of LB. The evidence suggests that LBs are potentially cost-effective in selecting treatments for patients with lung cancer. Moreover, for CRC and gastric as well as other types of cancer, LBs appear to be cost-effective in screening and early detection. This is in comparison with various alternatives, such as the SOC and no screening scenarios. However, it is important to mention that in some comparisons, LBs were used in combination with SOC, instead of replacing it. The authors anticipate economic benefits from these LB applications specifically, and caution against generalizing these results to other clinical applications across different cancer types. This underscores the importance of recognizing the context-specific nature of these findings.
Furthermore, it should also be noted that there were only two studies [38, 51] that investigated the role of LB in the monitoring of treatment response, and both demonstrated that LB is a cost-saving option. Of these, one study [51] determined that LB is likely to be a cost-effective option, while the other study [38] showed that LB is not cost-effective. It is likely that the variations observed are a result of divergent modeling considerations, including factors such as the population studied and the structure of the model employed. To this end, the review indicates a dearth of health-economic evidence pertaining to the use of LBs in other clinical applications, such as prognostication, risk of relapse, and monitoring of disease burden.
While the current evidence suggests that LB has health economic benefits, however, the majority of the studies included in this review used cohort-based models, which may not fully capture the complexity and patient-specific heterogeneity inherent in PM. Moreover, uncertainty in economic models and data gaps were found to be prevalent challenges in modeling the cost-effectiveness of LBs. In view of the fact that clinical trials demonstrating utility may not be feasible due to the complexity of PM and the need for patient-specific decision-making, the use of real-world data and advanced simulation methods (such as dynamic simulation modeling) may provide an alternative way to evaluate the cost-effectiveness of LB instead. Dynamic simulation modeling techniques, which can use patient-level data to design mathematical representations of complex systems and intervention scenarios, show promise in addressing these challenges.
Addressing these challenges in health economic modeling is crucial to guide reimbursement decisions and support the translation of LB in clinical practice. Future evaluations should consider alternative approaches to capture the complexity and individual patient factors as well as model companion diagnostics and a combination of tests. It is also important to model diagnostic accuracy and study specific outcomes, while incorporating patients’ and physicians’ preferences. Therefore, future research should focus on incorporating more advanced simulation methods and real-world data to address the challenges in modeling the cost-effectiveness of LB. Furthermore, the potential cost-effectiveness of LB in lung cancer and colorectal cancer supports the need for further research and evaluation of these cancer types.
4.1 Study Limitations
It is important that the current study’s findings should be interpreted in light of its limitations. Firstly, there is a possibility that not all health economic evaluations or budget impact analyses performed for LBs have been published. Therefore, conclusions from this literature review might be subjected to publication bias, where studies with positive outcomes are favored over those with negative results. Secondly, given that the CHEERS checklist was used to assess the quality of reporting, and as this checklist was not originally developed to score publications, it might have led to subjectivity in the appraisal methodology, particularly the classification of the overall reporting quality. Finally, given the qualitative nature of the analyses performed around health economic modeling challenges that may have been addressed or reported by the publications, bias may have been introduced when summarizing findings. It should be noted that the final results were narrated at the discretion of the reviewers. Furthermore, due to the use of varied analytical methods for evaluating health outcomes and costs associated with the interventions, data pooling was not feasible, and the reviewers were required to exercise discretion in summarizing the study results.
5 Conclusion
The review suggests that LBs could be a cost-effective approach for guiding treatment choices in lung cancer, as well as aiding in the screening and early detection of other cancers, including colorectal, gastric, breast, and brain cancers. This is in comparison with various alternatives, such as the SOC and no screening scenarios. However, it is important to mention that in some comparisons, LBs were used in combination with SOC instead of replacing it. Notably, only two studies assessed the cost-effectiveness of LBs in monitoring treatment response, with both suggesting potential cost savings. Moreover, the majority of health and budget impact studies, which were primarily focused on the use of LBs in treatment selection among patients with lung cancer, reported either cost savings or a minimal-to-moderate budget impact. These findings, however, should be interpreted considering their specific contexts, refraining from broad application across diverse cancer types.
Furthermore, to support the translation of LBs in clinical practice and to guide reimbursement decisions, future health economic evidence may be needed using advanced simulation methods to account for the potential challenges, including modeling patient-level processes, combinations of tests and treatments, diagnostic performance, and patients’ and physicians’ preferences. Careful consideration of these challenges is crucial, as they can increase uncertainty in economic models and affect how the results are interpreted.
References
Ferlay J, E.M., Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: cancer today. I 2020 [cited 2022 1 August]. https://gco.iarc.fr/today/home.
The global challenge of cancer. Nat Cancer. 2020;1(1):1–2.
Thomsen CB, et al. Monitoring the effect of first line treatment in RAS/RAF mutated metastatic colorectal cancer by serial analysis of tumor specific DNA in plasma. J Exp Clin Cancer Res. 2018;37(1):55.
Malczewska A, et al. NETest liquid biopsy is diagnostic of lung neuroendocrine tumors and identifies progressive disease. Neuroendocrinology. 2019;108(3):219–31.
Ilie M, Hofman P. Pros: can tissue biopsy be replaced by liquid biopsy? Transl Lung Cancer Res. 2016;5(4):420–3.
Cescon DW, et al. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer. 2020;1(3):276–90.
Thomas CM, Sweep CG. Serum tumor markers: past, state of the art, and future. Int J Biol Markers. 2001;16(2):73–86.
Cabarkapa S, et al. Prostate cancer screening with prostate-specific antigen: a guide to the guidelines. Prostate Int. 2016;4(4):125–9.
Baron AT, et al. Soluble epidermal growth factor receptor (sEGFR) [corrected] and cancer antigen 125 (CA125) as screening and diagnostic tests for epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):306–18.
Tan E, et al. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18(1):15–24.
Ilie M, et al. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med. 2014;2(11):107.
Nilsson RJ, et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2016;7(1):1066–75.
Toth K, et al. Detection of methylated septin 9 in tissue and plasma of colorectal patients with neoplasia and the relationship to the amount of circulating cell-free DNA. PLoS ONE. 2014;9(12): e115415.
Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–86.
Fernandez-Lazaro D, et al. Clinical perspective and translational oncology of liquid biopsy. Diagnostics (Basel). 2020;10(7):443.
Vymetalkova V, et al. Circulating cell-free DNA and colorectal cancer: a systematic review. Int J Mol Sci. 2018;19(11):3356.
Wan JCM, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–38.
Siravegna G, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14(9):531–48.
Mj IJ, et al. Health economic impact of liquid biopsies in cancer management. Expert Rev Pharmacoecon Outcomes Res. 2018;18(6):593–9.
Mj IJ, et al. Emerging use of early health technology assessment in medical product development: a scoping review of the literature. Pharmacoeconomics. 2017;35(7):727–40.
Sullivan SD, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
Mauskopf JA, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices—budget impact analysis. Value Health. 2007;10(5):336–47.
van Til JA, Ijzerman MJ. Why should regulators consider using patient preferences in benefit–risk assessment? Pharmacoeconomics. 2014;32(1):1–4.
Towse A, Garrison LP Jr. Economic incentives for evidence generation: promoting an efficient path to personalized medicine. Value Health. 2013;16(6 Suppl):S39-43.
Marshall DA, et al. Addressing challenges of economic evaluation in precision medicine using dynamic simulation modeling. Value Health. 2020;23(5):566–73.
Annemans L, Redekop K, Payne K. Current methodological issues in the economic assessment of personalized medicine. Value Health. 2013;16(6 Suppl):S20–6.
Degeling K, Koffijberg H, Mj IJ. A systematic review and checklist presenting the main challenges for health economic modeling in personalized medicine: towards implementing patient-level models. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):17–25.
Siebert U, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health. 2012;15(6):812–20.
Degeling K, et al. Accounting for parameter uncertainty in the definition of parametric distributions used to describe individual patient variation in health economic models. BMC Med Res Methodol. 2017;17(1):170.
Ontario H, F.L.G.-V.O.C.L.S.A.H.C. Lambrinos A. Cell-free circulating tumour DNA blood testing to detect EGFR T790M mutation in people with advanced non-small cell lung cancer: a health technology assessment. Ontario Health Technol Assess Ser.2020;20(5):1–176.
Yang SC, et al. Economic analysis of tissue-first, plasma-first, and complementary NGS approaches for treatment-naive metastatic lung adenocarcinoma. Front Oncol. 2022;12: 873111.
Ezeife DA, et al. The economic value of liquid biopsy for genomic profiling in advanced non-small cell lung cancer. Ther Adv Med Oncol. 2022;14:17588359221112696.
Zhao Z, et al. Cost-effectiveness of low-dose computed tomography with a plasma-based biomarker for lung cancer screening in China. JAMA Netw Open. 2022;5(5): e2213634.
Englmeier F, et al. Clinical benefit and cost-effectiveness analysis of liquid biopsy application in patients with advanced non-small cell lung cancer (NSCLC): a modelling approach. J Cancer Res Clin Oncol. 2022;149:1495–1511 (2023). 09 May 2022
Cheng M, Akalestos A, Scudder S. Budget impact analysis of EGFR mutation liquid biopsy for first- and second-line treatment of metastatic non-small cell lung cancer in Greece. Diagnostics (Basel). 2020;10(6):429.
Johnston KM, et al. Comprehensive genomic profiling for non-small-cell lung cancer: health and budget impact. Curr Oncol. 2020;27(6):e569–77.
Patel YP, et al. Health and budget impact of liquid-biopsy-based comprehensive genomic profile (CGP) testing in tissue-limited advanced non-small cell lung cancer (aNSCLC) patients. Curr Oncol. 2021;28(6):5278–94.
Rodriguez CA, et al. Monitoring treatment response in metastasic colorectal cancer: economic evaluation of PrediCTC versus computed tomography scan. Glob Reg Health Technol Assess. 2019;6(1):1-10.
Deibel A, et al. Evaluating key characteristics of ideal colorectal cancer screening modalities: the microsimulation approach. Gastrointest Endosc. 2021;94(2):379.
Ladabaum U, et al. Colorectal cancer screening with blood-based biomarkers: cost-effectiveness of methylated septin 9 DNA versus current strategies. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2013;22(9):1567–76.
To YH, et al. Circulating tumour DNA as a potential cost-effective biomarker to reduce adjuvant chemotherapy overtreatment in stage II colorectal cancer. Pharmacoeconomics. 2021;39(8):953–64.
Peterse EFP, et al. Comparing the cost-effectiveness of innovative colorectal cancer screening tests. J Natl Cancer Inst. 2021;113(2):154–61.
Kapoor R, et al. Evaluating the use of microRNA blood tests for gastric cancer screening in a stratified population-level screening program: an early model-based cost-effectiveness analysis. Value Health J Int Soc Pharmacoecon Outcomes Res. 2020;23(9):1171–9.
Izumi D, et al. Assessment of the diagnostic efficiency of a liquid biopsy assay for early detection of gastric cancer. JAMA Netw Open. 2021;4(8): e2121129.
So JBY, et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut. 2021;70(5):829–37.
Lipscomb J, et al. Evaluating the impact of multicancer early detection testing on health and economic outcomes: toward a decision modeling strategy. Cancer. 2022;128(Suppl 4):892–908.
Tafazzoli A, et al. The potential value-based price of a multi-cancer early detection genomic blood test to complement current single cancer screening in the USA. Pharmacoeconomics. 2022;40(11):1107–17.
Hackshaw A, et al. Estimating the population health impact of a multi-cancer early detection genomic blood test to complement existing screening in the US and UK. Br J Cancer. 2021;125(10):1432–42.
Sanchez-Calderon D, et al. Analysis of the cost-effectiveness of liquid biopsy to determine treatment change in patients with her2-positive advanced breast cancer in Colombia. Clin Outcomes Res. 2020;12:115–22.
van der Poort EKJ, et al. The early detection of breast cancer using liquid biopsies: model estimates of the benefits, harms, and costs. Cancers (Basel). 2022;14(12):2951.
Degeling K, et al. Comparison of timed automata with discrete event simulation for modeling of biomarker-based treatment decisions: an illustration for metastatic castration-resistant prostate cancer. Value Health. 2017;20(10):1411–9.
Bagrodia A, et al. Impact of circulating microRNA test (miRNA-371a-3p) on appropriateness of treatment and cost outcomes in patients with stage I non-seminomatous germ cell tumours. BJU Int. 2021;128(1):57–64.
Gray E, et al. Early economic evaluation to guide the development of a spectroscopic liquid biopsy for the detection of brain cancer. Int J Technol Assess Health Care. 2021;37: e41.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, Caulley L, Chaiyakunapruk N, Greenberg D, Loder E, Mauskopf J, Mullins CD, Petrou S, Pwu RF, Staniszewska S. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: A Report of the ISPOR CHEERS II Good Practices Task Force. Value Health. 2022;25(1):10-31. PMID: 35031088
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflict of interest
MF and MV receive a PhD (unrestricted) scholarship from Illumina under the University of Melbourne/Illumina partnership, The Advanced Genomics Collaboration (TAGC).
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author contribution
All authors contributed to the study conception, design, and protocol review. Search strategy prepared by M.F., and approved by H.K., S.Q.W., M.V., and M.I.J. The selection process was carried out collectively by all authors, with data extraction being conducted by MF. All authors contributed to the interpretation and discussion of the findings. The first manuscript draft was composed by MF and underwent critical review by all other authors. All authors have read and approved the final version.
Acknowledgments
The authors would like to thank Lindy Cochrane, Liaison Librarian, Nursing, Health Sciences, Population and Global Health at the University of Melbourne, for reviewing and advising on the selection of databases and search strategy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fagery, M., Khorshidi, H.A., Wong, S.Q. et al. Health Economic Evidence and Modeling Challenges for Liquid Biopsy Assays in Cancer Management: A Systematic Literature Review. PharmacoEconomics 41, 1229–1248 (2023). https://doi.org/10.1007/s40273-023-01292-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01292-5