Abstract
Background
Individual preferences for treatment options for major depressive disorder can impact therapeutic decision making, adherence, and ultimately outcomes.
Objectives
This systematic review of discrete choice experiments (DCEs) on patient preferences for major depressive disorder treatment assessed the range of DCE applications in major depressive disorder to document patient stakeholder involvement in DCE development and to identify the relative importance of treatment attributes.
Methods
We searched MEDLINE via Ovid (1946-present), EMBASE (Elsevier interface), Cochrane Central Register of Controlled Trials (Wiley interface), and PsycINFO (EBSCO interface) databases on 29 May, 2024. Covidence software facilitated the review, which four members completed independently. The review was conducted in two phases: title and abstract and then a full-text review. We used an established quality reporting tool to evaluate selected articles. The Covidence extraction tool was adapted for this study.
Results
A total of 19 articles were included in this review. Most studies elicited preferences for depression treatment (63.2%) and care delivery (10.5%). Two assessed willingness to pay. Individuals prefer a combination of medicine and counseling over each treatment alone. Treatment efficacy, relapse prevention, and symptom relief were among the most important attributes. Individuals were willing to accept larger risks to achieve symptom improvement. Few studies examined preference heterogeneity with latent subgroups.
Conclusions
Discrete choice experiments for major depressive disorder treatment preferences enable an assessment of trade-offs for first-line therapeutic options. Patient stakeholders are infrequently involved as collaborators in the DCE development. Few examined preference heterogeneity among subgroups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
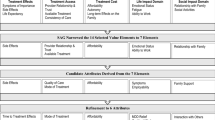
Most discrete choice experiments on major depressive disorder treatment elicited preferences mainly for medication and treatment delivery, but patient stakeholder involvement in discrete choice experiment development was rare. |
Preference coefficients and relative attribute importance were commonly reported but a few studies reported trade-offs between attributes and willingness to pay. |
Treatment efficacy, relapse prevention, and symptom relief were among the most important treatment attributes, but few studies explored heterogeneity by latent subgroups or demographic characteristics. |
Few studies included diverse racial/ethnic groups, which leaves a gap in understanding patient preferences for major depressive disorder treatment in these groups. |
1 Introduction
Major depressive disorder (MDD) is a chronic condition characterized by sadness, loss of interest, sleep disturbance, and a sense of worthlessness that causes significant impairment across multiple functional domains, for example, social or work [1]. Major depressive disorder affects a substantial proportion of the global population, which has only grown in numbers and severity. Globally, it is the most prevalent mental disorder, only second to anxiety, and it is a leading cause of years living with disability [2]. In 2020, the lifetime prevalence of depression in the USA was 18%, and among those aged 18–24 years it is as high as 21.5% [3]. Data from the National Survey on Drug Use and Health show that, among the US population aged 12 years and older, the past-year prevalence of depression increased from 7.3% in 2015 to 9.2% in 2020 [4]. The proportion experiencing severe depressive symptoms increased more than three-fold during the COVID-19 pandemic relative to the pre-pandemic period [5].
There are several therapeutic options for managing MDD. The most common are antidepressant medications and psychotherapy, used alone and in combination. Antidepressants are the first-line treatment for moderate/severe depression [6]. Electroconvulsive therapy is often used for individuals who do not respond to antidepressants, and it is typically reserved for treatment-resistant depression. However, as many as 50% of individuals are non-adherent to depression treatment [7, 8]. Reasons for non-adherence stem from patient-related factors and concerns about the safety of treatments [8, 9]. Perceptions of dependence on drugs and misunderstanding about MDD and antidepressants are concerns that can lead to poor adherence [9]. As therapeutic options for MDD carry a different array of benefits and risks, individual preferences are important considerations. Research has shown that individuals who do not receive their preferred treatment are less likely to adhere to treatment [10]. Understanding preferences for MDD treatment would provide insights into factors influencing adherence.
In the last two decades, there has been considerable growth in the application of stated preference methods to quantify individuals’ preferences for healthcare interventions. Discrete choice experiments (DCEs) are used to identify attributes of healthcare interventions, services, and outcomes that are important drivers of decision making [11]. A DCE is a stated preference method that elicits decisions among alternative options and can be used to assess trade-offs among attributes. Individuals are presented with choice sets, including two to three hypothetical alternatives that display a range of attributes [12]. Individuals consider the advantages and disadvantages of the attributes within each alternative and then select the alternative they most prefer. The theoretical underpinning is that individuals select the alternative that is of greatest utility to them. The steady increase since 2005 in the use and the types of applications of DCEs demonstrate its prominence in patient preference research [13]. TunneBen et al. identified 11 studies in their systematic review of DCEs for depression and anxiety on the literature published up through 2019 [14]. They found that attributes related to the process of care were most important, followed by cost and treatment outcomes [14]. As many of the included studies combined depression and anxiety, it was not possible to assess the findings specific to individuals with MDD. There has not been a recent systematic review of DCEs specific for MDD. The purpose of this systematic review was to assess the range of applications of DCEs specifically on MDD treatment, the inclusion of patient stakeholders in the development of the DCE, and the key attributes that are most important to individuals living with MDD.
2 Methods
We conducted a systematic review of the literature on DCEs for MDD treatment and management. Our review covered the range of MDD treatments or healthcare interventions for which patient preferences were elicited. Given the importance of meaningful patient engagement in research, we assessed the extent to which individuals living with MDD or other stakeholders were involved as collaborators in the development of the DCE. We adhered to the guidelines and standards for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15]. The systematic review was registered with PROSPERO (The International Prospective Register of Systematic Reviews) prior to conducting the review (No. CRD42023476472). This study did not require institutional review board approval.
2.1 Search Strategy
Our team identified search terms within three domains: DCE, MDD, and stated preferences. The list of search terms included terms in the subject heading, keywords, and article titles. All reviewers (SdR, AAR, AF, DEP) evaluated the list for redundant terms and those that were too broad and irrelevant for this review. A research librarian (EFG) used these terms to develop an optimal search strategy that combined subject headings and keywords and was limited to human studies (Electronic Supplementary Material [ESM]). We searched the following databases on 14 July, 2023: MEDLINE via Ovid (1946-present), EMBASE (Elsevier interface), Cochrane Central Register of Controlled Trials (Wiley interface), and PsycINFO (EBSCO interface). A search update was conducted in all databases on 29 May, 2024. We also reviewed the reference lists of the three key published reviews on DCEs [14, 16, 17]. The results of the database searches were uploaded into Covidence software to conduct the review (Covidence systematic review software; Veritas Health Innovation, Melbourne, VIC, Australia, www.covidence.org).
2.2 Screening Procedures
The screening was conducted in two phases. Phase one was the title and abstract review and phase two was a full-text review. Four individuals (SdR, AAR, AF, DEP) independently conducted the title and abstract review. Agreement from a minimum of two reviewers was needed to advance the article to phase two. The same four individuals independently conducted the full-text review. Agreement from a minimum of two reviewers was needed for the article to advance to data extraction. After each phase, conflicts were discussed, and decisions were made based on consensus among all four reviewers.
2.3 Selection Criteria
We retained only those articles that employed a DCE, reported empirical data, targeted MDD, and elicited patient preferences. Studies that described a conjoint analysis or adaptive conjoint analysis were retained, given their similarity in the preference elicitation method. We did not include best-worst scaling because this was distinctly different, and this review was specific to DCE methods. We excluded articles if it was not possible to distinguish MDD from other conditions (i.e., samples had a combination of diagnoses, such as anxiety and bipolar depression), if depression was due to a medical condition (e.g., post-partum depression), if it focused on provider preferences, or if was a conference abstract.
2.4 Quality Assessment
Using an existing quality assessment tool, one author (AAR) assessed the risk of bias, and one author (SDR) reviewed the information. We selected the tool used by Mandeville et al., [18] given it had been used in a systematic review of DCEs. The criteria provide a measure of the quality of the reporting and identify items that could have a meaningful impact on the validity of the findings. We adapted this tool and completed it within the Covidence platform. There were four items related to the choice task design: choice of attributes and levels grounded in qualitative work with a target population, no conceptual overlap between attributes, uni-dimensional attributes, and the inclusion of an opt-out or status quo option or justification of a forced choice. Three items assessed the study conduct, including piloting conducted amongst the target population, target population(s) appropriate for a research objective, and a sampling frame representative of a target population. Four items assessed the quality of the analysis, including any pooled analysis from different subgroups appropriate, econometric model appropriate for choice task design, econometric model accounts for serial correlation of choices, and relative attribute effects compared using a common metric.
2.5 Data Extraction and Analysis
We adapted the Covidence screening tool for data extraction, which was pre-tested prior to extracting any information from selected articles. Three reviewers (AAR, AF, DEP) independently extracted data and the fourth reviewer (SdR) evaluated and resolved all conflicts. Each paper was extracted independently by two reviewers.
Publication information extracted from each article included the author affiliation, country/origin of the study, publication year, funding source, and conflict of interest. The methodological details we extracted included the study goal and aims, purpose of preference elicitation, pilot test conducted, mode of data collection, treatments assessed, statistical models, and primary and secondary outcomes. We also extracted information related to the DCE, including the number of attributes and levels per attribute, number of choice tasks, number of profiles per choice task, and number of attributes per profile, DCE design, and the design efficiency. We recorded details about patient involvement in the DCE development, including the formative research, which involved the foundational steps to identify and select attributes and attribute levels. Data extracted from the study results included study participant age, sex, and race/ethnicity, the sample size, type of preference utility metric, relative attribute importance or other ranking, trade-off measures, latent classes, other subgroup analyses, and assessment of a correlation between preferences and demographic characteristics.
Data on the study characteristics were summarized descriptively. We summarized data on the purpose of preference elicitation and the treatment modality. The type of formative work conducted and the extent of stakeholder involvement in the formative work were characterized for each study. Attributes domains were categorized as effects, cost, access, and patient driven, and documented for each study along with the focus of the DCE and preference measures. Within the study, we summarized the details of the DCE by the number of attributes, attribute levels, choice sets, profiles per choice set, and attributes per profile.
3 Results
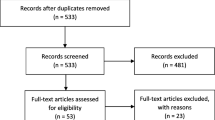
The search strategy produced 1551 articles and after removing 586 duplicates there were 965 articles that underwent title and abstract screening (Fig. 1). Of those, 902 were excluded, leaving 63 for a full-text review. During the full-text review, we excluded 44 articles because the DCEs were conducted in the wrong population (i.e., not a patient sample), designs were not a DCE, or they were conference abstracts. A total of 19 articles were included in this review.
3.1 Study Characteristics
A summary of the study characteristics is in Table 1. Most studies were conducted with participants aged 18 years or older (89.5%) and 47.4% had sample sizes of 200 or more. Race was not mentioned in nine (47.4%) of the studies, while some included participants of multiple races. Most studies were conducted in the USA (42.1%) or Europe (36.8%). Over two thirds of studies were published in 2011 or later. Most studies elicited preferences for depression treatment (63.2%). Two studies included measures of willingness to pay (WTP).
3.2 Quality Assessment
Overall, all studies met the majority of the quality criteria based on the template adapted from Mandeville et al. [18]. Only seven studies [22,23,24,25,26,27] met the criteria for attribute and level selection based on qualitative work with the target population. Five studies did not meet criteria for pilot testing [19, 21, 27,28,29]. The quality assessment is shown in Table S2 of the ESM.
3.3 DCE Development and Stakeholder Involvement
Most of the studies used surveys (n = 18), one used focus groups [28] and five used interviews [20, 22, 26, 27, 30] in the DCE development, and particularly during attribute identification (Table S3 of the ESM). One study did not explicitly mention formative work to develop the DCE attributes [31]. One study described the formative work [22], which involved stakeholder collaborators, and one described the protocol [30] with plans to engage stakeholder collaborators.
Two (10.5%) of the identified studies included patient stakeholders as collaborators in the attribute development of the DCE [22, 25]. In the other 17 studies, attribute development was conducted mainly using a literature review or focus groups with expert providers. Fifty-three percent reported conducting a pilot test of the DCE instrument (Table S3 of the ESM) [22, 23, 25, 26, 30,31,32,33,34,35].
Formative work in the DCE development varied from development to attribute-level identification and selection. All but one study [31] described the formative work underpinning the DCE development. Overall, 13 (68.4%) studies mentioned DCE formative work for attribute development and attribute-level identification and selection (Table S3 of the ESM).
3.4 Treatment Modalities
The included studies covered a range of MDD treatment modalities (Fig. 2). Four of the nine (47.4%) studies that focused on medication treatment also included medication and psychotherapy [19, 22, 23, 31]. The nine DCEs that focused on treatment delivery elicited preferences for provider characteristics, treatment and/or healthcare setting, and mobile health technologies [19, 20, 23,24,25,26, 32, 34,35,36]. Two studies included electroconvulsive therapy along with lifestyle changes and peer support along with medication and psychotherapy [22, 23].
Treatment modalities included in discrete choice experiments for major depressive disorder. Note: categories are not mutually exclusive; medication = antidepressant treatments; psychotherapy = behavioral therapy, counseling, mindfulness-based cognitive therapy; treatment delivery = provider preferences, genetic testing, treatment plans, mHealth apps; other = peer support, lifestyle changes, messaging
3.5 Design and Structure of the DCE Choice Tasks
Nine DCEs (47.4%) had a fractional factorial design [19, 25, 27, 29,30,31,32, 35, 36]. Six studies did not explicitly mention the design [20, 23, 24, 26, 28, 33]. Three were a full profile [22, 34, 37] and one used an adaptive conjoint analysis [21]. Five studies mentioned the DCE design was D-efficient [22, 23, 26, 29, 34], of which two stated it was optimized [29] or maximized [34], and one stated a 100% D-efficient design. The remaining studies did not explicitly mention the DCE efficiency.
The DCE structure across studies is summarized in Table 2. Five studies incorporated two to three different DCEs in the survey [19, 23, 32, 38]. On average, DCEs included 5.3 attributes, with a range from 2 to 10. Most attribute levels ranged from two to four, with a small number that had five, seven, or eight levels. Choice sets ranged from a high of 20 to a low of five, with an average of 11.5 choice tasks per DCE. More recently published articles tended to include fewer choice sets. Most DCEs presented two to three profiles per choice set (mean = 2.0). The number of attributes per profile was, on average, 5.7, but this varied from two to 10.
3.6 DCE Analysis and Attribute Domains
The DCEs were analyzed using a variety of analytic models (Table 3). Preference heterogeneity was modeled using segmentation methods, such as a latent class analysis, or by segmenting the sample by demographic characteristics. Eight studies conducted subgroup analyses [20, 24,25,26,27, 29, 34, 35]. Groenewoud et al. [35] examined preference heterogeneity by healthcare orientation (outcome vs trust-oriented), disease severity, and educational attainment. Lokkerbol et al. [34] created a median split in the sample to create three analytic subgroups: age, educational attainment, and impairment. Preferences for mobile technology were assessed by technology acceptance, age, nationality, and educational attainment [24]. Sonik et al. [25] examined provider preferences by distinct subgroups defined by race/ethnicity, sex, and prior discrimination. Age and sex subgroup analyses examined preference heterogeneity for depression treatment outcomes [27]. Six studies correlated preferences with demographic characteristics by including demographic variables in the analytic model [19, 24, 25, 28, 34, 36].
The main findings from the included studies are summarized by the DCE focus, attribute domains, and preference measures (Table 4). The attribute domains across all studies included treatment effects (n = 11, 57.9%), treatment costs (n = 9, 47.4%), treatment access (n = 15, 78.9%), and patient-driven factors (n = 2, 10.5%).
3.7 Preferences for Depression Treatment Attributes
Across studies, there were some similarities in preferences for depression treatment (Table 4). Individuals prefer medication and counseling combined over each treatment alone [19, 25]. White participants preferred medicine over counseling [25, 36]. Individual psychotherapy is preferred over group therapy [19, 26, 34, 37], but mindfulness-based cognitive therapy (MBCT) is preferred in a group setting [37]. Overall findings for MBCT revealed that reducing relapse had a greater influence on preferences than the delivery method, type of interaction, or the waiting period and strong negative preferences for individual and face-to-face sessions [37]. Regarding mHealth technology for depression management, the accuracy of the device was the strongest and user benefit was the least influential on preference [24].
Higher costs corresponded with a lower preference for the treatment or service [23, 29, 31, 32, 36]. One study found that the WTP was large to eliminate MDD symptoms, but the side effects of treatment reduced the WTP [31].
A total of ten studies reported the relative attribute importance [20, 21, 23, 26,27,28, 31, 33, 34, 37]. Treatment efficacy, relapse prevention, and symptom relief were among the most important treatment attributes [21, 23, 33]. Depression treatment outcomes of greatest relative importance are energy/fatigue and side effects [27]. Bell et al. [28] elicited preferences for messaging that would motivate care seeking and found that six attributes explained 71% of relative attribute importance. These include messaging about the understanding of symptoms, identifying treatments other than medicine, assuring that depression affects many people, acknowledging depression as a medical condition, addressing the shame in disclosing symptoms, and presenting information on the effectiveness of medication [28].
Studies that focused on depression treatment access and delivery found relatively important attributes to be distance to the treatment and psychiatry specialty [20]. Others found that face-to-face was the most important relative to individual sessions, waiting time, or number of sessions per week, in relative order [34].
3.8 Trade-Offs Among Depression Treatment Attributes
Several studies reported the trade-offs among attributes. Individuals were willing to accept larger risks with ketamine treatment to achieve symptom improvement [33]. Results from an adaptive choice conjoint instrument completed by individuals recruited from community pharmacies in the Netherlands show that psychotherapy and selective serotonin reuptake inhibitor antidepressants had a high benefit-to-risk ratio [21]. However, this was reversed with increasing age [21]. When asked about preferences for mHealth technology for managing depression, individuals were willing to trade accuracy for privacy and were willing to accept a smaller reduction in accuracy for a higher benefit [24].
3.9 Heterogeneity in Preferences for Depression Treatment
Few studies examined preference heterogeneity. The few that did distinguished preference heterogeneity using latent class analysis models [23, 26, 37]. Fifer et al. [23] reported a two-class solution in preferences for depression treatment plans. One class prioritized medication, brain stimulation treatment, and the provider type while the second class prioritized treatment effectiveness, the provider type, and medication [23]. The DCE for MBCT yielded four classes with distinct preferences [37]. One class had strong preferences for reducing relapse, and thus focused on treatment effectiveness [37]. The second and third classes prioritized the delivery interface but differed on the modality, with one strongly preferring individual 1:1 delivery and the other demonstrating a strong preference for a face-to-face session. The fourth class strongly preferred scheduling MBCT sessions during an individual’s own time rather than during work hours [37]. Waumans et al. [26] identified three classes, which differed with respect to individual versus group therapy and the frequency of visits [26].
4 Discussion
This systematic review found 19 articles specific to DCEs measuring treatment preferences for individuals living with MDD. We found that the purpose of the DCE preference elicitation covered topics including specific treatments, such as antidepressant medication and psychotherapy, the provision of care, including mHealth technologies and genetic screening, as well as provider characteristics. Treatment effectiveness, such as a reduction in symptoms or a relapse, and treatment side effects were common across studies. Preferences for treatment costs were assessed in eight of the studies [22, 23, 25, 29, 31, 32, 35, 36], and one described a protocol that included a cost attribute [30]. Discrete choice experiments on the features of depression, such as loss of interest, fatigue, guilt, or pain, were rare, with only one study identified [27].
Treatment costs are influential in preferences for MDD treatment. Consistently, studies in this systematic review reported decreases in preference with cost increases [23, 29, 32, 36]. This carries important implications for valuing medical innovations and the adoption of such into practice [39]. Including a direct cost attribute in the DCE was typical in the MDD studies in our systematic review. Indirect costs of care are also an important consideration, but this was not explicitly measured in the included studies. The valuation of MDD treatments and care plans is important, considering that 30% of individuals are treatment resistant [40]. Brain stimulation [41] can be a critical option for those who are resistant to first-line medication treatments, but these interventions are costly [42]. The high proportion of treatment resistance and patient preference sensitivity to cost suggests that less costly second-line alternatives could ensure treatment uptake and ultimately better outcomes.
The included studies were published from 2001 through 2024, with the majority published in 2011 and later. Several studies have demonstrated the increase in DCE applications in healthcare beginning around 2000 but the rapid growth started occurring in 2008 and onward [13, 43, 44]. A review of conjoint analysis applications from 2007 or earlier show very few studies with a clinical focus on mental disorders [44]. Notably, only two studies included in our systematic review were published before 2005. There is a need to better understand preferences among individuals living with MDD, and especially among subgroups with different degrees of symptom severity or impairment in home, work, and social functioning.
The methodological sophistication of the designs has increased over time [13, 44], but our systematic review found variability in the design description and the inclusion of alternatives. Only five studies described the experimental design efficiency, yet this is a critical consideration and one of the essential phases in DCE development [11, 45]. Several ISPOR Task Force Reports emphasizing the importance of the experimental design [11, 45] were published prior to many of the articles included in this review. The lack of detail about the experimental design indicates the need to better articulate this for transparency in the precision of the estimated parameters. We found that the average number of attributes across the 19 included studies was five, with five to 20 choice tasks. This is consistent with an earlier review of the design of conjoint analysis applications [43]. The DCEs with fewer choice sets tended to be in the more recently published studies, which may reflect changes in the software applications used for stated preference research. Certainly, this reduces the cognitive burden and, hopefully, increases the trustworthiness of the responses.
Patient stakeholders were rarely involved as collaborators in the attribute development phases of identification, selection, or refinement. Only two studies reported patient engagement in the development of the DCE [22, 25]. Attribute identification is fundamental to the conceptual framework underpinning the DCE. Ideally, the attributes and attribute levels should reflect as closely as possible the key decisions that those with the condition make in a real-life situation. Often, this relies on qualitative research methods to elicit the attributes [46]. A two-stage process for attribute identification and language refinement is recommended [46] in the development of attributes for DCEs. It is possible that stakeholder groups were meaningfully engaged in the DCE development, but this was not transparent in the articles. Our systematic review identified this as a gap in DCE development for MDD.
Preference heterogeneity was not a common metric reported in the included studies. Eight studies reported subgroup differences by participant characteristics [20, 24,25,26,27, 29, 34, 35] and three studies explicitly described latent subgroups [23, 26, 37]. Further, only two studies examined variation in preference by race/ethnicity [25, 36]. Importantly, depression is more common in African-American and Hispanic groups than in White individuals [47] and preferences for treatment also differ by race/ethnicity [48]. Understanding the differences in preferences for MDD treatment is fundamental to developing personalized treatment plans that individuals are more likely to adhere to. Application of DCEs to evaluate preference heterogeneity for MDD treatments warrants further study to address this existing gap in research.
The systematic review of DCEs on treatment preferences for MDD included 19 studies with varied applications, which limits the inferences we were able to make from the findings. This systematic review is limited to studies published in English. Although our search strategy included four databases widely used in systematic reviews, we may have missed reports in the gray literature. Several factors associated with the selected papers may limit the representativeness of treatment preferences of individuals living with MDD. More than half of the studies included in this review had sample sizes of less than 200, and five had samples of less than 100. Most studies were conducted in the USA or Europe. European-based studies were limited to five countries: Denmark, Germany, Netherlands, Spain, and the UK. The geographic range of studies affects the racial diversity of the samples. Few studies focused on emerging technologies, such as mobile health applications, which restricts the ability to assess preferences for digital options for disease management and care delivery. Only one study addressed the features of depression, and so we were unable to assess preferences for the characteristics of depression that are important to individuals living with MDD.
5 Conclusions
This systematic review identified DCEs that focused on a range of MDD treatment interventions, and many evaluated the combination of medication with some form of psychotherapy. This enables an assessment of first-line treatment and provides valuable information about how individuals trade-off the benefits and risks of treatment. Despite the expansion of DCEs in the last two decades, studies on the measurement of treatment preferences for MDD remain scarce. Our review found limitations in the report of experimental designs to fully evaluate the precision of estimated coefficients, the inclusion of treatment outcomes, the meaningful engagement of patient stakeholders in the development of DCEs, and in the inclusion of diverse racial/ethnic samples. These are important gaps in the methodological application of DCEs for MDD. Future studies using DCE applications to evaluate preferences for MDD treatment can overcome these limitations and gaps. With better evidence of what is important to individuals living with MDD, it is hoped that this could result in treatment plans that align with individuals’ preferences and, ultimately, better adherence and outcomes.
References
Maj M, Stein DJ, Parker, G, et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry. 2020;19(3):269–93.
Global Burden of Disease Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–50.
Lee B, Wan Y, Carlson SA, et al. National, state-level, and county-level prevalence estimates of adults aged ≥18 years self-reporting a lifetime diagnosis of depression: United States, 2020. MMWR Morb Mortal Wkly Rep. 2023;72(24):644–50.
Goodwin RD, Dierker LC, Wu M, et al. Trends in U.S. depression prevalence from 2015 to 2020: the widening treatment gap. Am J Prev Med. 2022;63(5):726–33.
Ettman CK, Abdalla SM, Cohen GH, et al. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3(9): e2019686.
Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5(5):CD010558.
Stein-Shvachman I, Karpas DS, Werner P. Depression treatment non-adherence and its psychosocial predictors: differences between young and older adults? Aging Dis. 2013;4(6):329–36.
Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5–6):41–6.
Ho SC, Jacob SA, Tangiisuran B. Barriers and facilitators of adherence to antidepressants among outpatients with major depressive disorder: a qualitative study. PLoS ONE. 2017;12(6): e0179290.
Leung LB, Ziobrowski HN, Puac-Polanco V, et al. Are veterans getting their preferred depression treatment? A national observational study in the Veterans Health Administration. J Gen Intern Med. 2022;37(13):3235–41.
Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77.
Soekhai V, de Bekker-Grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26.
TunneBen M, Hiligsmann M, Stock S, et al. Patients’ preferences for the treatment of anxiety and depressive disorders: a systematic review of discrete choice experiments. J Med Econ. 2020;23(6):546–56.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Affengruber L, Wagner G, Dobrescu A, et al. Values and preferences of patients with depressive disorders regarding pharmacologic and nonpharmacologic treatments: a rapid review. Ann Intern Med. 2023;176(2):217–23.
Eiring O, Landmark BF, Aas E, et al. What matters to patients? A systematic review of preferences for medication-associated outcomes in mental disorders. BMJ Open. 2015;5(4): e007848.
Mandeville KL, Lagarde M, Hanson K. The use of discrete choice experiments to inform health workforce policy: a systematic review. BMC Health Serv Res. 2014;14:367.
Dwight-Johnson M, Lagomasino IT, Aisenberg E, et al. Using conjoint analysis to assess depression treatment preferences among low-income Latinos. Psychiatr Serv. 2004;55(8):934–6.
Fortney J, Thill JC, Zhang M, et al. Provider choice and utility loss due to selective contracting in rural and urban areas. Med Care Res Rev. 2001;58(1):60–75.
Wouters H, Van Dijk L, Van Geffen EC, et al. Primary-care patients’ trade-off preferences with regard to antidepressants. Psychol Med. 2014;44(11):2301–8.
dosReis S, Bozzi LM, Butler B, et al. Preferences for treatments for major depressive disorder: formative qualitative research using the patient experience. Patient. 2023;16(1):57–66.
Fifer S, Puig A, Sequeira V, et al. Understanding treatment preferences of Australian patients living with treatment-resistant depression. Patient Prefer Adherence. 2021;15:1621–37.
Simblett SK, Pennington M, Quaife M, et al. Patient preferences for key drivers and facilitators of adoption of mHealth technology to manage depression: a discrete choice experiment. J Affect Disord. 2023;331:334–41.
Sonik RA, Creedon TB, Progovac AM, et al. Depression treatment preferences by race/ethnicity and gender and associations between past healthcare discrimination experiences and present preferences in a nationally representative sample. Soc Sci Med. 2020;253: 112939.
Waumans RC, Muntingh ADT, Veldwijk J, et al. Treatment preferences of adolescents and young adults with depressive symptoms: a discrete choice experiment. Appl Health Econ Health Policy. 2024;22(3):401–13.
Zimmermann TM, Clouth J, Elosge M, et al. Patient preferences for outcomes of depression treatment in Germany: a choice-based conjoint analysis study. J Affect Disord. 2013;148(2–3):210–9.
Bell RA, Paterniti DA, Azari R, et al. Encouraging patients with depressive symptoms to seek care: a mixed methods approach to message development. Patient Educ Couns. 2010;78(2):198–205.
Herbild L, Gyrd-Hansen D, Bech M. Patient preferences for pharmacogenetic screening in depression. Int J Technol Assess Health Care. 2008;24(1):96–103.
Xie P, Li HQ, Peng WL, et al. Eliciting depression patients’ preferences for medication management: a protocol for discrete choice experiment. Patient Prefer Adherence. 2024;18:289–300.
Morey E, Thacher JA, Craighead WE. Patient preferences for depression treatment programs and willingness to pay for treatment. J Ment Health Policy Econ. 2007;10(2):73–85.
Dwight-Johnson M, Lagomasino IT, Hay J, et al. Effectiveness of collaborative care in addressing depression treatment preferences among low-income Latinos. Psychiatr Serv. 2010;61(11):1112–8.
Fairchild AO, Katz EG, Reed SD, et al. Patient preferences for ketamine-based antidepressant treatments in treatment-resistant depression: results from a clinical trial and panel. Neurol Psychiatry Brain Res. 2020;37:67–78.
Lokkerbol J, Geomini A, van Voorthuijsen J, et al. A discrete-choice experiment to assess treatment modality preferences of patients with depression. J Med Econ. 2019;22(2):178–86.
Groenewoud S, Van Exel NJ, Bobinac, A, et al. What influences patients’ decisions when choosing a health care provider? Measuring preferences of patients with knee arthrosis, chronic depression, or Alzheimer’s disease, using discrete choice experiments. Health Serv Res. 2015;50(6):1941–72.
Dwight Johnson M, Apesoa-Varano C, Hay J, et al. Depression treatment preferences of older white and Mexican origin men. Gen Hosp Psychiatry. 2013;35(1):59–65.
Lau MA, Colley L, Willett BR, et al. Employee’s preferences for access to mindfulness-based cognitive therapy to reduce the risk of depressive relapse: a discrete choice experiment. Mindfulness. 2012;3:318–26.
Dwight-Johnson M, Lagomasino IT, Simpson GM. Underuse of evidence-based pharmacotherapies for affective disorders. Psychiatr Serv. 2003;54(8):1076–8.
Allen JD, Stewart MD, Roberts SA, et al. The value of addressing patient preferences. Value Health. 2017;20(2):283–5.
Gong Q, Wu Q, Scarpazza C, et al. Prognostic prediction of therapeutic response in depression using high-field MR imaging. Neuroimage. 2011;55(4):1497–503.
Holtzheimer PE 3rd, Nemeroff CB. Advances in the treatment of depression. NeuroRx. 2006;3(1):42–56.
Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: a review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009;2(3):339–52.
Marshall D, Bridges JF, Hauber B, et al. Conjoint analysis applications in health: how are studies being designed and reported? An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–56.
Bridges J, Kinter E, Kidane L, et al. Things are looking up since we started listening to patients: recent trends in the application of conjoint analysis in health 1970–2007. Patient. 2008;1(4):273–82.
Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13.
Coast J, Al-Janabi H, Sutton EJ, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21(6):730–41.
Dunlop DD, Song J, Lyons JS, et al. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93(11):1945–52.
Givens JL, Houston TK, Van Voorhees BW, et al. Ethnicity and preferences for depression treatment. Gen Hosp Psychiatry. 2007;29(3):182–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
Susan dosReis, Dafne Espinal Pena, Alexandra Fincannon, Emily F. Gorman, and Alejandro Amill-Rosario have no conflicts of interests that are directly relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the materials presented in this manuscript. The authors have no financial or proprietary interests in any material discussed in this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
SdR conceptualized the study design and implementation, conducted the reviews, participated in consensus meetings, adjudicated the data extraction discrepancies, supervised the data analysis, and drafted and edited the manuscript. DEP completed the reviews, participated in consensus meetings, completed the data extraction, prepared preliminary data tables, and reviewed and edited the manuscript. AF completed the reviews, participated in consensus meetings, contributed to the data extraction, and reviewed and edited the manuscript. EFG assisted with developing the search strategy, implemented the search strategy, and uploaded the information into Covidence, consulted on the quality-review tool, and reviewed and edited the manuscript. AA-R conducted the reviews, participated in consensus meetings, contributed to the data extraction, prepared the data files, constructed the data tables, completed the PROSPERO registration, implemented the quality review, and drafted and edited the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dosReis, S., Espinal Pena, D., Fincannon, A. et al. Discrete Choice Experiments to Elicit Patient Preferences for the Treatment of Major Depressive Disorder: A Systematic Review. Patient (2024). https://doi.org/10.1007/s40271-024-00706-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s40271-024-00706-6