Abstract
Introduction
Biological therapies are valuable treatments for severe psoriasis. Children aged under 12 years are underrepresented in therapeutic trials for these drugs. The objective of the ‘BiPe Jr’ cohort study was to evaluate the drug survival, effectiveness, tolerance and switching patterns of biological therapies in children under 12 years of age with psoriasis.
Methods
We conducted a multicentre retrospective study of children with psoriasis who received at least one injection of a biological agent, even off-licence, before the age of 12 years in France and Italy, collecting the data between April and August 2021. The data collected were from March 2012 up to August 2021.
Results
In total, 82 children (mean age: 9.1 years; females: 61.0%) received 106 treatments. The drugs administered were adalimumab (n = 49), etanercept (n = 37), ustekinumab (n = 15), anakinra (n = 2), infliximab (n = 2) and secukinumab (n = 1). The most common form of psoriasis was plaque psoriasis (62.9%). The Physician Global Assessment and the Psoriasis Area Severity Index (PASI) scores decreased significantly from baseline to 3 months after treatment initiation for the three main biological drugs; PASI went from 14.1 ± 9.4 to 4.1 ± 11.3 for adalimumab (p = 0.001), 14.9 ± 9.3 to 5.1 ± 4.0 for etanercept (p = 0.002) and 11.6 ± 8.3 to 2.6 ± 2.2 for ustekinumab (p = 0.007). A trend towards higher 2-year maintenance rates was observed for ustekinumab and adalimumab, compared with etanercept (p = 0.06). 52 children discontinued their biological therapy, most frequently due to inefficacy (n = 28) and remission (n = 14). Seven serious adverse events (SAEs) were reported, including four severe infections.
Discussion
Our analyses of drug survival and treatment patterns, combined with those of previous studies conducted in older children, indicate that there is a trend towards higher 2-year survival rates of ustekinumab and adalimumab. The SAEs identified were rare, but highlight the need for increased vigilance concerning infections. Overall, the biological therapies showed good effectiveness and safety profiles when used in daily practice for the treatment of young children with psoriasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Overall, the main biological therapies evaluated in this study (i.e. adalimumab, etanercept and ustekinumab) in children with psoriasis showed good effectiveness and safety profiles. |
Results suggest a trend towards higher 2-year continuation rates of ustekinumab and adalimumab compared with etanercept. |
Serious adverse events were uncommon but highlight the need for increased vigilance concerning infections. |
1 Introduction
Psoriasis management has changed considerably since the advent of biological treatments, and continues to evolve with new drugs being licensed regularly. Biological drugs are indicated for the treatment of severe forms of psoriasis, and their effectiveness has been proven in both adult and paediatric populations [1,2,3]. However, data on the safety and effectiveness of these agents are lacking in younger children (i.e. patients aged under 12 years). Although national guidelines for the treatment of psoriasis in paediatric patients have been proposed in several countries, the exact role of biological agents in the management of children with severe psoriasis has not yet been clearly defined [3,4,5].
The conventional systemic agents used in clinical practice for the treatment of childhood psoriasis may include acitretin, cyclosporine and methotrexate, as well as fumaric acid esters in some countries [2,3,4,5,6,7]. In France and Italy, methotrexate is not licensed for use in children, and cyclosporine is only licensed for use in patients aged over 16 years [8]. Thus, in younger children, therapeutic choices in case of inefficacy of local treatments rapidly lead to the use of biological therapies. Data on the long-term and daily practice effectiveness of these drugs in young patients are therefore needed in order to better define the role of these highly effective drugs in the management of this population.
Five biological drugs are now licensed for use in children: two anti-tumour necrosis factor (TNF)-α agents (adalimumab and etanercept), an anti-interleukin 12-23 agent (ustekinumab) and two anti-interleukin 17 agents (secukinumab and ixekizumab). These agents are licensed for use in children aged 6 years or above, except for adalimumab, which can be used from the age of 4 years. However, prior to 2020, only the two anti-TNFα agents were authorized for use in children under 12 years.
Although the range of biological treatments available for use in young children with psoriasis is increasing, children under 12 years are underrepresented in clinical trials, and even when these patients are included, their data are generally not analysed independently [9,10,11,12]. One exception was the open-label CADMUS Jr study, in which 44 patients (aged ≥ 6 to < 12 years of age) were included to evaluate the effectiveness, safety, pharmacokinetic and biomarker results of ustekinumab treatment [13]. A retrospective cohort study evaluating the use of biological drugs in all children under the age of 18 years has also been conducted in daily practice settings in France (the BiPe study). Although this study assessed data from 134 children using 184 lines of therapy, less than a third of the children included were under the age of 12 years and no subpopulation analyses were made [14]. To fill this data gap, we have now performed a study on an extended BiPe cohort, involving children from both Italy and France being treated for psoriasis with biological agents, but including only children under 12 years of age (the BiPe Jr cohort). The aims of this study were to evaluate the effectiveness, tolerance and patterns of biological treatments of this young population in daily practice.
2 Materials and Methods
2.1 Study Design
This retrospective multicentre study was conducted by dermatologists practicing in 12 French and three Italian hospitals. All dermatologists who were members of the French (Société Française de Dermatologie Pédiatrique) and Italian (Società Italiana di Dermatologia Pediatrica) societies of paediatric dermatology, and the French Research Group on Psoriasis (GrPso) were invited to participate. They were invited to fill in a case report form about the characteristics and treatments of their paediatric patients with psoriasis. All data were collected anonymously, from April to August 2021. Updated data from children involved in the previous study (BiPe) who met the inclusion criteria for the BiPe Jr cohort were also included.
2.2 Inclusion and Exclusion Criteria
Children with psoriasis were included if they were under 12 years of age at the initiation of the biotherapy and had received at least one dose of a biological drug. Data from children who had received biotherapies through off-label prescriptions (i.e. due to clinical presentation or licencing age restrictions) were included. All types of cutaneous psoriasis were included (plaque psoriasis, guttate psoriasis, scalp psoriasis, acropulpitis, palmoplantar psoriasis whether in plaques or pustular, generalized pustular psoriasis, erythrodermia, napkin psoriasis). Children were excluded if they were receiving a biological agent as part of a therapeutic trial or if they were receiving biological therapy exclusively for the treatment of psoriatic arthritis. If a child who was included passed the age of 12 years, data related to their treatment after the age of 12 were not analysed.
2.3 Data Collected
The collected data were from March 2012 up to August 2021. At initiation of treatment with a first biological agent (baseline), the demographic data collected were the age, sex, body weight and height and medical history of the patients, including details of any treatments other than those being used for psoriasis, as well as details of any family history of psoriasis. Data on psoriasis characteristics were also collected at baseline and included the age of onset, clinical type, presence of nail and articular involvement, and current and previous treatments for psoriasis, as well as details of psoriasis severity based on Psoriasis Assessment Severity index (PASI) and Physician Global Assessment (PGA) scores. PGA and PASI were evaluated only for plaque psoriasis.
Data collected after treatment initiation with the first biological agent until either discontinuation of all biological agents or the patient reached > 12 years of age (follow-up) included the date of discontinuation of a biological treatment and of the initiation of subsequent treatments, details of associated treatments and causes of discontinuation, details of any serious adverse events (SAEs), and PASI and PGA scores at 3 (± 1) months (M3).
2.4 Outcomes
The main outcomes were analyses of the treatment patterns, including the frequency of prescription of each of the first-line biological agents, and the switching of these first-line agents to subsequent biological drugs. Treatment effectiveness, drug survival and reasons for discontinuation were also compared between agents.
2.5 Definitions
Body mass index categories and SAEs were defined as described previously for the BiPe cohort [14]. Treatment effectiveness was assessed for the three most commonly prescribed treatments by (1) the evolution of PGA and PASI scores between baseline and M3, (2) the number and percentage of children with PGA scores of 0 or 1 at M3 and (3) the number and percentage of children with a reduction in PASI scores from baseline of 50% (PASI 50) or 75% (PASI 75). Remission was defined as a PGA or PASI score of 0 reached after treatment initiation. Loss of efficacy was defined as a worsening of the psoriasis after a transient improvement. Primary inefficacy was defined as the absence of improvement of the psoriasis since treatment initiation.
2.6 Switching of Biological Treatments
A switch to another agent was defined as a change in biological treatment because of side effects, intolerance or absence of effectiveness, with a maximum gap of 4 months between the two treatments. If the gap between treatments was longer than 4 months, this was considered as a discontinuation and restart of biological therapy. Only switches performed before the patients reached the age of 12 years were included.
Sankey diagrams [15, 16] were used to represent and assess the flow between successive biological treatment steps and their frequency. In addition, sunburst diagrams [16] were used to illustrate successive biological treatment steps for each patient, allowing assessments of therapeutic sequences at an individual level. Only the first three lines of biological therapy were considered in these analyses.
For the most commonly prescribed agent, a comparison of drug survival was conducted between when the agent was used as a first-line biological therapy and when it was used as a second-line or third-line biological treatment.
2.7 Statistical Analysis
Quantitative data were expressed as means ± standard deviation and qualitative data as frequency and percentages. Comparisons of means between treatment groups were performed using the Student t-test. Comparisons of frequencies were performed using the chi-square test or Fisher exact test when necessary. The probability of continuing treatment with the initially prescribed agent was assessed using the Kaplan–Meier method. Curve comparisons were performed using the log-rank test. A p-value below 0.05 was considered statistically significant. All statistical analyses were performed using the R software, version 3.6.3 (http://www.r-project.org/, Vienna, Austria).
3 Results
3.1 Study Population
Eighty-two children were included in the study, cumulating in 106 lines of biological treatment. The clinical and psoriasis characteristics of the patients at baseline are detailed in Table 1. Fifty of the children were girls (61%), and the mean age at initiation of biological therapy was 9.1 ± 0.6 years. Among the patients who received their first biological therapy when they were below the age indicated in the licence for use for the agent, the age at initiation varied from 1.9 years for etanercept to 4.5 years for ustekinumab. Eleven children (16.7%) were overweight and five (7.6%) were obese. Plaque psoriasis was the most common psoriasis presentation (n = 49, 60.5%) followed by palmoplantar plaque psoriasis (n = 16, 19.8%) and guttate psoriasis (n = 9, 11.1%). The other clinical psoriasis forms reported were pustular psoriasis (n = 4), scalp psoriasis (n = 2) and erythrodermia (n = 1). The most common systemic treatments prescribed prior to initiation of biological therapy were acitretin (76.5%), methotrexate (43.8%) and cyclosporine (33.8%). There were no major statistical differences in prior treatments between biological therapy groups, except for a lower frequency of use of methotrexate before biological therapy in the patients who received ustekinumab (Table 1).
3.2 Biotherapies
Sixty-five children (79.3%) had only one biological therapy before the age of 12 years, 14 (17%) had two lines, one (1.2%) had three lines and one other child (1.2%) had six lines. The child who received six lines of therapy had a severe form of palmoplantar psoriasis. The following 106 drugs were prescribed: adalimumab (49 times), etanercept (37 times), ustekinumab (15 times), infliximab (twice), anakinra (twice) and secukinumab (once). The concomitant systemic treatments at initiation of biological therapy were acitretin (17.0%), methotrexate (5.7%) and cyclosporine (0.9%). Data are detailed in Table 2.
3.3 Drug Survival and Causes of Discontinuation
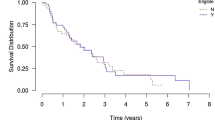
The cumulative duration of biological treatments in this cohort was 106.7 years, which correspond approximately to 15.6 months per patient. Two-year survival rates for the most commonly prescribed biological agents, adalimumab, etanercept and ustekinumab, are represented in Fig. 1a. Drug survival rates appeared higher for ustekinumab and adalimumab compared with etanercept, but these differences were not statistically significant (p = 0.06).
Kaplan–Meier curves of 2-year drug survival a for the three most frequently prescribed biological agents, adalimumab, etanercept and ustekinumab; and b for adalimumab when it was prescribed as a first-line biological therapy (Line 1) versus when it was prescribed as a second-line or third-line biological therapy (Line 23)
Biotherapies were discontinued in 52 cases. The three most common reasons for discontinuation were a loss of efficacy (19.8%), which was twice as frequent with etanercept as with adalimumab and ustekinumab (32.4% vs < 15%); remission of the psoriasis (13.2%) and primary inefficacy (8.5%). In four cases (3.8%), adverse events were given as the reason for stopping treatment. Data on drug discontinuation are shown in Table 3.
3.4 Effectiveness
Comparisons of mean PGA and PASI scores between baseline and M3 revealed that the use of all three of the most commonly prescribed agents led to significant reductions in psoriasis severity (Table 4). At baseline, the mean PASI scores seemed lower for the ustekinumab group (11.6 ± 8.3 vs 14.1 ± 9.4 for adalimumab and 14.9 ± 9.3 for etanercept). However, the PGA scores were similar between the three groups (3.8 ± 0.8 for adalimumab, 3.7 ± 0.9 for etanercept and 3.6 ± 0.9 for ustekinumab). PGA 0–1 at M3 was reached more frequently with adalimumab than with the other common treatments (72.0% vs 37.5% for ustekinumab, and 31.2% for etanercept; p = 0.02). PASI 50 was reached in 76.5% of children on adalimumab, 77.8% of children on ustekinumab and 62.5% of children on etanercept. PASI 75 and PASI 90 were more frequently reached for children on adalimumab (64.7% and 35.3%, respectively) compared with children on ustekinumab (44.4% and 11.1%, respectively) or etanercept (37.5% and 0%, respectively). Higher scores tended to be observed among patients treated with adalimumab, although no significant differences were observed between treatments (Table 4).
3.5 Treatment Patterns and Switches
Prescription patterns for the first three lines of biological drugs prescribed are represented in Fig. 2a and b. Distinct patterns of intraclass and interclass switches between first-line and second-line treatments were observed. Most notably, when etanercept was the first biological treatment prescribed, the switch to a second agent exclusively involved adalimumab. A similar trend was observed in cases where biological therapy was discontinued and restarted: another anti-TNFα agent was always reintroduced when etanercept was used as the first-line therapy. In contrast, the switches occurring when adalimumab was used as the first therapy always involved an interclass change, with the second-line treatment always being ustekinumab. Conversely, when the first biological therapy was ustekinumab, the second-line treatment was adalimumab.
Treatment patterns for biological therapies, including switches between agents, and discontinuation and reintroduction treatments. Only data for the first three lines of biological therapy are presented. a A Sankey diagram showing the flow and relative frequency of successive biological treatments. Each column represents a line of biological treatment. Treatments are ordered according to frequency, with the uppermost biological agent in each line of treatment being the most frequent. Switches between biological agents are shown and treatment discontinuation is represented by an intermediary column. b A sunburst diagram displaying the successive treatment steps for individual patients in a circular representation. The inner circle represents initial treatment
For the most frequently prescribed treatment, adalimumab, no significant differences in drug survival were observed between patients naive to biological therapy (i.e. receiving adalimumab as a first-line biological agent) and non-naive patients (i.e. those receiving adalimumab as a second-line or third-line biological agent) (p = 0.63; Fig. 1b).
3.6 Serious Adverse Events
SAEs are detailed in Table 5. Seven serious adverse events were reported, and six of these were considered as potentially linked to a biological treatment. Infections were the most common types of SAE (n = 4). An acute renal failure was reported in a child under adalimumab, not considered to be linked to the biological drug by the doctors in charge of the patient, but the drug was still discontinued afterwards. Most of the SAEs reported were seen in patients receiving anti-TNFα agents (adalimumab, n = 4; etanercept, n = 2), with the other SAE being observed in a patient prescribed anakinra.
4 Discussion
This retrospective multicentre study of 106 lines of biological treatments in 82 children with psoriasis provides valuable information on the safety and real-life effectiveness of these treatments. Most of the treatments received by our cohort of patients had been licensed for use in children (i.e. adalimumab, etanercept and ustekinumab). These three drugs were by far the most frequently prescribed biological drugs in our cohort and we focused our analyses on them. Overall, our analyses revealed a favourable safety profile and good effectiveness of these biological drugs.
The analysis of drug survival in our cohort of young patients suggested a trend towards higher maintenance of treatment with ustekinumab and adalimumab than with etanercept. Although the differences in drug survival observed in our current study were not statistically significant (p = 0.06), higher drug survival rates for ustekinumab and adalimumab compared with etanercept were observed in children aged < 18 years in the BiPe cohort study [14]. However, in contrast to the findings in the BiPe cohort, in the current study we did not observe any differences in drug survival between naive and non-naive children who received adalimumab [14]. Wan et al., using commercial insurance claims data, recently analysed the treatment of children with psoriasis in the United States from 2001 to 2016. They found that among new users, drug survival was greater for etanercept and ustekinumab than for methotrexate. Among biological agents, survival was found to be better for ustekinumab than for anti-TNFα agents [17].
The most frequent cause of treatment discontinuation identified in our study was inefficacy (loss of efficacy in 19.8% of cases and primary inefficacy in 8.5% of cases). These findings are similar to those of the BiPe cohort study, in which the two most common causes of discontinuation were loss of efficacy (19.2%) and primary inefficacy (8.9%) [14]. However, in our BiPe Jr cohort, the second most frequent cause of treatment discontinuation was remission (13.2%). A remission under these drugs appears to be a possible outcome in children, raising the question of a possible withdrawal, at least temporarily, as the psoriasis improves. However, we don’t know if the remission was due to the treatment or the spontaneous evolution of the psoriasis. Furthermore, as the children in our study were only followed up until they reached 12 years of age, we have no information on the need to reintroduce treatments later on. Longer follow-up and prospective cohort studies are needed to confirm these findings.
The biological drugs used appeared to be effective in our young cohort. Although effectiveness was assessed both by comparing mean PGA and PASI scores at baseline and 3 months and by comparing the number of children reaching PASI 50 and PASI 75 at 3 months, the results obtained need to be interpreted with caution due to missing data, particularly for children with non-plaque forms of psoriasis. However, analysis of the available data revealed a trend towards better effectiveness for adalimumab (PASI 75: 64.7%) compared with ustekinumab (PASI 75: 44.4%) and etanercept (PASI 75: 37.5%). Adalimumab treatment was also associated with the highest rate of children reaching PGA 0 or 1 at 3 months: 72.0% for adalimumab compared with 44.4% for ustekinumab and 37.5% for etanercept. The lower PASI score at baseline for the group under ustekinumab may also explain why this group less frequently reached PASI 75 than adalimumab (with no significant statistical difference), even though they both had a similar maintenance rate. Few other studies have assessed the effectiveness of these treatments in children in real-life practice. However, etanercept and adalimumab were found to be effective and well tolerated in real-life retrospective studies of children (aged from 1 to 16 years) with severe plaque psoriasis [18, 19]. Other studies have assessed the effectiveness and tolerance of biological drugs only in clinical trial settings [9,10,11,12,13], which do not reflect daily practice. Indeed, in the BiPe study, we showed that the majority of children treated with biological agents in real-life practice would not have been included in the phase III trials: 54.5% were ineligible for at least one of the randomized controlled trials based on the presence of one or more of the exclusion criteria. The most common criteria leading to exclusion were the clinical type of psoriasis, the disease severity being lower than required, and the use of prior or concomitant psoriasis treatments [20].
The results of our safety analyses were reassuring, with only a small number of SAEs being reported, and most of them being reversible. However, discontinuation of treatment was needed for four children because of adverse events. The profile of SAEs observed in our BiPe Jr cohort was similar to that described previously in older children and adults [7, 9,10,11,12,13,14, 21]. The majority of the SAEs reported so far with biological therapies have been associated with infections, justifying the need in children for preventative measures, including vaccinations, as is recommended in adults [22]. Another SAE reported in our study was body weight gain in a child receiving adalimumab. Body weight gain is a well-known side effect observed in adults treated with anti-TNFα agents, as well as in children with inflammatory bowel diseases receiving these therapies [23,24,25]. In adults with psoriasis, dietary interventions may help to limit the amount of body weight gained, and thus a similar approach could be proposed for children [23].
In our study, 19.4% of the children switched biological agents at least once, a level close to that observed in the BiPe cohort (22%) [26]. The majority of switches between biological agents involved only etanercept or adalimumab, and nearly all children treated with ustekinumab did not require switching to another biological treatment. However, it should be noted that ustekinumab was only recently licensed for use in children aged < 12 years and therefore tended to be introduced in older children for whom no follow-up data were analysed after they reached the age of >12 years. Our analysis of treatment switching highlighted two major treatment patterns: a high frequency of intraclass (anti-TNFα agent) switches, always involving changing from etanercept to adalimumab; and the occurrence of systematic interclass switches from adalimumab to ustekinumab. These treatment patterns may well reflect the chronology of changes to licences for use of biological agents in paediatric psoriasis: etanercept was the first agent to be licensed for use in children < 12 years of age, followed by adalimumab and ustekinumab. A few studies have assessed the effectiveness of specific patterns of switching biological agents, including both intraclass and interclass switches; however, these studies were only conducted on small numbers of patients and involved adult populations [27,28,29,30,31].
Relation between psoriasis, obesity and biologics is complex: (1) overweight/obesity is a significant comorbidity associated with psoriasis [32, 33]; (2) TNFα inhibitors can induce body weight gain [23]; (3) for some of these drugs, the management of overweight, but especially obese children is a challenge, as the standard dosage is adapted to weight but not to body fat, and the dosage may be inappropriate. This could explain the frequency of non-responders. For example, in our study, the percentage of overweight or obese children was a little bit higher in the etanercept group. It was also the drug that showed the worst efficacy in our analysis. We didn’t analyse conventional treatments in this study, thus we can hardly conclude on their place. A limitation of biological drugs compared with conventional treatments is the cost, which leads to the suggestion of first trying a conventional treatment, even if not licenced for children, provided that the child doesn’t present any contraindication. Indeed, the safety and efficacy profiles of methotrexate are reassuring in several published studies. The lack of data on the newly licenced biological drugs in young children < 12 years of age limits the recommendations on the use of secukinumab and ixekizumab and there isn’t enough data to establish strong guidelines on the place of ustekinumab. However, regarding the trend of better effectiveness and maintenance rate of adalimumab over etanercept and also its lower frequency of injections, we recommend trying adalimumab first. It is worth noting that tolerance and survival rates may be influenced by the frequency of administration, which varies depending on the biological drug. Ustekinumab has the lowest frequency of administration (every 12 weeks) compared with adalimumab and etanercept (every other week and every week, respectively).
The main limitation of our study was its retrospective design, which had the potential to introduce memory bias and led to missing data, most notably for severity scores. Due to the recent authorization of secukinumab and ixekizumab for children, data on their real-life prescription are scarce and need to be further assessed. Few children were prescribed ustekinumab, probably due to the recent extension of its licenced age, reducing the statistical power to detect differences between the three main biological drugs. Another limitation comes from the fact that the data were collected from different years, and thus, depending on the years the children were followed-up, some biological drugs were not available, which limited the alternative treatments for these patients. Therefore, the survival rate comparisons need to be interpreted with caution. Moreover, the evaluation of effectiveness was limited by the inadequacy of available assessment tools for evaluating non-plaque forms of psoriasis. Further studies on the real-life use of biological drugs are therefore needed to address these issues. Although our study has provided valuable insights into the role played by biological agents in the treatment of young children with psoriasis, two key points need to be addressed in the near future: (1) what will be the role of the anti-interleukin 17 agents, which were licensed in 2021, in the treatment of these patients, both as first-line biological treatments and as subsequent therapies after treatment switching; (2) specific guidelines on switching biological agents (most notably the interest in intraclass vs interclass switches) and strategies to improve prescribing practices are needed to improve the management of young patients with psoriasis. The findings of the current study will contribute to the implementation of these strategies.
5 Conclusion
Our retrospective French-Italian cohort study on the use of biological agents in children under 12 years of age provided several key insights for the management of these patients. Our findings suggested a tendency for higher, but not statistically significantly greater, survival rates for ustekinumab and adalimumab compared with etanercept. In addition, our study indicated that the use of biological drugs in younger children is safe and effective. Although infrequent, the most common SAEs reported involved infections in patients receiving anti-TNFα agents, emphasizing the need to be vigilant about the risks of infection in this population. Our study will contribute to the generation of much needed guidelines for the use and switching of biological agents in children with psoriasis.
References
Nast A, Gisondi P, Ormerod AD, et al. European S3—guidelines on the systemic treatment of psoriasis vulgaris—update 2015–Short version—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29:2277–94.
Mahé E, Amy-De La-Bretêque M, Phan C. Perspectives on the pharmacological management of psoriasis in pediatric and adolescent patients. Expert Rev Clin Pharmacol. 2021;14:807–19.
Menter A, Cordoro KM, Davis DMR, et al. Joint American academy of dermatology-national psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161–201.
Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents—short version part 2. J Dtsch Dermatol Ges. 2019;17:959–73.
Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group. Eur J Pediatr. 2017;176:1339–54.
Charbit L, Mahé E, Phan A, et al. Systemic treatments in childhood psoriasis: a French multicentre study on 154 children. Br J Dermatol. 2016;174:1118–21.
Bronckers IMGJ, Seyger MMB, West DP, et al. Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153:1147–57.
Mahé E, Corgibet F, Maccari F, et al. Prescriptions hors AMM (autorisation de mise sur le marché) dans le psoriasis de l’enfant. Ann Dermatol Venereol. 2020;147:429–38.
Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–51.
Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594–603.
Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40–9.
Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35:938–47.
Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664–72.
Phan C, Beauchet A, Burztejn AC, et al. Biological treatments for paediatric psoriasis: a retrospective observational study on biological drug survival in daily practice in childhood psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1984–92.
Lamer A, Laurent G, Pelayo S, et al. Exploring patient path through Sankey diagram: a proof of concept. Stud Health Technol Inform. 2020;270:218–22.
Roux J. Parcours de soins des patients atteints de sclérose en plaques à partir des données médico-administratives en France. 2018. https://tel.archives-ouvertes.fr/tel-02379451.
Wan J, Shin DB, Gelfand JM. Treatment utilization and drug survival of systemic medications among commercially insured children with psoriasis. Pediatr Dermatol. 2021;38:1169–77.
Di Lernia V, Bianchi L, Guerriero C, et al. Adalimumab in severe plaque psoriasis of childhood: a multi-center, retrospective real-life study up to 52 weeks observation. Dermatol Ther. 2019;32:e13091.
Di Lernia V, Guarneri C, Stingeni L, et al. Effectiveness of etanercept in children with plaque psoriasis in real practice: a one-year multicenter retrospective study. J Dermatolog Treat. 2017;18:1–3.
Phan C, Beauchet A, Burztejn AC, et al. Evaluation of children with psoriasis from the BiPe cohort: are patients using biotherapies in real life eligible for phase III clinical studies? Paediatr Drugs. 2019;21:169–75.
Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280–7.
Richard MA; Groupe de recherche sur le psoriasis de la Société Française de Dermatologie. Psoriasis: évaluation initiale et bilan thérapeutique pratique. Ann Dermatol Venereol. 2019;146:440–9.
Mahé E, Reguiai Z, Barthelemy H, et al. Evaluation of risk factors for body weight increment in psoriatic patients on infliximab: a multicentre, cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28:151–9.
Wu MY, Yu CL, Yang SJ, Chi CC. Change in body weight and body mass index in psoriasis patients receiving biologics: a systematic review and network meta-analysis. J Am Acad Dermatol. 2020;82:101–9.
Mazhar F, Battini V, Pozzi M, et al. Changes in anthropometric parameters after anti-TNFα therapy in inflammatory bowel disease: a systematic review and meta-analysis. BioDrugs. 2020;34:649–68.
Phan C, Beauchet A, Reguiai Z, et al. Switching biologics in children with psoriasis: results from the BiPe cohort. Pediatr Dermatol. 2022;39:35–41.
Bonifati C, Morrone A, Cristaudo A, Graceffa D. Effectiveness of anti-interleukin 23 biologic drugs in psoriasis patients who failed anti-interleukin 17 regimens. A real-life experience. Dermatol Ther. 2021;34:e14584.
Sherman S, Solomon Cohen E, Amitay-Laish I, et al. IL-17A inhibitor switching—efficacy of ixekizumab following secukinumab failure. A single-center experience. Acta Derm Venereol. 2019;99:769–73.
Chiricozzi A, Conti A, Burlando M, et al. Switching from secukinumab to ustekinumab in psoriasis patients: results from a multicenter experience. Dermatol Basel Switz. 2019;235:213–8.
Damiani G, Conic RRZ, de Vita V, et al. When IL-17 inhibitors fail: Real-life evidence to switch from secukinumab to adalimumab or ustekinumab. Dermatol Ther. 2019;32:e12793.
Tai YC, Tsai TF. Switching biologics in psoriasis—practical guidance and evidence to support. Expert Rev Clin Pharmacol. 2020;13:493–503.
Badaoui A, Tounian P, Mahé E. Psoriasis and metabolic and cardiovascular comorbidities in children: a systematic review. Arch Pediatr. 2019;26:86–94.
Phan K, Lee G, Fischer G. Pediatric psoriasis and association with cardiovascular and metabolic comorbidities: systematic review and meta-analysis. Pediatr Dermatol. 2020;37:661–9.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics Declarations
In France and Italy, an ethics declaration is not necessary for retrospective data. We only required “non-opposition for participating to the study”.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of interest
V. Di Lernia: AbbVie and Novartis. J. Gottlieb: AbbVie, Celgene, Lilly, Novartis, Janssen Cilag, and UCB. N. Quiles-Tsimaratos: AbbVie, Celgene, Janssen Cilag, Leo Pharma, Lilly, Novartis, Pfizer, and UCB. H. Barthelemy: AbbVie, JanssenCilag, Leo Pharma, and Pfizer. I. Neri: Janssen Cilag, Sanofi, and Lilly. E. Mahé: AbbVie, Amgen, Celgene, Janssen Cilag, Leo Pharma, Lilly, and Novartis.
Ethics approval
We required the “non-opposition for participating to the study”.
Consent to participate
We required the “non-opposition for participating to the study”.
Consent for publication
We required the “non-opposition for participating to the study”.
Availability of data and materials
Data available on request to E. Mahé.
Code availability
Not applicable.
Author contributions
All authors: interpretation of data; revised critically the work, approved the version to be published. Vito Di Lernia, Anne-Claire Bursztejn, Juliette Mazereeuw-Hautier, Jérémy Gottlieb, Audrey Lasek, Hélène Aubert, Catherine Droitcourt, Cristina Bulai-Livideanu, Anna Belloni Fortina, Francesca Caroppo, Nathalie Quiles-Tsimaratos, Stéphanie Mallet, Hugues Barthélémy, Eve Puzenat, Danielle Bouilly-Auvray, Iria Neri,and Céline Phan: acquisition of data. Alain Beauchet and Raphaëlle Curmin: statistical analysis. J Zitouni and Emmanuel Mahé: conception, design, acquisition, part of analysis, creation of network, wrote the article.
Rights and permissions
About this article
Cite this article
Zitouni, J., Beauchet, A., Curmin, R. et al. Effectiveness and Safety of Adalimumab, Etanercept and Ustekinumab for Severe Psoriasis in Children Under 12 Years of Age: A French-Italian Daily Practice Cohort (BiPe Jr). Pediatr Drugs 24, 281–292 (2022). https://doi.org/10.1007/s40272-022-00501-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00501-6