Abstract
Juvenile localized scleroderma (jLS) is an orphan disease that can lead to cosmetic disfiguration and orthopedic problems. Two recent publications review the current recommendations regarding diagnosis, assessment, follow up and treatment of pediatric localized scleroderma cases, both of which suggest the Localized Scleroderma Cutaneous Assessment Tool as an important instrument to assess activity and damage. This review focuses on the systemic treatment of jLS. Systemic treatment includes synthetic and biologic disease-modifying antirheumatic drugs. Systemic therapy is indicated if the lesion crosses any joint, or leads to potential cosmetic disfiguration or orthopedic problems. The only controlled trial of systemic treatment has shown the efficacy of methotrexate, which is the first choice of treatment. It appears superior to phototherapy according to a recently published meta-analysis. In case of methotrexate intolerance, mycophenolate mofetil is an option. In case of methotrexate nonresponse, addition of mycophenolate mofetil, tocilizumab or abatacept seems to be effective. Future treatment options derived and extrapolated from adult trials regarding treatment of skin involvement of systemic scleroderma or fibrosis are promising, as the final pathway in the skin seems to be similar in both diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
LoSCAT is the most validated and used instrument to assess cutaneous activity. |
It is important to assess extracutaneous activity such as arthritis, uveitis, and CNS involvement regularly. |
The aim of therapy is to reach inactive disease; therefore, therapy should be escalated to reach the state of inactive disease as quickly as possible to prevent damage. |
1 Introduction
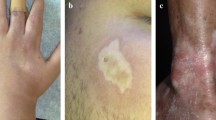
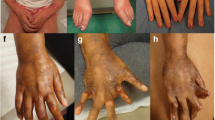
Juvenile localized scleroderma (jLS) is an orphan disease, with an estimated incidence rate of 3.4 per million children per year (95% confidence interval [CI] 2.7–4.1) [1]. The estimated prevalence per year ranged from 3.2 to 3.6 per 10,000 children in the US claims database [2]. The most used and recognized classification for jLS was published in 2006 by Laxer and Zulian [3]. It is an autoimmune disease, where profibrotic mechanisms play a key role [4]. The term LS includes a spectrum of sclerosing diseases of the skin, but may also involve neighboring tissues such as fascia, muscle, bone, and underlying tissues. Unlike systemic scleroderma, however, there is no significant involvement of internal organs in LS, other than musculoskeletal, ocular, and CNS involvement. There are similarities in the end pathways of systemic and localized scleroderma and the skin biopsy findings are similar. Fibrosis occurs similarly in both systemic and localized scleroderma, with extensive extracellular matrix formation and autoimmune dysfunction thought to be key pathogenic processes. Supporting the theory of a common pathway are, for example, the demonstration of CCL18 chemokine and other cytokines as biomarkers, reflecting activity of inflammation and angiogenesis [5, 6].
Treatment can consist of local or systemic therapy, and several groups have proposed recommendations for systemic treatment in jLS. The Hamburg Scleroderma Consensus Group (HSCG) consists of pediatric rheumatologists from the Pediatric Rheumatology European Society (PRES) Scleroderma working group and pediatric dermatologists with a research interest in jLS. The HSCG recommended treatment with systemic medication if lesions are crossing a joint, lead to cosmetic changes, and/or lead to a potential limb length discrepancy [7]. The Single Hub and Access point for pediatric Rheumatology in Europe (SHARE) [8] developed recommendations for follow up and treatment for jLS [9] and absolutely agreed with the HSCG recommendations. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) group also made suggestions regarding indications for systemic treatment in their consensus treatment plan [10]. According to CARRA, systemic treatment is needed in patients with high severity and moderate severity disease. High severity patients were defined as “presentation with generalized or pansclerotic morphea, craniofacial linear scleroderma (en coup de sabre), or other subtype with evidence of high morbidity (e.g. central nervous system involvement, extremity shortening, joint contracture)”. Moderate severity was defined as “circumscribed deep morphea or linear scleroderma of the trunk or extremity without evidence of high morbidity”. According to the SHARE [8] and HSCG [7] recommendations, patients needing systemic treatment should be followed by a pediatric rheumatologist in cooperation with a pediatric dermatologist specializing in jLS.
In this review, the focus is on systemic treatment of jLS, which is most commonly prescribed by pediatric rheumatologists [11]. The selection of papers was based on a PubMed search of papers published in the last 10 years with the keywords ‘localized scleroderma’, ‘children’, and ‘treatment’. Papers that reported controlled trials or case series were included. Some case reports in which a new rescue therapy was reported were also included.
2 What is the Aim of the Current Therapy?
The aim of jLS treatment is to attain inactive disease, and to prevent damage and disease progression. Mertens et al. [5] suggest that a “window of therapeutic opportunity” exists for jLS; thus, early effective treatment before damage occurs is important. International guidance also recommended early control of both cutaneous and extracutaneous manifestations [7, 9]. Extracutaneous manifestations associated with jLS should be treated according to treatment guidelines for the given association (e.g., uveitis, arthritis, seizures) (evidence grade 4 D) [7].
3 How Do We Assess Disease Activity?
The Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) [12] was established to assess cutaneous activity (using the modified Localized Scleroderma Skin Severity Index [mLoSSI]) and damage (using the Localized Scleroderma Skin Damage Index [LoSDI]) in jLS. It contains two visual analog scales from 0 to 100 to rate physician global assessment of disease activity and global disease damage. The use of LoSCAT is recommended at every follow-up by both the HSCG and the SHARE groups [7, 9]. According to the HSCG, LoSCAT is an “acceptable tool for capturing cutaneous disease activity and damage” (evidence grade 2, B). The LoSCAT has been shown to be responsive to change in disease [13]. Doppler ultrasound seems to be a promising tool to assess jLS lesion activity, especially on the face [14].
Activity of extracutaneous involvement should also be assessed. For arthritis, a full joint count, including the temporomandibular joint, is suggested at every clinic assessment, as arthritis or even enthesitis can occur, even in anatomical regions with no skin lesions. Uveitis screening is suggested for ‘white anterior uveitis’ every 6 months in patients with facial skin involvement and every 12 months with non-facial skin involvement (evidence grade 4, D) [7]. For patients with lesions of the head, assessment of CNS involvement should occur at baseline with MRI of the CNS and after that depending on clinical presentation [7].
4 What are the Systemic Treatment Options?
Under systemic treatment in this review, we consider synthetic and biologic disease-modifying antirheumatic drugs (DMARDs) [15]. Most data focus on conventional DMARDs (csDMARDs) such as methotrexate and mycophenolate mofetil (MMF). There are case series or reports suggesting the efficacy of biologic DMARDs (bDMARDs). There is nearly no data regarding targeted synthetic DMARDs (tsDMARDs); these are presented as future options in Section 5.
4.1 Methotrexate (MTX)
The mechanism of action of low-dose (15 mg/m2 body surface area/week) methotrexate (MTX) has not been completely clarified, although multiple intracellular levels of the effect of MTX have been suggested, such as the immunomodulating effect on interleukin-6 production and other cytokines. MTX is the only medication for which a prospective, placebo-controlled trial has been conducted and proved the efficacy of the drug in the target lesions [16]. In the prospective study of Zulian et al. [16], oral MTX 15 mg/m2 body surface area/week over 12 months was used, but with a maximum dose of 20 mg/week, which is less than the currently used maximum dose of 25 mg/week. The response to treatment was defined as the absence of new lesions, skin score rate (SSR) ≤ 1, and a decrease in lesion temperature of at least 10% compared with baseline. Treatment failure was defined as the occurrence of new lesions, SSR > 1, or increased lesion temperature. All analyses in this study were done on an intent-to treat basis. Seventy patients were randomized, with three subtypes of jLS (linear, generalized, and mixed) [3]. The study showed the superiority of the MTX arm over placebo. In thermography, after 9 and 12 months there was a significant improvement in the MTX arm compared with the placebo arm. Thermography is not a universally available assessment tool; it is quite expensive and can be falsely positive in burned out lesions with subcutaneous atrophy. As pointed out in the editorial accompanying this trial, the lack of significant change in several of the parameters studied was likely related to the small size of the study population [17]. The definition of response did not include some parameters of the LoSCAT [12, 18]. In the long-term extension of this study, with a median observation period of 30 months, 32 of the 58 patients still under follow-up were in clinical remission off medication [19]. In this study, extracutaneous involvement of the disease was not evaluated.
Based on the controlled study data and positive clinical experience, MTX is recommended as the first-choice systemic treatment for jLS (Fig. 1). It is the suggested treatment in the CARRA consensus treatment plans [10]. These treatment arms were developed by a core group of pediatric rheumatologists, dermatologists, and a lay advisor, who were engaged by CARRA to develop standardized treatment plans and assessment parameters for jLS using consensus methods/nominal group techniques. A meta-analysis showed MTX therapy had superior efficacy over phototherapy for treating children with jLS [20]. MTX is used in around 90–95% of patients as first choice of therapy in clinical practice [21, 22].
MTX is known to be effective in juvenile idiopathic arthritis (JIA)-associated uveitis [23, 24], which resembles jLS-associated uveitis. It is also the first-choice treatment for jLS-associated arthritis. jLS-associated uveitis behaves similarly to JIA-associated uveitis and is an asymptomatic ‘white uveitis’ [25]. MTX seems to be effective in jLS-associated CNS involvement as well [26].
MTX can be used as monotherapy or with a bridging regimen of oral or intravenous pulsed methylprednisolone at the initiation of MTX treatment. The CARRA consensus treatment plan [10] offers both options and is based on the personal preference of the treating physician [27]. The prospective study of Zulian et al. [16] used a bridging regimen of oral glucocorticoids. In the proposal of the HSCG [7], bridging therapy with methylprednisolone is recommended for a minimum of 3 months while MTX induction occurs (evidence grade 4, D) [7]. The debate is still ongoing regarding how long and what dose of glucocorticoid bridging is really needed.
4.1.1 When Should MTX be Discontinued in Case of Reaching Inactive Disease?
In two-thirds of cases, MTX therapy seems to achieve inactive disease [22]; however, there remains a question of when MTX can be weaned or stopped. The HSCG [7] suggested that systemic treatment should not be stopped before 12 months of inactive disease. Prospective data is missing to support this suggestion. Some patients even flare on an effective dose of MTX or MMF treatment according to data from the National Registry of Childhood Onset Scleroderma (NRCOS) [28]. Linear lesions on the limbs seem to be a risk factor for recurrence of the disease [29] and disease can reoccur after years of inactivity [30].
4.1.2 When Should MTX Treatment be Changed or Another Treatment Added?
In the national UK audit regarding assessment and treatment of jLS, 95.5% of the 149 patients were treated with MTX as first-line therapy and 34.2% received two or more DMARDs [22]. It seems that only two-thirds of patients responded to MTX, which is a similar response rate to the prospective study of Zulian et al. [16]. In the case of nonresponse after 3 months of treatment, or if disease inactivity is not reached after 6 months of treatment, the HSCG recommendations [7] suggest that systemic treatment should be changed (evidence grade 4, D). The HSCG suggests several options [7], although there is currently limited evidence for second-line therapies for MTX non-responders. Options include MMF, either switching to or in addition to MTX (evidence grade 3, C), or adding bDMARDs such as abatacept and infliximab (evidence grade 4, D—extrapolated from adult case series, with no pediatric evidence available).
4.2 Mycophenolate Mofetil (MMF)
MMF targets the T lymphocyte pathway [31]. There is limited data published about the effectiveness of MMF in jLS. MMF can be used in cases of MTX intolerance [32] or MTX nonresponse [33]. According to the UK audit, it was the most commonly used second-line treatment in clinical practice, used in 89.5% of cases [22]. The CARRA consensus treatment plans [10] suggest MMF in the case of MTX intolerance or nonresponse. The applied dose is according to the suggestion of the US Food and Drug Administration (FDA) dosing recommendations for pediatric renal disease. MMF dosing is according to the following protocol: < 1.25 m2 body surface area: 600 mg/m2 twice daily; 40–50 kg or 1.25–1.5 m2 body surface area: 750 mg twice daily; > 50 kg or > 1.5 m2 body surface area: 1000 mg twice daily.
In the HSCG recommendation, it is stated that “There is currently limited evidence for second line therapies for MTX non-responders. Options include MMF (either switching or in addition to MTX) (evidence grade 3, C)”. MMF presumably has the same effectiveness as MTX monotherapy, but it has less effect on arthritis, the most significant extracutaneous involvement of the disease [34]. It seems to have similar effects to MTX on uveitis [35].
4.3 Tocilizumab
Tocilizumab is a monoclonal antibody raised against the soluble receptor for interleukin (IL)-6. It is approved to treat juvenile idiopathic polyarticular and systemic arthritis in pediatric rheumatology. Data from basic research suggests that IL-6 inhibition can be an effective target in LS, as serum levels of soluble IL-6R have been found to be increased in patients with LS compared with healthy controls. Increased serum levels of soluble IL-6R correlated with the number of linear lesions and the number of involved body areas [36, 37]. In an adult phase II study of systemic sclerosis, tocilizumab showed good efficacy regarding skin involvement [38, 39]. In the three published case series, including 18 therapy-resistant jLS cases where mostly MTX and MMF had already been tried, subcutaneous or intravenous tocilizumab showed a promising response rate [40,41,42]. The applied dose of tocilizumab was adopted from the polyarticular JIA treatment protocol [43] or from the systemic JIA treatment schedule [44]. Further studies are needed to determine whether the higher dosing schedule used in systemic JIA treatment is needed. It could be that tocilizumab would show a greater efficacy if applied after MTX partial or nonresponse as a second-line agent. Tocilizumab is a promising effective choice for extracutaneous involvement of jLS including arthritis and uveitis [45, 46]. In one of the case series, a patient with CNS involvement [40] responded. Another recent review found similar results [47].
4.4 Abatacept
Abatacept is a soluble recombinant fusion protein that inhibits T-cell activation by binding to CD80 and CD86, thereby blocking interaction with CD28. Abatacept seems to be a promising drug to treat skin involvement in systemic scleroderma [48, 49]. The recently published, double-blind, phase II trial [50] for early systemic sclerosis in adults showed promising results. A decline in the modified Rodnan Skin Score (mRSS) over 12 months was clinically and significantly higher in abatacept versus placebo for the inflammatory (p < 0.001) and normal-like skin gene expression subsets (p = 0.03). Abatacept also showed a promising effect in 18 adult patients [51,52,53] with localized scleroderma. These results can be extrapolated to pediatric patients as the pathophysiology of the disease is assumed to be the same in adult and pediatric populations. No pediatric data has been published yet. Abatacept as a subcutaneous injection was recently licensed for polyarticular JIA, in weight-adjusted doses [54]. This makes abatacept a more applicable therapy option for MTX nonresponders.
4.5 Rituximab
Rituximab is a chimeric monoclonal antibody targeting human CD20 on mature B cells. Rituximab is a promising unlicensed treatment for skin involvement in systemic sclerosis [55]. There is only one adult case report published for localized scleroderma [56, 57]. Rituximab is approved for the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides. It has only recently been licensed for pediatric use in granulomatosis with polyangiitis and microscopic polyangiitis in the US; therefore, it is not a first-choice therapy in children with MTX-resistant jLS.
4.6 Infliximab
Infliximab is a chimeric anti-tumor necrosis factor (TNF) monoclonal antibody. Infliximab has not proved to be effective in the skin involvement of systemic scleroderma [57, 58]. Three case reports demonstrated a positive result in localized scleroderma, one of them in a child [59] and two in adults [60, 61]. The adult patients were treated with several other drugs simultaneously, so it is hard to judge which led to the positive result.
4.7 Imatinib
Imatinib is a tyrosine kinase inhibitor that failed to show efficacy in a controlled trial for systemic scleroderma [62]. There are four published case reports in localized scleroderma [63,64,65], one of them in a child [66]. Imatinib is only licensed for children with chronic myeloid leukemia (CML). It does not seem a real therapeutic option for children with localized scleroderma.
5 Future Potential Therapies
There are several drugs in the pipeline to treat systemic scleroderma with an effect on skin involvement. As the final pathway in the skin has lots of similarities between localized and systemic scleroderma, it might be reasonable to extrapolate the effects in systemic scleroderma to localized scleroderma. The drugs in the pipeline are belimumab (a B-cell activating factor [BAFF] inhibitor), riociguat (an activator of soluble guanylate cyclase [sGC], which catalyzes the production of cyclic guanosine monophosphate [cGMP]), dabigatran (a selective thrombin inhibitor), coagulation Factor XIII, fresolimumab (a G class immunoglobulin [IgG]-4 kappa targeting transforming growth factor-β), and tyrosine kinase inhibitors such as tofacitinib and baricitinib [48].
6 Conclusion
jLS is an orphan disease. The treatment goals are to stop progression of the disease, prevent damage, improve quality of life, and achieve inactive disease. MTX with or without bridging glucocorticoids for the first 3 months is first-line treatment. If MTX intolerance occurs, MMF can be considered. Treatment options for MTX nonresponse include adding MMF, tocilizumab, or abatacept to MTX, or substituting MTX with tocilizumab or abatacept. Further studies are needed to explore the best possible therapy. The CARRA consensus treatment plan is one of the research projects aiming to gain more knowledge regarding the efficacy of different treatment options. In future studies, it is important to incorporate the response of extracutaneous involvement and quality of life of patients in the assessment of the efficacy of the therapy.
References
Herrick AL, Ennis H, Bhushan M, Silman AJ, Baildam EM. Incidence of childhood linear scleroderma and systemic sclerosis in the UK and Ireland. Arthritis Care Res (Hoboken). 2010;62(2):213–8.
Beukelman T, Xie F, Foeldvari I. The prevalence of localised scleroderma in childhood assessed in the administrative claims data from the United States. J Scleroderma Relat Disord. 2018;3(2):189–90.
Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18(6):606–13.
Saracino AM, Denton CP, Orteu CH. The molecular pathogenesis of morphoea: from genetics to future treatment targets. Br J Dermatol. 2017;177(1):34–46.
Mertens JS, de Jong E, van den Hoogen LL, Wienke J, Thurlings RM, Seyger MMB, et al. The identification of CCL18 as biomarker of disease activity in localized scleroderma. J Autoimmun. 2019;101:86–93.
Torok KS, Kurzinski K, Kelsey C, Yabes J, Magee K, Vallejo AN, et al. Peripheral blood cytokine and chemokine profiles in juvenile localized scleroderma: T-helper cell-associated cytokine profiles. Semin Arthritis Rheum. 2015;45(3):284–93.
Constantin T, Foeldvari I, Pain CE, Palinkas A, Hoger P, Moll M, et al. Development of minimum standards of care for juvenile localized scleroderma. Eur J Pediatr. 2018;177(7):961–77.
Wulffraat NM, Vastert B, Consortium S. Time to share. Pediatr Rheumatol Online J. 2013;11(1):5.
Zulian F, Culpo R, Sperotto F, Anton J, Avcin T, Baildam EM, et al. Consensus-based recommendations for the management of juvenile localised scleroderma. Ann Rheum Dis. 2019;2(78):1019–24.
Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64(8):1175–85.
Hawley DP, Pain CE, Baildam EM, Murphy R, Taylor AE, Foster HE. United Kingdom survey of current management of juvenile localized scleroderma. Rheumatology (Oxford). 2014;53(10):1849–54.
Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA Jr. Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford). 2010;49(2):373–81.
Kelsey CE, Torok KS. The Localized Scleroderma Cutaneous Assessment Tool: responsiveness to change in a pediatric clinical population. J Am Acad Dermatol. 2013;69(2):214–20.
Murray AK, Moore TL, Manning JB, Dinsdale G, Wilkinson J, Bhushan M, et al. Non-invasive imaging of localised scleroderma for assessment of skin blood flow and structure. Acta Derm Venereol. 2016;96(5):641–4.
Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewe R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis. 2014;73(1):3–5.
Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998–2006.
Foeldvari I. Methotrexate in juvenile localized scleroderma: adding to the evidence. Arthritis Rheum. 2011;63(7):1779–81.
Arkachaisri T, Vilaiyuk S, Li S, O’Neil KM, Pope E, Higgins GC, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. J Rheumatol. 2009;36(12):2819–29.
Zulian F, Vallongo C, Patrizi A, Belloni-Fortina A, Cutrone M, Alessio M, et al. A long-term follow-up study of methotrexate in juvenile localized scleroderma (morphea). J Am Acad Dermatol. 2012;67(6):1151–6.
Marrani E, Foeldvari I, Lopez JA, Cimaz R, Simonini G. Comparing ultraviolet light A photo(chemo)therapy with methotrexate protocol in childhood localized scleroderma: evidence from systematic review and meta-analysis approach. Semin Arthritis Rheum. 2018;48(3):495–503.
Wu EY, Li S, Torok KS, Virkud YV, Fuhlbridge RC, Rabinovich CE. Baseline description of the Juvenile Localized Scleroderma Subgroup from the Childhood Arthritis and Rheumatology Research Alliance Legacy Registry. ACR Open Rheumatol. 2019;1:119–24.
Lythgoe H, Almeida B, Bennett J, Bhat C, Bilkhu A, Brennan M, et al. Multi-centre national audit of juvenile localised scleroderma: describing current UK practice in disease assessment and management. Pediatr Rheumatol Online J. 2018;16(1):80.
Foeldvari I, Wierk A. Methotrexate is an effective treatment for chronic uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2005;32(2):362–5.
Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom-Guillaume S, Edelsten C, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018;77(8):1107–17.
Zannin ME, Martini G, Athreya BH, Russo R, Higgins G, Vittadello F, et al. Ocular involvement in children with localised scleroderma: a multi-centre study. Br J Ophthalmol. 2007;91(10):1311–4.
Anderson LE, Treat JR, Licht DJ, Kreiger PA, Knight AM. Remission of seizures with immunosuppressive therapy in Parry–Romberg syndrome and en coup de sabre linear scleroderma: case report and brief review of the literature. Pediatr Dermatol. 2018;35(6):e363–5.
Li SC, Fuhlbrigge RC, Laxer RM, Pope E, Ibarra MF, Stewart K, et al. Developing comparative effectiveness studies for a rare, understudied pediatric disease: lessons learned from the CARRA juvenile localized scleroderma consensus treatment plan pilot study. Pediatr Rheumatol Online J. 2019;17(1):43.
Kurzinski KL, Zigler CK, Torok KS. Prediction of disease relapse in a cohort of paediatric patients with localized scleroderma. Br J Dermatol. 2019;180(5):1183–9.
Mertens JS, Seyger MM, Kievit W, Hoppenreijs EP, Jansen TL, van de Kerkhof PC, et al. Disease recurrence in localized scleroderma: a retrospective analysis of 344 patients with paediatric- or adult-onset disease. Br J Dermatol. 2015;172(3):722–8.
Litaiem N, Bacha T, Drissi H, Zeglaoui F. An evaluation of long-term outcomes and recurrence rates in patients with morphea. Int J Dermatol. 2019;58(4):E90–2.
Ozgen M, Koca SS, Dagli AF, Gundogdu B, Ustundag B, Isik A. Mycophenolate mofetil and daclizumab targeting T lymphocytes in bleomycin-induced experimental scleroderma. Clin Exp Dermatol. 2012;37(1):48–54.
Mertens JS, Marsman D, van de Kerkhof PC, Hoppenreijs EP, Knaapen HK, Radstake TR, et al. Use of mycophenolate mofetil in patients with severe localized scleroderma resistant or intolerant to methotrexate. Acta Derm Venereol. 2016;96(4):510–3.
Martini G, Ramanan AV, Falcini F, Girschick H, Goldsmith DP, Zulian F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology (Oxford). 2009;48(11):1410–3.
Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheumatol. 2005;52(9):2873–81.
Rathinam SR, Gonzales JA, Thundikandy R, Kanakath A, Murugan SB, Vedhanayaki R, et al. Effect of corticosteroid-sparing treatment with mycophenolate mofetil vs methotrexate on inflammation in patients with uveitis: a randomized clinical trial. JAMA. 2019;322(10):936–45.
Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Arch Dermatol Res. 1995;287(2):193–7.
Nagaoka T, Sato S, Hasegawa M, Ihn H, Takehara K. Serum levels of soluble interleukin 6 receptor and soluble gp130 are elevated in patients with localized scleroderma. J Rheumatol. 2000;27(8):1917–21.
Khanna D, Denton CP, Lin CJF, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis. 2018;77(2):212–20.
Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–40.
Foeldvari I, Anton Lopez J, Friswell M, Bica B, de Inocencio J, Aquilani A, et al. Tocilizumab is a promising treatment option for therapy resistant juvenile localized scleroderma. J Scleroderma Relat Disord. 2017;2(3):203–7.
Martini G, Campus S, Raffeiner B, Boscarol G, Meneghel A, Zulian F. Tocilizumab in two children with pansclerotic morphoea: a hopeful therapy for refractory cases? Clin Exp Rheumatol. 2017;35 Suppl 106(4):211–3.
Lythgoe H, Baildam E, Beresford MW, Cleary G, McCann LJ, Pain CE. Tocilizumab as a potential therapeutic option for children with severe, refractory juvenile localized scleroderma. Rheumatology (Oxford). 2018;57(2):398–401.
Brunner HI, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase 3, randomised, double-blind withdrawal trial. Ann Rheum Dis. 2015;74(6):1110–7.
De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2385–95.
Tappeiner C, Mesquida M, Adan A, Anton J, Ramanan AV, Carreno E, et al. Evidence for tocilizumab as a treatment option in refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2016;43(12):2183–8.
Calvo-Rio V, Santos-Gomez M, Calvo I, Gonzalez-Fernandez MI, Lopez-Montesinos B, Mesquida M, et al. Anti-interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthritis Rheumatol. 2017;69(3):668–75.
Pena-Romero AG, Garcia-Romero MT. Diagnosis and management of linear scleroderma in children. Curr Opin Pediatr. 2019;31(4):482–90.
Bruni C, Praino E, Allanore Y, Distler O, Gabrielli A, Iannone F, et al. Use of biologics and other novel therapies for the treatment of systemic sclerosis. Expert Rev Clin Immunol. 2017;13(5):469–82.
Elhai M, Meunier M, Matucci-Cerinic M, Maurer B, Riemekasten G, Leturcq T, et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann Rheum Dis. 2013;72(7):1217–20.
Khanna D, Spino C, Johnson S, Chung L, Whitfield M, Denton CP, et al. Abatacept in early diffuse cutaneous systemic sclerosis—results of a phase 2 investigator-initiated, multicenter, double-blind randomized placebo-controlled trial. Arthritis Rheumatol. 2019. https://doi.org/10.1002/art.41055.
Adeeb F, Anjum S, Hodnett P, Kashif A, Brady M, Morrissey S, et al. Early- and late-stage morphea subtypes with deep tissue involvement is treatable with Abatacept (Orencia). Semin Arthritis Rheum. 2017;46(6):775–81.
Stausbol-Gron B, Olesen AB, Deleuran B, Deleuran MS. Abatacept is a promising treatment for patients with disseminated morphea profunda: presentation of two cases. Acta Derm Venereol. 2011;91(6):686–8.
Fage SW, Arvesen KB, Olesen AB. Abatacept improves skin-score and reduces lesions in patients with localized scleroderma: a case series. Acta Derm Venereol. 2018;98(4):465–6.
Brunner HI, Tzaribachev N, Vega-Cornejo G, Louw I, Berman A, Calvo Penades I, et al. Subcutaneous abatacept in patients with polyarticular-course juvenile idiopathic arthritis: results from a phase III open-label study. Arthritis Rheumatol. 2018;70(7):1144–54.
Kafaja S, Clements P. Management of widespread skin thickening in diffuse systemic sclerosis. Curr Treat Opt Rheumatol. 2016;2(1):49–60.
Chimenti MS, Teoli M, Di Stefani A, Giunta A, Esposito M, Perricone R. Resolution with rituximab of localized scleroderma occurring during etanercept treatment in a patient with rheumatoid arthritis. Eur J Dermatol. 2013;23(2):273–4.
Elhai M, Boubaya M, Distler O, Smith V, Matucci-Cerinic M, Alegre Sancho JJ, et al. Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: a prospective cohort study. Ann Rheum Dis. 2019;9(78):979–87.
Denton CP, Engelhart M, Tvede N, Wilson H, Khan K, Shiwen X, et al. An open-label pilot study of infliximab therapy in diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2009;68(9):1433–9.
Ferguson ID, Weiser P, Torok KS. A case report of successful treatment of recalcitrant childhood localized scleroderma with infliximab and leflunomide. Open Rheumatol J. 2015;9:30–5.
Diab M, Coloe JR, Magro C, Bechtel MA. Treatment of recalcitrant generalized morphea with infliximab. Arch Dermatol. 2010;146(6):601–4.
Resorlu H, Kilic S, Isik S, Gokmen F. Successful infliximab therapy in a patient with comorbid spondyloarthritis, primary biliary cirrhosis and generalized morphea. Acta Clin Belg. 2017;72(5):365–8.
Prey S, Ezzedine K, Doussau A, Grandoulier AS, Barcat D, Chatelus E, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167(5):1138–44.
Alcantara-Reifs CM, Garnacho-Saucedo GM, Salido-Vallejo R, de la Corte-Sanchez S, Garcia-Nieto AV. Imatinib treatment of therapy resistant generalized deep morphea. Dermatol Ther. 2015;28(5):271–3.
Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63(5):e102–4.
Coelho-Macias V, Mendes-Bastos P, Assis-Pacheco F, Cardoso J. Imatinib: a novel treatment approach for generalized morphea. Int J Dermatol. 2014;53(10):1299–302.
Inamo Y, Ochiai T. Successful combination treatment of a patient with progressive juvenile localized scleroderma (morphea) using imatinib, corticosteroids, and methotrexate. Pediatr Dermatol. 2013;30(6):e191–3.
Acknowledgements
I thank Dr. Hanna Lythgoe from University Children’s Hospital in Manchester, UK for the thoughtful language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this review.
Conflict of interest
In the last 2 years, the author was a participant of an advisory board of Pfizer, Novartis, and BMS and worked as an advisor for Genentech, Bayer, Lilly, MEDAC, Sanofi, and Inventa.
Rights and permissions
About this article
Cite this article
Foeldvari, I. Update on the Systemic Treatment of Pediatric Localized Scleroderma. Pediatr Drugs 21, 461–467 (2019). https://doi.org/10.1007/s40272-019-00363-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-019-00363-5