Abstract
Background and Objective
Voriconazole pharmacokinetics are highly variable in pediatric patients, and the optimal dosage has yet to be determined. The purpose of this study was to describe voriconazole pharmacokinetic and pharmacodynamic targets achieved and evaluate the efficacy and safety of voriconazole for critically ill pediatrics.
Methods
This is a single-center retrospective study conducted at a pediatric intensive care unit at a tertiary/quaternary hospital. Pediatrics admitted to the pediatric intensive care unit and who received voriconazole for a proven or suspected fungal infection with at least one measured trough concentration were included. The primary outcomes included the percentage of pediatric patients who achieved the pharmacokinetic and pharmacodynamic targets. Secondary outcomes included assessing the correlation between voriconazole trough concentrations and clinical/microbiological outcomes. All statistical analyses were performed using the R statistical software and Microsoft Excel. Multiple logistic regression was used to assess the predictors of both clinical and microbiologic cures. Multiple linear regression was used to determine significant factors associated with trough concentrations.
Results
A total of 129 voriconazole trough concentrations were measured from 71 participants at steady state after at least three doses of voriconazole. The mean (± standard deviation) of the first and second trough concentrations were 2.9 (4.2) and 2.3 (3.3) mg/L, respectively. Among the first trough concentrations, only 33.8% were within the therapeutic range (1–5 mg/L), 46.5% were below the therapeutic range, and 19.7% were above the therapeutic range. A clinical cure occurred in 78% of patients, while a microbiologic cure occurred in 80% of patients.
Conclusions
Voriconazole trough concentrations vary widely in critically ill pediatric patients and only a third of the patients achieved therapeutic concentrations with initial doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A high variability in voriconazole trough concentrations between patients was found. |
The initial measured trough concentrations in 71 patients were 33.8% within the therapeutic range, 46.5% below the therapeutic range, and 19.7% above the therapeutic range. |
Patient dosing should be individualized by measuring the therapeutic drug concentration of voriconazole. |
1 Introduction
Invasive fungal infections are associated with high morbidity and mortality in immunosuppressed pediatric patients [1]. Patients with prolonged neutropenia, allogeneic hematopoietic stem cell transplant, solid organ transplant, inherited or acquired immunodeficiencies, and corticosteroid use are considered at risk of invasive fungal infections [2]. The incidence of these infections has increased in recent decades [3,4,5]. For example, the incidence of invasive aspergillosis worldwide has risen lately to > 300,000 cases from the previously reported 200,000 cases, with an associated mortality rate of 30–80% [6]. This is mainly due to the use of immunosuppressive drugs and chemotherapy. Appropriate treatment and dosing with antifungal therapy are essential to improve treatment outcomes and reduce the mortality risk [7, 8].
Voriconazole is a broad-spectrum triazole antifungal agent commonly used for both prophylaxis and the treatment of invasive fungal infections in pediatric patients. Voriconazole has complex pharmacokinetics, the drug has nonlinear pharmacokinetics and high between-subject variability [9]. In addition, voriconazole has a narrow therapeutic window. Therapeutic drug monitoring has been suggested as a tool to improve the treatment of voriconazole and has been shown to improve the efficacy and safety of voriconazole [8, 10], although it is important to note that the package insert for voriconazole does not recommend therapeutic drug monitoring. High voriconazole trough concentrations are associated with toxicities such as hepatotoxicity and visual and/or auditory hallucinations [9, 11, 12]. In contrast, low concentrations can increase the risk of treatment failure [9, 12,13,14]. The suggested therapeutic range is different from center to center but is in the range of 1–5.5 mg/L. Based on previous studies, approximately 50% of pediatric patients still do not achieve voriconazole therapeutic trough concentrations [3, 8, 15]. Therefore, therapeutic drug monitoring is crucial in determining the efficacy and safety of voriconazole, especially in pediatric patients in pediatric intensive care unit (PICU) settings [16,17,18,19,20].
Voriconazole pharmacokinetics in pediatrics are different than in adults [13]. Because of a higher hepatic clearance and a first-pass effect, pediatric patients need larger weight-based doses or more frequent doses of voriconazole [20]. Additionally, the pediatric population undergoes significant developmental and maturational changes, which may significantly influence the pharmacokinetic variability in this population. Moreover, pediatric patients exhibit different voriconazole pharmacokinetics when compared with adults. One of these differences was evaluated by pharmacokinetic (PK) modeling studies, which showed that pediatric patients demonstrated a three-fold lower area under the concentration–time curve after receiving 4 mg/kg of voriconazole every 12 h when compared with the adult population receiving the same dose [21]. For critically ill pediatric patients, the variability and complexity of pharmacokinetics are expected to be higher. This is because of several factors such as critical diseases, inflammatory status, augmented renal clearance, and therapeutic interventions (e.g., extracorporeal organ support systems or whole-body hypothermia) [22].
To our knowledge, no study has evaluated voriconazole pharmacokinetics and pharmacodynamics in PICUs. Therefore, the purpose of this study was to describe voriconazole PK/pharmacodynamic (PK/PD) targets achieved and to evaluate the efficacy and safety of voriconazole for the pediatric population admitted in the PICU setting.
2 Materials and Methods
2.1 Study Design and Population
This is a single-center retrospective study conducted at the PICU at the King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia. Inclusion criteria included any pediatric patient (1 month to 14 years of age) admitted to the PICU who received voriconazole orally or intravenously for at least 3 days for a proven or probable fungal infection and had at least one measured trough concentration. Following the Hospital Formulary and Drug Therapy Guide for dosing voriconazole, the target initial dose was 9 mg/kg/dose every 12 hfollowed by monitoring of serum trough concentrations to maintain trough concentrations of 2–6 mg/L. Exclusion criteria included patients less than 1 month of age or more than 14 years of age, patients with missing dosing information, or patients without a voriconazole trough concentration during the study time period. The study period was from January 2019 to August 2022. Before July 2020, blood samples of voriconazole concentrations were sent out for assay to the Lab Bioscientia in Germany or Mayo Clinic Laboratories in the USA. Starting from October 2020, blood samples of voriconazole concentrations were assayed in-house at the King Faisal Specialist Hospital and Research Center in Riyadh, Saudi Arabia. For all centers, the assay method was conducted using liquid chromatography-tandem mass spectrometry.
2.2 Data Collection
Data were collected from the hospital’s electronic medical records. Data collected consisted of age, gender, height, weight, laboratory values, indication for voriconazole, dose, dosing frequency, trough concentrations, presence of neutropenia, treatment duration, drugs known to interact with voriconazole (proton pump inhibitors, macrolides, amiodarone, cyclosporine, tacrolimus, nifedipine, sildenafil, phenobarbital, and rifampicin), treatment outcome, dose changes and their timing, time of the level draw, comorbidities, and a description of the fungal infection (proven or probable fungal infections). A proven fungal infection was defined as a fungus detected by the culture of a tissue specimen or by radiographic imaging. A probable fungal infection was defined as clinical documentation of a fungal suspicion based on the clinical presentation and/or radiographic imaging by the attending physician. Neutropenia was defined as an absolute neutrophil count of ≤ 500 cells/mm3 or < 1000 cells/mm3 with an anticipated decline to < 500 cells/mm3 within 48 h, according to the Infectious Diseases Society of America guidelines [23]. The World Health Organization criterion was used for defining obesity in children < 2 years of age, and the Centers for Disease Control and Prevention was used for defining obesity in children ≥ 2 years of age. Children ≥ 2 years of age were classified as obese if the body mass index was ≥ 95th percentile, while children < 2 years of age were classified as obese if weight-for-length was ≥ 97.7th percentile [24, 25].
2.3 Outcomes
The primary outcomes included describing the percentage of pediatric patients who achieved the PK/PD target in the PICU setting. Secondary outcomes included assessing the correlation between voriconazole trough concentrations and the clinical/microbiological outcomes of pediatric patients in the PICU setting. Additional secondary outcomes focused on describing the voriconazole dosing regimens, adjustments, and safety.
For the primary outcomes, we assessed the percentage of patients who achieved the voriconazole therapeutic target trough concentration. The therapeutic range for voriconazole was defined as having a trough drawn at a minimum of 3 days post-starting treatment between 1 and 5.5 mg/L [9]. We defined a proper dose adjustment as increasing the dose for patients with low trough voriconazole concentrations or decreasing the dose for patients with high voriconazole trough concentrations. We also looked at factors contributing to high or low voriconazole trough concentrations. That included dose, weight, presence of drug interactions, and laboratory values such as alanine transaminase, aspartate transaminase, alkaline phosphatase, C-reactive protein, and albumin levels [26,27,28,29]. For drug interactions, interacting drugs were classified as either enzyme inhibitors or inducers.
For the secondary outcomes, we assessed the correlation between voriconazole trough concentrations and clinical or microbiologic outcomes and toxicity. For this analysis, we used the first trough concentration drawn for all patients. The clinical and microbiologic outcomes were evaluated for patients with invasive fungal infections. Invasive fungal infections were classified according to the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium treatment guidelines [30]. Treatment success was defined according to clinical, mycological, or radiologic responses. Complete response indicates the resolution of all clinical signs and symptoms attributable to the infection and radiographic resolution. Treatment failure was defined as a persistent infection > 14 days, or a progressing infection. Microbiological success was defined as the absence of the original pathogen in the culture of the baseline sample as reported in the subsequent cultures. All-cause mortality was defined as death occurring within the study period while the patient was taking voriconazole.
For toxicity, we looked at the relationship between voriconazole trough concentrations and toxicity. That included any documented voriconazole-associated toxicities such as neurological toxicity (e.g., visual disturbance, peripheral neuropathy, or audio or visual hallucinations) and hepatic toxicity.
2.4 Statistical Analysis
The baseline characteristics were analyzed with descriptive statistics. Quantitative variables were summarized as mean (standard deviation). Qualitative variables were summarized as frequencies and percentages. All of the statistical analysis was performed using R statistical software and Microsoft Excel. Multiple logistic regression was used to assess the predictors of both clinical and microbiologic cures. Multiple linear regression was used to determine significant factors associated with trough concentrations. The factors tested in the multiple linear regression model included dose, alanine transaminase, albumin levels, aspartate transaminase, alkaline phosphatase, bilirubin, C-reactive protein, and the presence of cytochrome P450 2C19 enzyme inducers or inhibitors. A p value < 0.05 was considered significant.
3 Results
3.1 Clinical Information
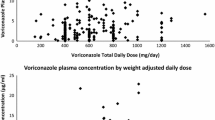
A total of 129 voriconazole trough concentrations were measured from 71 participants. Male individuals constituted 43 patients (60.6%) of the study group. The mean (± standard deviation) age (years), weight (kg), and height (cm) were 6.78 (± 1.19), 20.19 (± 12.07), and 111.97 (± 28.14), respectively (Table 1). The average body mass index was 14.93 ± 2.98. Thirty-three patients (44.5%) were underweight, four patients (5.6 %) were overweight, three patients (4.2%) were obese, and 31 patients (43.7%) were healthy weight. There were 71.8% of participants who had proven infections, 8.5% of participants had a probable infection, and 19.7% participants had a possible infection. For patients with culture-confirmed fungal infections, the most common isolated organisms were Aspergillus terreus and Aspergillus flavus. There were 45.1% of participants with neutropenia at the beginning of treatment. The average voriconazole dose was (7.33 ± 3.76) mg/kg. Fifty-nine participants had an appropriate initial dose (defined as 6–9 mg/kg). Conversely, 12 participants had an inappropriate initial dose. Ten out of 12 participants had drug interactions with the following medications (clarithromycin, cyclosporine, rifampin, tacrolimus, acyclovir, and amlodipine) and the second dose was adjusted accordingly if the patient did not have an adequate response, was unable to tolerate the dose, or adequate trough concentrations were not achieved. One of the remaining two participants started with 5.64 mg/kg without justification and the second dose was adjusted to 7.11 mg/kg. No explanation was found for the second patient. Voriconazole doses were given every 12 h. Participants were classified into the following groups according to the possible drug interactions: participants with a drug inducer (1%), participants with a drug inhibitor (66%), participants with each of two inducers and an inhibitor (1%), and participants with no drug interaction (32%). Baseline demographics of the 71 patients are presented in Tables 1 and 2.
3.2 Voriconazole Pharmacokinetics/Pharmacodynamics
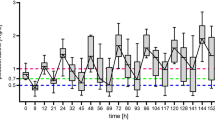
The first trough measurement was performed for all patients; however, the second trough measurement was performed for only 58 patients. The mean of the first trough plasma concentrations was 2.90 ± 4.22 mg/L (mean ± standard deviation) and ranged between 0.1 and 22.90 mg/L. The mean of the second trough plasma concentrations was 2.30 ± 3.3 mg/L (mean ± standard deviation) and ranged between 0.26 and 15.96 mg/L. Among the first measured trough concentrations, only 33.80% were within the therapeutic range (1–5 mg/L), 46.48% were below the therapeutic range, and 19.71% were above the therapeutic range. For the second measured trough concentrations, only 34.48% were within the therapeutic range, 53.45% were below the therapeutic range, and 12.07% were above the therapeutic range (Table 3). When assessing the correlation between voriconazole trough concentrations and patients demographics and variables (presence of drug interaction, dose, weight, C-reactive protein, albumin, alanine transaminase, and aspartate transaminase), only the dose was identified as a significant factor (R2 = 0.12, p < 0.05). Patients taking enzyme inhibitors had higher trough concentrations (3.14 vs 2.4 mg/L) versus patients not taking enzyme inhibitors. However, results were not statistically significant (p = 0.3).
3.3 Clinical and Microbiologic Outcomes
For patients who received voriconazole for treatment, a clinical cure occurred in 73% (41 out of 56) of patients. The average first voriconazole trough concentration was 3.5 mg/L in patients who achieved a clinical cure and 2.4 mg/L in patients who did not achieve a clinical cure; however, the results were not statistically significant (p = 0.39). For patients who achieved a clinical cure, 53% had low trough concentrations < 1 mg/L, while for patients who did not achieve a clinical cure, 66% had low trough concentrations < 1 mg/L (p = 0.38). A microbiologic assessment was available for 44 patients, a cure occurred in 80% of them. The average first voriconazole trough concentration was 3.7 mg/L in patients who achieved a microbiological cure and 1.6 mg/L in patients who did not achieve a microbiological cure; however, the results were not statistically significant (p = 0.23). For patients who did not achieve a clinical cure, 54% had low trough concentrations of < 1 mg/L, while for patients who did not achieve a microbiological cure, 45% had low trough concentrations of < 1 mg/L (p = 0.6).
3.4 Toxicity
Suspected voriconazole toxicity occurred in nine patients. We had seven patients with elevations in liver enzymes (three times upper the normal limit) and five of them switched from voriconazole to liposomal amphotericin B. The average voriconazole trough concentrations in patients with elevated liver enzymes was 5.4 mg/L versus 2.6 mg/L in patients who did not have elevations (p = 0.09). Three of the seven patients who had elevated liver enzymes had trough concentrations > 5.5 mg/L. The other two toxicities were one patient with QT prolongation and another patient with a type II heart block. Both toxicities occurred in patients who received another drug with cardiac side effects. The patient who developed QT prolongation was taking voriconazole and clarithromycin, while the patient who developed the heart block was taking voriconazole and levofloxacin.
4 Discussion
To our knowledge, this is the first study describing a voriconazole PK/PD target achieved and evaluating the efficacy and safety of voriconazole for the pediatric population admitted in the PICU setting. In our analysis, voriconazole trough concentrations displayed very high variability with a coefficient of variation above 100%. Additionally, only 33% were within the therapeutic range at the first measured trough and 34% at the second measured trough. Similarly, some studies have reported that around 34–36% of pediatric patients achieved the target concentration during the first measured trough [31, 32]. Recent studies have shown a higher percentage (about 55%) of pediatric patients reaching the therapeutic trough concentrations with initial doses [8, 33].
In our study, we noticed patients with lower trough concentrations had a higher treatment failure, and patients with higher troughs had increased voriconazole toxicity. It is important to note that our study’s results were not statistically significant. It could be because of the small sample size. Additionally, our patient population was critically ill pediatric patients, a heterogeneous group with high variability. This could have impacted our results. However, several previous studies in adult and pediatric patients have demonstrated this effect [9, 12, 13, 34, 35]. For example, in pediatric patients, the study by Choi et al. demonstrated that patients who experienced a treatment failure at week 6 of voriconazole treatment were more likely to have voriconazole concentrations below 1 mg/L (failure vs success, 42.1% vs 19.7%; p = 0.012) [36]. In another study, it was shown that steady-state voriconazole concentrations >3.6 mg/L were associated with an increased risk of hepatoxicity [37].
Given the high variability observed in voriconazole trough concentrations and its narrow therapeutic window, it is important to understand the variables impacting its pharmacokinetics to optimize its dosing. These factors include weight, age, cytochrome P450 2C19 enzyme inhibitors or inducers, genetic polymorphisms in cytochrome P450 2C19, and liver function [9]. In addition to the factors above, critical illness also impacts drugs pharmacokinetics [38]. For instance, several factors can affect drug distribution, such as endothelial dysfunction contributing to increased volume distribution, altered protein binding, fluid resuscitation, organ dysfunction, fluctuating regional blood flow, and cardiac output [39]. Healthcare practitioners need to consider all factors that might influence voriconazole pharmacokinetics for initial dosing. An approach that can handle these complexities to improve voriconazole dosing in pediatrics is model-informed precision dosing. This approach can be used for initial dosing or to adjust doses when trough concentrations are available using a Bayesian approach. The advantage of this approach is that it can take all variables known to impact voriconazole pharmacokinetics into account to calculate individualized dosing. It can also be used to optimize the monitoring of voriconazole using Bayesian pharmacokinetics [40, 41]. However, limited studies still evaluate the clinical usefulness of using this tool in clinical practice.
Our study has some limitations. First, the nature of the retrospective design may have resulted in some missing or undocumented information. As a result, we could not establish a causal relationship between the outcomes and voriconazole. To account for this, we did employ the use of multiple logistic and linear regression analyses to assess the predictors of both clinical and microbiologic cures and to determine significant factors associated with trough concentrations. Second, this study was conducted at a single center. Therefore, the results may not be generalizable to other institutions. Third, our study included a limited sample size. However, our sample size was comparable to other PK/PD studies [3, 15].
5 Conclusions
We noticed voriconazole trough concentrations vary widely in critically ill pediatric patients and only a third of the patients achieved therapeutic concentrations with initial doses.
References
Ferreras-Antolín L, Sharland M, Warris A. Management of invasive fungal disease in neonates and children. Pediatr Infect Dis J. 2019;38(6S Suppl. 1):S2–6.
Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1-60.
Hu L, Dai TT, Zou L, Li TM, Ding XS, Yin T. Therapeutic drug monitoring of voriconazole in children from a tertiary care center in China. Antimicrob Agents Chemother. 2018;62(12):e00955-e1018.
Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5):563–71.
Singh G, Pitoyo CW, Aditianingsih D, Rumende CM. Risk factors for early invasive fungal disease in critically ill patients. Indian J Crit Care Med. 2016;20(11):633–9.
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases: estimate precision. J Fungi (Basel). 2017;3(4):57.
Zhang MK, Rao ZG, Ma T, Tang M, Xu TQ, He XX, et al. Appropriate empirical antifungal therapy is associated with a reduced mortality rate in intensive care unit patients with invasive fungal infection: a real-world retrospective study based on the MIMIC-IV database. Front Med (Lausanne). 2022;9: 952611.
Valle-T-Figueras JM, Renedo Miró B, Benítez Carabante MI, Díaz-de-Heredia C, Vima Bofarull J, Mendoza-Palomar N, et al. Voriconazole use in children: therapeutic drug monitoring and control of inflammation as key points for optimal treatment. J Fungi (Basel). 2021;7(6):456.
Owusu Obeng A, Egelund EF, Alsultan A, Peloquin CA, Johnson JA. CYP2C19 polymorphisms and therapeutic drug monitoring of voriconazole: are we ready for clinical implementation of pharmacogenomics? Pharmacotherapy. 2014;34(7):703–18.
John J, Loo A, Mazur S, Walsh TJ. Therapeutic drug monitoring of systemic antifungal agents: a pragmatic approach for adult and pediatric patients. Expert Opin Drug Metab Toxicol. 2019;15(11):881–95.
Duehlmeyer S, Klockau C, Yu D, Rouch J. Characterization of therapeutic drug monitoring practices of voriconazole and posaconazole at a pediatric hospital. J Pediatr Pharmacol Ther. 2021;26(1):26–32.
Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, et al. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71(7):1772–85.
Tucker L, Higgins T, Egelund EF, Zou B, Vijayan V, Peloquin CA. Voriconazole monitoring in children with invasive fungal infections. J Pediatr Pharmacol Ther. 2015;20(1):17–23.
Miyakis S, van Hal SJ, Ray J, Marriott D. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect. 2010;16(7):927–33.
Boast A, Curtis N, Cranswick N, Gwee A. Voriconazole dosing and therapeutic drug monitoring in children: experience from a paediatric tertiary care centre. J Antimicrob Chemother. 2016;71(7):2031–6.
Chen J, Chan C, Colantonio D, Seto W. Therapeutic drug monitoring of voriconazole in children. Ther Drug Monit. 2012;34(1):77–84.
Lin XB, Huang F, Tong L, Xia YZ, Wu JJ, Li J, et al. Pharmacokinetics of intravenous voriconazole in patients with liver dysfunction: a prospective study in the intensive care unit. Int J Infect Dis. 2020;93:345–52.
Pieper S, Kolve H, Gumbinger HG, Goletz G, Würthwein G, Groll AH. Monitoring of voriconazole plasma concentrations in immunocompromised paediatric patients. J Antimicrob Chemother. 2012;67(11):2717–24.
Mori M, Fukushima K, Miharu M, Goto H, Yoshida M, Shoji S. A retrospective analysis of voriconazole pharmacokinetics in Japanese pediatric and adolescent patients. J Infect Chemother. 2013;19(1):174–9.
Gerin M, Mahlaoui N, Elie C, Lanternier F, Bougnoux ME, Blanche S, et al. Therapeutic drug monitoring of voriconazole after intravenous administration in infants and children with primary immunodeficiency. Ther Drug Monit. 2011;33(4):464–6.
Walsh TJ, Driscoll T, Milligan PA, Wood ND, Schlamm H, Groll AH, et al. Pharmacokinetics, safety, and tolerability of voriconazole in immunocompromised children. Antimicrob Agents Chemother. 2010;54(10):4116–23.
Gijsen M, Vlasselaers D, Spriet I, Allegaert K. Pharmacokinetics of antibiotics in pediatric intensive care: fostering variability to attain precision medicine. Antibiotics (Basel). 2021;10(10):1182.
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-93.
Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. Available online at: https://www.cdc.gov/healthyweight/bmi/calculator.html. Accessed 14 Jan 2022.
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva:World Health Organization (2006). Available online at: https://www.who.int/childgrowth/standards/technical_report/en/. Accessed Sept 2023.
Bury D, Tissing WJE, Muilwijk EW, Wolfs TFW, Brüggemann RJ. Clinical pharmacokinetics of triazoles in pediatric patients. Clin Pharmacokinet. 2021;60(9):1103–47.
Shi C, Xiao Y, Mao Y, Wu J, Lin N. Voriconazole: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2019;58(6):687–703.
van den Born DA, Märtson AG, Veringa A, Punt NC, van der Werf TS, Alffenaar JC, et al. Voriconazole exposure is influenced by inflammation: a population pharmacokinetic model. Int J Antimicrob Agents. 2023;61(4): 106750.
Li X, Lai F, Jiang Z, Li M, Chen Z, Cheng J, et al. Effects of inflammation on voriconazole levels: a systematic review. Br J Clin Pharmacol. 2022;88(12):5166–82.
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–76.
Bartelink IH, Wolfs T, Jonker M, de Waal M, Egberts TC, Ververs TT, et al. Highly variable plasma concentrations of voriconazole in pediatric hematopoietic stem cell transplantation patients. Antimicrob Agents Chemother. 2013;57(1):235–40.
Kang HM, Lee HJ, Cho EY, Yu KS, Lee H, Lee JW, et al. The clinical significance of voriconazole therapeutic drug monitoring in children with invasive fungal infections. Pediatr Hematol Oncol. 2015;32(8):557–67.
Lempers VJ, Meuwese E, Mavinkurve-Groothuis AM, Henriet S, van der Sluis IM, Hanff LM, et al. Impact of dose adaptations following voriconazole therapeutic drug monitoring in pediatric patients. Med Mycol. 2019;57(8):937–43.
Hanai Y, Hamada Y, Kimura T, Matsumoto K, Takahashi Y, Fujii S, et al. Optimal trough concentration of voriconazole with therapeutic drug monitoring in children: a systematic review and meta-analysis. J Infect Chemother. 2021;27(2):151–60.
Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–11.
Choi SH, Lee SY, Hwang JY, Lee SH, Yoo KH, Sung KW, et al. Importance of voriconazole therapeutic drug monitoring in pediatric cancer patients with invasive aspergillosis. Pediatr Blood Cancer. 2013;60(1):82–7.
Shen K, Gu Y, Wang Y, Lu Y, Ni Y, Zhong H, et al. Therapeutic drug monitoring and safety evaluation of voriconazole in the treatment of pulmonary fungal diseases. Ther Adv Drug Saf. 2022;13:20420986221127504.
Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient: concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11.
Charlton M, Thompson JP. Pharmacokinetics in sepsis. BJA Educ. 2019;19(1):7–13.
Hope WW, Vanguilder M, Donnelly JP, Blijlevens NM, Brüggemann RJ, Jelliffe RW, et al. Software for dosage individualization of voriconazole for immunocompromised patients. Antimicrob Agents Chemother. 2013;57(4):1888–94.
Hope W, Johnstone G, Cicconi S, Felton T, Goodwin J, Whalley S, et al. Software for dosage individualization of voriconazole: a prospective clinical study. Antimicrob Agents Chemother. 2019;63(4):e02353-e2418.
Acknowledgements
We thank Ms. Rania Aljaber, a senior pharmacy specialist from the pharmacy automation team at the King Faisal Specialist Hospital and Research Center, for her assistance in retrieving the patients’ data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the conduct of this study.
Conflicts of interest/competing interests
Khalid W. Taher, Razan Almofada, Sufyan Alomair, Ahmed A. Albassam, and Abdullah Alsultan have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was approved by the King Faisal Specialist Hospital & Research Centre Institutional Review Board (No. 2221242).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The King Faisal Specialist Hospital & Research Centre provided the study data; however, there are restrictions on its availability. These data were used under license for the current study and are not available publicly. However, upon reasonable request and with permission from the King Faisal Specialist Hospital & Research Centre, the authors could provide data.
Code availability
Not applicable.
Authors’ contributions
RA and SA worked on the data collection. AAA and AA worked on the data analysis. All authors worked on designing the study, writing the manuscript, and reviewing the final version of the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taher, K.W., Almofada, R., Alomair, S. et al. Therapeutic Drug Monitoring of Voriconazole in Critically Ill Pediatric Patients: A Single-Center Retrospective Study. Pediatr Drugs 26, 197–203 (2024). https://doi.org/10.1007/s40272-023-00616-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-023-00616-4