Abstract
Background
A significant limitation of the traditional randomized controlled trials is that strong preferences for (or against) one treatment may influence outcomes and/or willingness to receive treatment. Several trial designs incorporating patient preference have been introduced to examine the effect of treatment preference separately from the effects of individual interventions. In the current study, we summarized results from studies using doubly randomized preference trial (DRPT) or fully randomized preference trial (FRPT) designs and examined the effect of treatment preference on clinical outcomes.
Methods
The current systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies using DRPT or FRPT design were identified using electronic databases, including PubMed, Cochrane Library, EMBASE, and Google Scholar between January 1989 and November 2018. All studies included in this meta-analysis were examined to determine the extent to which giving patients their preferred treatment option influenced clinical outcomes. The following data were extracted from included studies: study characteristics, sample size, study duration, follow-up, patient characteristics, and clinical outcomes. We further appraised risk of bias for the included studies using the Cochrane Collaboration’s risk of bias tool.
Results
The search identified 374 potentially relevant articles, of which 27 clinical trials utilized a DRPT or FRPT design and were included in the final analysis. Overall, patients who were allocated to their preferred treatment intervention were more likely to achieve better clinical outcomes [effect size (ES) = 0.18, 95% confidence interval (CI) 0.10–0.26]. Subgroup analysis also found that mental health as well as pain and functional disorders moderated the preference effect (ES = 0.23, 95% CI 0.11–0.36, and ES = 0.09, 95% CI 0.03–0.15, respectively).
Conclusions
Matching patients to preferred interventions has previously been shown to promote outcomes such as satisfaction and treatment adherence. Our analysis of current evidence showed that allowing patients to choose their preferred treatment resulted in better clinical outcomes in mental health and pain than giving them a treatment that is not preferred. These results underline the importance of incorporating patient preference when making treatment decisions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The concept of patient-centered care has gained universal recognition in the discussion of best practices in modern healthcare [1]. Shared decision-making (SDM), an essential component of the patient-centered care model, is designed to empower patients with the best available evidence to make informed clinical decisions [2]. SDM requires an exchange of information between clinicians and patients; however, one of the greatest challenges for healthcare providers in implementation of SDM is consistently engaging patients in the clinical decision-making process [1]. Incorporating patient treatment preference into clinical decision-making has been demonstrated to increase patient satisfaction, promote treatment adherence, and subsequently, improve clinical outcomes [3].
While several clinical trials showed no difference in outcomes between patients who received a preferred treatment and those who did not receive a preferred one [4,5,6], there is a growing body of evidence demonstrating additional benefits, separate from the direct effects of treatment, of the effects of treatment preference. Le et al. [7] reported that among patients diagnosed with posttraumatic stress disorder (PTSD) who received either prolonged exposure therapy (PET) or pharmacotherapy, there was a significant improvement in health-related quality of life outcomes in patients who received their preferred treatment modality compared to those treated with a non-preferred option. In terms of improving adherence, a randomized preference trial by Kwan et al. [5], comparing treatment modalities for depression, found that patients allocated to a non-preferred treatment arm were more likely to drop out of the study and attend fewer expected visits compared to preference-matched patients. In addition to improvement in adherence and quality of life, preference has been shown to improve clinical outcomes. Kocsis et al. [8] found that patients diagnosed with depression who received their preferred treatment had higher rates of remission and better clinical outcomes, demonstrated by lower scores on the Hamilton Rating Scale for Depression.
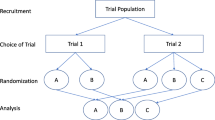
Randomized controlled trials (RCTs), the gold standard in clinical research, remain the most robust method to obtain quality data concerning efficacy and safety. They, however, fail to account for patients who decline randomization due to a strong preference for (or against) a particular treatment option, allowing only participants with weak or no treatment preference to be investigated [9]. Strong preference for a certain treatment may jeopardize both the internal and external validity of trial results [10]. Several trial designs, incorporating patient preference, have been introduced to examine the effects of treatment preference separately from the direct effects of individual interventions [11]. A doubly randomized preference trial (DRPT) or two-stage randomized preference trial design first randomly assigns patients to either the choice or no-choice arm. Thereafter, within the choice arm, patients are given an opportunity to select their preferred treatment; while within the no-choice arm, patients are again randomly assigned to a treatment group [12]. In a fully randomized preference trial (FRPT), patient treatment preferences are revealed at baseline; patients are then, nonetheless, randomly assigned to a treatment group [13]. The FRPT design differs from the DRPT design, which allows patients in the choice arm to receive their preferred treatment. Another preference trial design is called the partially randomized preference trial (PRPT). In a PRPT, only patients with a strong treatment preference are allowed to receive their preferred treatment, while those without a specific treatment preference are randomly assigned to a treatment [14]. Of the three trial designs discussed, the DRPT and FRPT designs are able to estimate an unbiased treatment preference effect (PE) [11]. Previous meta-analysis studies examining the effect of treatment preference had either lacked data from trials using DRPT and/or FRPT designs, included studies evaluating non-clinical outcomes, or included study results from PRPT trials [10, 15,16,17,18]. Therefore, the current meta-analysis was restricted to clinical trials using the DRPT and FRPT designs in order to examine the unbiased effect of treatment preference on clinical outcomes.
2 Methods
The current systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. All included studies received ethical and institutional review board (IRB) approval from their respective institutions.
2.1 Literature Search
Studies were identified using electronic databases, including PubMed, Cochrane Library, EMBASE, and Google Scholar. The literature review included works conducted between January 1989 and November 2018 to encompass all preference trials completed subsequent to the introduction of the DRPT design by Rucker [12] and the FRPT design by Torgerson et al. [13]. Search terms included “patient preference” or “patient choice” in combination with “two-stage” or “doubly randomized” or “fully randomized”. Further search strategies included a citation search using Rucker et al. [12] and Torgerson et al. [13], as these were the first papers to describe the DRPT and FRPT designs, respectively.
2.2 Eligibility Criteria
All studies included in this meta-analysis were examined to determine the extent to which giving patients their preferred treatment option influenced clinical outcomes. Figures 1 and 2 depict the DRPT and FRPT designs. The effect of treatment preference, i.e., PE, was defined as the difference in clinical outcomes between patients who received their preferred treatment and those who did not receive their preferred treatment, as follows [11]:
where μAA represents observed mean outcome of patients who prefer treatment A and receive treatment A. Likewise, μBB denotes observed mean outcome of patients who prefer and receive treatment B. On the contrary, μAB represents observed mean outcome of patients who prefer treatment B but receive treatment A, and μBA denotes observed mean outcome of patients who prefer treatment A but receive treatment B.
The DRPT design also allows for estimation of the effect of treatment choice. The effect of treatment choice, i.e., the choice effect, is defined as the difference in clinical outcomes between patients who are given an opportunity to choose their treatment and those who are randomly assigned to treatment, denoted as [20]:
where μA and μB represent the observed overall mean outcome of patients randomly assigned to treatment A and B, respectively, regardless of preference. The PE differs from the choice effect in that the no-choice arm includes a proportion of patients who were randomized to preferred treatment by chance.
Other types of clinical trials such as traditional RCT and PRPT designs were excluded as they did not estimate the true effect of treatment preference [11]. Studies measuring nonclinical outcomes, such as academic examination scores or patient satisfaction, were also excluded. Two independent investigators independently screened study titles and abstracts for eligibility. Full text versions of appropriate trials were retrieved and further examined for inclusion. Discrepancies between investigators were resolved through a consensus discussion.
2.3 Risk of Bias Assessment and Data Extraction
Risk of bias was assessed using the Cochrane Collaboration’s risk of bias tool, which evaluates the quality of RCTs based on the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases [21]. Using these criteria, the Cochrane tool classifies each trial as having a low, unclear, or high risk of bias. AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews) [22] was used to evaluate the methodological quality of the 2008 Preference Collaboration Review Group meta-analysis [18]. The following data were extracted from included studies: study characteristics, sample size, study duration, follow-up, patient characteristics (age, gender), and clinical outcomes.
2.4 Statistical Analysis
We estimated the standardized effect size (ES) using Cohen’s d for the mean differences in the clinical outcomes of patients who received a preferred treatment compared to those who received a non-preferred treatment for each included study [23]. Cohen’s d was calculated with values of 0.2, 0.5, and 0.8, corresponding to small, medium, and large ES, respectively. The choice effect was also estimated for studies using a DRPT design. An overall ES was calculated across studies. Subgroup analyses were conducted for distinct preference trial designs and disease categories.
The fixed-effects model assumes that there is one true ES between studies and that all differences in observed treatment outcomes across included studies are solely due to sampling error [24]. In the current meta-analysis, heterogeneity was likely to be observed due to differences in study design and disease state; thus, a random effects model was appropriate to account for variance between studies. The I2 statistic was evaluated to quantify the variability in PE estimates due to heterogeneity between studies rather than chance. I2 was calculated with values of 25%, 50%, and 75%, corresponding to low, moderate, and high heterogeneity, respectively. Data was analyzed using the R programming package Metafor [25].
3 Results
3.1 Search Results and Study Selection
The initial electronic database search identified 374 potentially relevant articles, of which 98 duplicates were excluded. The screening phase identified 36 RCTs, published in English, utilizing an FRPT or DRPT design. Of the 36 preference trials identified, seven measured non-clinical outcomes, eight did not report the required statistics to calculate an ES for the PE, and two pairs of studies analyzed data from the same sample (Fig. 3). Data from studies reporting multiple clinical outcomes or data from multiple studies evaluating the same sample population were aggregated and an overall ES was applied, resulting in 19 distinct ESs. Included trials measured various outcomes such as Hamilton Rating Scale for Depression scores, remission of depression, pain and function scores, quality of life, and weight loss. The pooled ES of eight studies using an FRPT design reported by the Preference Collaborative Review Group in 2008 was also included [18].
A total of 27 preference trials examining the effects of patient preference on clinical outcomes met the established inclusion criteria of this study. Six studies utilized the DRPT design, allowing patients randomized to the choice arm to receive their preferred treatment. The remaining 21 studies utilized the FRPT design, randomly assigning patients to interventions without regard to patient treatment preference identified at baseline.
Of the 27 included studies, nine investigated the effect of patient preference on outcomes related to mental health disorders and 14 studies evaluated the PE related to pain and functional disorders. The remaining four studies evaluated the impact of patient preference on weight-loss treatment options. Initial analysis of all studies produced a high I2 value of 94.9%. After excluding the four weight-loss trials (rationale discussed below), heterogeneity improved significantly and the I2 value was reduced to a moderate 52.1%.
3.2 Quality Assessment
Included studies were classified as randomized trials; however, allocation was either not concealed or the method of concealment was not described in eight trials [5, 6, 8, 26,27,28,29,30]. All included studies were classified as having a moderate or high risk of performance bias, primarily due to the inability to blind treatment assignment. The choice arm in DRPT designs is inherently unblinded as patients choose their intervention. Inability to blind is also a characteristic of many FRPTs such as in psychotherapy versus pharmacotherapy trials. All studies reported attrition rates; however, they were unbalanced between groups in several studies, leading to potential for attrition bias (see Appendix Figs. 1 and 2 in the Electronic Supplementary Material). There was no evidence of publication bias observed when evaluating the funnel plot of included studies (see Appendix Fig. 3 in the Electronic Supplementary Material). The primary methodological limitation of the Preference Collaborate Review Group meta-analysis identified by AMSTAR-2 was lack of specific detail regarding quality assessment of included studies.
3.3 Overall Preference Effect
Overall, patients who received their preferred treatment intervention were more likely to achieve better clinical outcomes, with an overall preference ES = 0.18, 95% confidence interval (CI) 0.10–0.26; p < 0.0001 (Fig. 4). The I2 test for heterogeneity produced a value of 52.1%, indicating a moderate level of heterogeneity among studies included in the pooled analysis.
3.4 Preference Effect by Disease Category
Subgroup analyses revealed that treatment related to pain and functional disorders (ES = 0.09, 95% CI 0.03–0.15; p = 0.0032) as well as to mental health disorders (ES = 0.23, 95% CI 0.11–0.36; p = 0.0003) was improved when treatment preference and treatment allocation were congruent (Fig. 4). Analysis of both disease categories produced a small ES; however, matching patients to preferred interventions showed a larger magnitude of benefit in the treatment of mental-health–related conditions in comparison to pain- and function-related conditions.
Eight out of nine trials in the mental health subgroup compared psychotherapy to pharmacotherapy (Table 1). Mergl et al. [31] reported significantly lower scores on the 17-item Hamilton Rating Scale for Depression (HAMD-17) in patients with mild to moderate depression who received their preferred intervention in the psychotherapy group. However, no significant improvement of HAMD-17 scores was observed in patients who preferred and received pharmacotherapy with sertraline [31]. This indicated that preference considerations were more meaningful when considering psychotherapy in patients with depression. On the contrary, Le et al. [7] found that in patients diagnosed with PTSD, the treatment PE was significant in patients who received their preferred pharmacotherapy, sertraline, but only observed a nonsignificant improvement in a subgroup of patients who received their preferred psychotherapy, PET [7].
3.5 Preference Effect by Trial Design
We also estimated overall preference ESs by trial design, i.e., FRPT versus DRPT, to determine whether PEs were moderated by study design. The analysis between the two designs was rather similar with both FRPT (ES = 0.14, 95% CI 0.05–0.22; p = 0.001) and DRPT (ES = 0.27, 95% CI 0.06–0.47; p = 0.009) demonstrating a small, but statistically significant benefit in patients who received their preferred treatment in both trial designs (see Appendix Fig. 4 in the Electronic Supplementary Material). Two included studies utilizing the DRPT design used an alternative randomization method, choosing to randomize patients once into three groups: (1) treatment A, (2) treatment B, and (3) choice arm [26, 31]. Using this simplified method rather than the proposed consecutive randomizations using a 1:1 ratio might have resulted in a loss of precision [11].
3.6 Choice Effect
The choice effect was estimated only for studies using the DRPT design. When examining the difference in clinical outcomes between patients who were given an opportunity to choose their preferred treatment and those who were assigned to a treatment, we observed a small but statistically significant ES (ES = 0.14, 95% CI 0.0–0.28; p = 0.046; see Appendix Fig. 5 in the Electronic Supplementary Material). The magnitude of the treatment-choice effect was marginally lower relative to treatment PE; nonetheless, results indicate that simply having the opportunity to choose a treatment might confer a benefit.
3.7 Treatment and Selection Effects
ESs for the treatment and selection effects (SEs) were also estimated for comparison. The treatment effect (TE) is defined as the difference in observed mean outcomes between interventions [11]. Three out of four DRPT studies included in the statistical analysis observed a larger preference ES compared to TE size [7, 26, 31] (Table 1). Several FRPT studies also reported a larger ESs for PE compared to TE [5, 29, 30] (Table 1). The SE describes how outcomes might differ in participants who would select a particular treatment if given the opportunity. Only the DRPT design can estimate an unbiased SE [11]. There were several significant differences in clinical outcomes between patients who preferred one treatment and those who would prefer the other. Specifically, preferring PET as opposed to preferring pharmacotherapy and preferring a self-directed program (SD) versus preferring a group program (G) would result in better Sickness Impact Profile (SIP) total score in women with diagnosed cardiac disease and health-related quality of life in patients with PTSD, respectively (Table 1). For weight-loss trials, patients preferring calorie- and fat-restricted lacto-ovo-vegetarian diet (LOV-D) as compared to preferring standard calorie- and fat-restricted diet (STD-D) and preferring low-fat diet (LFD) versus preferring low-carbohydrate diet (LCD) were more likely to lose more weight (Table 1).
4 Discussion
4.1 Summary of Results
The current meta-analysis was conducted to estimate the effect of patient preference on clinical outcomes in 23 FRPT and DRPT. Unlike previous meta-analysis studies [10, 15,16,17,18], this study investigated the effect of treatment preference in clinical trials using the FRPT and DRPT study designs, as they are the only trial designs that can estimate an unbiased PE [11]. Intuitively, receipt of a preferred treatment would improve treatment adherence and patient satisfaction, as indicated in the literature [5, 32]. More importantly, our study directly examined the effect of treatment preference on clinical outcomes, separately from the TE, and showed that matching patients to their preferred treatment options improved clinical outcomes, with a small, but statistically significant overall preference ES of 0.18 (95% CI 0.10–0.26; p < 0.0001). The observed magnitude of benefit was particularly apparent in trials examining mental health conditions. These results are consistent with previous literature showing that patients who are more receptive and expect significant improvement gain greater benefit from psychotherapy [33].
4.2 Strengths and Limitations of Preference Trials
RCTs provide the foundation for evidence-based medicine; however, the effects of patient preference, when unaccounted for could threaten internal and external validity. When comparing new interventions to standard of care, patients consenting to randomization often prefer the experimental treatment. It would be illogical to risk randomization in this scenario, assuming the preferred standard treatment could be readily obtained [34]. Patients may also decline randomization because of a strong preference for (or against) a particular intervention, limiting study participants to those with weak or no preference [9]. Both scenarios illustrate the potential of patient preference to negatively impact external validity by influencing recruitment.
Negative patient mentality caused by discrepancy between preferred and allocated treatment has been termed resentful demoralization [35]. Blinding is the primary approach used to limit the effects of preference; therefore, resentful demoralization is particularly concerning in the present context, where in many of the included studies, blinding was deemed impossible or impractical. Resentful demoralization may reduce internal validity by effecting outcomes directly (impact on attrition and adherence) or indirectly through psychological responses, such as negative placebo-like effects, providing an inaccurate measure of the true TE [36, 37].
Several preference trials produced no strong evidence of a difference in clinical outcomes between groups of patients who received their preferred treatment and those who received a non-preferred treatment [4,5,6, 26, 28,29,30, 38,39,40,41,42,43,44]. Outcomes of weight-loss trials were not positively influenced by patient preference regardless of study design [27, 28, 40, 41] (see Appendix Fig. 6 in the Electronic Supplementary Material). These consistently negative results suggest that preference may not play a significant role in the treatment of obesity. Yancy et al. [40] observed no difference in weight loss between patients allowed to choose their preferred diet compared to those randomized to a diet option. Furthermore, in the Paving the Road to Everlasting Food and Exercise Routine (PREFER) trial conducted by Burke et al. [27], patients who received their diet of choice actually performed worse than patients assigned to a non-preferred diet. Intuitively, allowing a patient to choose his or her preferred treatment at worst should result in no difference in clinical outcomes compared to receiving a non-preferred treatment. The significantly negative results might indicate potential flaws in the study design and/or implementation. In the PREFER trial, the authors hypothesized that the lack of benefit seen in patients who received their preferred treatment might have been caused by differences in motivation and confidence. The no-choice group might have been determined to succeed despite their assignment, while the choice group might have been overly confident of positive results due to receiving their preferred intervention. In addition, participants might have merely forgotten their original preference, diminishing the influence of preference throughout the study. Yancy et al. [40] suggested the lack of benefits seen in the choice group might be due to increased palatability and subsequent increased caloric intake of foods in the preferred diet. An alternative explanation was the “personal trainer” effect, where participants are more adherent when given explicit direction [40]. Similar to the Yancy et al. [40] and Burke et al. [27] studies using the DRPT design, the other two weight-loss trials utilizing the FRPT design did not observe benefits when patients received their preferred interventions [28, 41]. As a result, we elected to exclude the weight-loss trials from the pooled analysis because of their inconsistency and other unobservable factors that might have influenced study results.
Preference trial designs offer several advantages in estimating the effect of patient treatment preference in clinical trials; however, they are not free of limitations. A potential limitation of preference trials is a flaw in the assumption that a chosen treatment accurately represents patients’ true preference. A true treatment preference requires a level of health literacy adequate to comprehend the treatment options, including the expected risks and benefits. Additionally, in preference trials, treatment preference was assumed to remain constant throughout the trial. However, in reality, patient preference may change after treatment begins [45]. Another significant limitation of preference trial designs is lack of data on strength of preference. It is reasonable to hypothesize that strength of preference is correlated to the observed magnitude of the treatment PE; however, this information is often not collected. Finally, it might be possible that reasoning for preferring a treatment option may vary. Some patients may choose an intervention primarily because of perceived efficacy, while others will take disruption of daily activities and other considerations of convenience into account [46, 47].
The current study had several limitations. PRPTs cannot estimate an unbiased PE because the mean outcomes of patients receiving treatment incongruent with their preference (denoted as μAB and μBA above) cannot be estimated. The exclusion of the PRPT design and trials with nonclinical outcomes left us a sample of 27 clinical trials available for analysis. A few studies using the DRPT design identified in our literature search compared choice and no-choice groups overall [48, 49]; however, to estimate PE, outcomes must be presented for interventions individually. Exclusion of these trials because of omitted data led to a disproportionate number of FRPTs included in this analysis. Also, due to the different study designs (DRPT vs FRPT) and disease categories, there was a moderate level of heterogeneity. This was addressed to some extent by excluding the weight-loss intervention trials [27, 28, 40, 41] from statistical analyses and use of a random effects model. By excluding the outlier weight-loss studies from the statistical analysis, the I2 value was reduced from 94.9 to 52.1%. However, use of a random effects model assigns less relative weight to large studies and more relative weight to small studies compared to the fixed-effects model [24]. This may have caused studies with smaller sample sizes to be given disproportionate weight in determining the overall ES. Finally, included clinical trials appeared to be at moderate/high risk of bias, primarily due to inability to blind either patients or trial personnel.
5 Conclusion
In line with the SDM model, involving patients in choice of therapy by relaying risks and benefits of treatment options, and supporting patients in exploring individualized treatment priorities and goals is imperative to achieving patient-centered care. The current study adds to the growing body of evidence that beyond being essential for patient autonomy, incorporating patient preference into clinical decision-making improves clinical outcomes.
References
Barry MJ, Edgman-Levitan S. Shared decision making—the pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–1. https://doi.org/10.1056/NEJMp1109283.
Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–7. https://doi.org/10.1007/s11606-012-2077-6.
Sidani S, Epstein DR, Bootzin RR, Moritz P, Miranda J. Assessment of preferences for treatment: validation of a measure. Res Nurs Health. 2009;32(4):419–31. https://doi.org/10.1002/nur.20329.
George SZ, Robinson ME. Preference, expectation, and satisfaction in a clinical trial of behavioral interventions for acute and sub-acute low back pain. J Pain. 2010;11(11):1074–82. https://doi.org/10.1016/j.jpain.2010.02.016.
Kwan BM, Dimidjian S, Rizvi SL. Treatment preference, engagement, and clinical improvement in pharmacotherapy versus psychotherapy for depression. Behav Res Ther. 2010;48(8):799–804. https://doi.org/10.1016/j.brat.2010.04.003.
Leykin Y, Derubeis RJ, Gallop R, Amsterdam JD, Shelton RC, Hollon SD. The relation of patients’ treatment preferences to outcome in a randomized clinical trial. Behav Ther. 2007;38(3):209–17. https://doi.org/10.1016/j.beth.2006.08.002.
Le QA, Doctor JN, Zoellner LA, Feeny NC. Effects of treatment, choice, and preference on health-related quality-of-life outcomes in patients with posttraumatic stress disorder (PTSD). Qual Life Res. 2018;27(6):1555–62. https://doi.org/10.1007/s11136-018-1833-4.
Kocsis JH, Leon AC, Markowitz JC, Manber R, Arnow B, Klein DN, et al. Patient preference as a moderator of outcome for chronic forms of major depressive disorder treated with nefazodone, cognitive behavioral analysis system of psychotherapy, or their combination. J Clin Psychiatry. 2009;70(3):354–61.
Torgerson D, Sibbald B. Understanding controlled trials: what is a patient preference trial? BMJ. 1998;316(7128):360.
King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al. Impact of participant and physician intervention preferences on randomized trials: a systematic review. J Am Med Assoc. 2005;293(9):1089–99. https://doi.org/10.1001/jama.293.9.1089.
Walter SD, Turner R, Macaskill P, McCaffery KJ, Irwig L. Beyond the treatment effect: evaluating the effects of patient preferences in randomised trials. Stat Methods Med Res. 2014;26(1):489–507. https://doi.org/10.1177/0962280214550516.
Rucker G. A two-stage trial design for testing treatment, self-selection and treatment preference effects. Stat Med. 1989;8(4):477–85.
Torgerson DJ, Klaber-Moffett J, Russell IT. Patient preferences in randomised trials: threat or opportunity? J Health Services Res Policy. 1996;1(4):194–7.
Brewin CR, Bradley C. Patient preferences and randomised clinical trials. BMJ. 1989;299(6694):313–5.
Swift JK, Callahan JL. The impact of client treatment preferences on outcome: a meta-analysis. J Clin Psychol. 2009;65(4):368–81. https://doi.org/10.1002/jclp.20553.
Swift JK, Callahan JL, Vollmer BM. Preferences. J Clin Psychol. 2011;67(2):155–65. https://doi.org/10.1002/jclp.20759.
Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506–17. https://doi.org/10.1016/j.cpr.2014.06.002.
Preference Collaborative Review Group. Patients’ preferences within randomised trials: systematic review and patient level meta-analysis. Br Med J. 2008;337:1864. https://doi.org/10.1136/bmj.a1864.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Public Libr Sci Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Marcus SM, Stuart EA, Wang P, Shadish WR, Steiner PM. Estimating the causal effect of randomization versus treatment preference in a doubly randomized preference trial. Psychol Methods. 2012;17(2):244–54. https://doi.org/10.1037/a0028031.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. https://doi.org/10.1136/bmj.j4008.
Wilson DB. Practical meta-analysis effect size calculator [Online calculator]. https://www.campbellcollaboration.org/research-resources/research-for-resources/effect-size-calculator.html. Accessed 10 Jan 2019.
Borenstein M. Introduction to meta-analysis. Chichester: Wiley; 2009.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010. https://doi.org/10.18637/jss.v036.i03.
McCaffery KJ, Turner R, Macaskill P, Walter SD, Chan SF, Irwig L. Determining the impact of informed choice: separating treatment effects from the effects of choice and selection in randomized trials. Med Decis Making. 2011;31(2):229–36. https://doi.org/10.1177/0272989x10379919.
Burke LE, Warziski M, Styn MA, Music E, Hudson AG, Sereika SM. A randomized clinical trial of a standard versus vegetarian diet for weight loss: the impact of treatment preference. Int J Obes. 2008;32(1):166–76. https://doi.org/10.1038/sj.ijo.0803706.
Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69(4):717–21. https://doi.org/10.1037/0022-006X.69.4.717.
Lin P, Campbell DG, Chaney EF, Liu CF, Heagerty P, Felker BL, et al. The influence of patient preference on depression treatment in primary care. Ann Behav Med. 2005;30(2):164–73. https://doi.org/10.1207/s15324796abm3002_9.
Sherman KJ, Cherkin DC, Ichikawa L, Avins AL, Delaney K, Barlow WE, et al. Treatment expectations and preferences as predictors of outcome of acupuncture for chronic back pain. Spine. 2010;35(15):1471–7. https://doi.org/10.1097/BRS.0b013e3181c2a8d3.
Mergl R, Henkel V, Allgaier AK, Kramer D, Hautzinger M, Kohnen R, et al. Are treatment preferences relevant in response to serotonergic antidepressants and cognitive-behavioral therapy in depressed primary care patients? Results from a randomized controlled trial including a patients’ choice arm. Psychother Psychosom. 2011;80(1):39–47. https://doi.org/10.1159/000318772.
Loh A, Simon D, Wills CE, Kriston L, Niebling W, Harter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67(3):324–32. https://doi.org/10.1016/j.pec.2007.03.023.
Delsignore A, Schnyder U. Control expectancies as predictors of psychotherapy outcome: a systematic review. Br J Clin Psychol. 2007;46(Pt 4):467–83. https://doi.org/10.1348/014466507x226953.
Bradley C. Designing medical and educational intervention studies. A review of some alternatives to conventional randomized controlled trials. Diabetes Care. 1993;16(2):509–18.
Cook T, Campbell D. Quasi-experimentation: design and analysis issues for field settings. Chicago: Rand McNally; 1979.
Bower P, King M, Nazareth I, Lampe F, Sibbald B. Patient preferences in randomised controlled trials: conceptual framework and implications for research. Soc Sci Med. 2005;61(3):685–95. https://doi.org/10.1016/j.socscimed.2004.12.010.
Janevic MR, Janz NK, Dodge JA, Lin X, Pan W, Sinco BR, et al. The role of choice in health education intervention trials: a review and case study. Soc Sci Med (1982). 2003;56(7):1581–94.
Dunlop BW, Kelley ME, Aponte-Rivera V, Mletzko-Crowe T, Kinkead B, Ritchie JC, et al. Effects of patient preferences on outcomes in the predictors of remission in depression to individual and combined treatments (PReDICT) study. Am J Psychiatry. 2017;174(6):546–56. https://doi.org/10.1176/appi.ajp.2016.16050517.
Stewart MJ, Maher CG, Refshauge KM, Herbert RD, Nicholas MK. Patient and clinician treatment preferences do not moderate the effect of exercise treatment in chronic whiplash-associated disorders. Eur J Pain. 2008;12(7):879–85. https://doi.org/10.1016/j.ejpain.2007.12.009.
Yancy WS Jr, Mayer SB, Coffman CJ, Smith VA, Kolotkin RL, Geiselman PJ, et al. Effect of allowing choice of diet on weight loss: a randomized trial. Ann Intern Med. 2015;162(12):805–14. https://doi.org/10.7326/m14-2358.
Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eat Behav. 2009;10(4):220–7. https://doi.org/10.1016/j.eatbeh.2009.07.004.
Adamson SJ, Sellman DJ, Dore GM. Therapy preference and treatment outcome in clients with mild to moderate alcohol dependence. Drug Alcohol Rev. 2005;24(3):209–16. https://doi.org/10.1080/09595230500167502.
Gum AM, Areán PA, Hunkeler E, et al. Depression treatment preferences in older primary care patients. Gerontologist. 2006;46(1):14–22. https://doi.org/10.1093/geront/46.1.14.
Cockayne S, Hicks K, Kangombe AR, Hewitt C, Concannon M, Thomas K, et al. The effect of patients’ preference on outcome in the EVerT cryotherapy versus salicylic acid for the treatment of plantar warts (verruca) trial. J Foot Ankle Res. 2012;5(1):28. https://doi.org/10.1186/1757-1146-5-28.
Bowling A, Rowe G. “You decide doctor”. What do patient preference arms in clinical trials really mean? J Epidemiol Commun Health. 2005;59(11):914–5. https://doi.org/10.1136/jech.2005.035261.
Corbett MS, Watson J, Eastwood A. Randomised trials comparing different healthcare settings: an exploratory review of the impact of pre-trial preferences on participation, and discussion of other methodological challenges. BioMed Central Health Services Res. 2016;16(1):589. https://doi.org/10.1186/s12913-016-1823-6.
McKay JR, Alterman AI, McLellan AT, Snider EC, O’Brien CP. Effects of random versus nonrandom assignment in a comparison of inpatient and day hospital rehabilitation for male alcoholics. J Consult Clin Psychol. 1995;63(1):70–8. https://doi.org/10.1037/0022-006X.63.1.70.
Rokke PD, Tomhave JA, Jocic Z. The role of client choice and target selection in self-management therapy for depression in older adults. Am Psychol Assoc. 1999;14:155–69.
Noel PH, Larme AC, Meyer J, Marsh G, Correa A, Pugh JA. Patient choice in diabetes education curriculum. Nutritional versus standard content for type 2 diabetes. Diabetes Care. 1998;21(6):896–901.
Long Q, Little RJ, Lin X. Causal inference in hybrid intervention trials involving treatment choice. J Am Stat Assoc. 2008;103(482):474–84. https://doi.org/10.1198/016214507000000662.
Clark NM, Janz NK, Dodge JA, Mosca L, Lin X, Long Q, et al. The effect of patient choice of intervention on health outcomes. Contemp Clin Trials. 2008;29(5):679–86. https://doi.org/10.1016/j.cct.2008.04.002.
Zoellner LA, Roy-Byrne PP, Mavissakalian M, Feeny NC. Doubly randomized preference trial of prolonged exposure versus sertraline for treatment of PTSD. Am J Psychiatry. 2019;176(4):287–96. https://doi.org/10.1176/appi.ajp.2018.17090995.
Moffett JK, Torgerson D, Bell-Syer S, Jackson D, Llewlyn-Phillips H, Farrin A, et al. Randomised controlled trial of exercise for low back pain: clinical outcomes, costs, and preferences. BMJ. 1999;319(7205):279. https://doi.org/10.1136/bmj.319.7205.279.
Thomas E, Croft PR, Paterson SM, Dziedzic K, Hay EM. What influences participants’ treatment preference and can it influence outcome? Results from a primary care-based randomised trial for shoulder pain. Br J Gen Pract. 2004;54(499):93–6.
Carr JL, Klaber Moffett JA, Howarth E, Richmond SJ, Torgerson DJ, Jackson DA, et al. A randomized trial comparing a group exercise programme for back pain patients with individual physiotherapy in a severely deprived area. Disabil Rehabil. 2005;27(16):929–37. https://doi.org/10.1080/09638280500030639.
Moffett JK, Jackson DA, Richmond S, Hahn S, Coulton S, Farrin A, et al. Randomised trial of a brief physiotherapy intervention compared with usual physiotherapy for neck pain patients: outcomes and patients’ preference. BMJ. 2005;330(7482):75. https://doi.org/10.1136/bmj.38286.493206.82.
Moffett JK, Jackson DA, Gardiner ED, Torgerson DJ, Coulton S, Eaton S, et al. Randomized trial of two physiotherapy interventions for primary care neck and back pain patients: ‘McKenzie’ vs brief physiotherapy pain management. Rheumatology. 2006;45(12):1514–21. https://doi.org/10.1093/rheumatology/kel339.
Salter GC, Roman M, Bland MJ, MacPherson H. Acupuncture for chronic neck pain: a pilot for a randomised controlled trial. BioMed Central Musculoskelet Disord. 2006;7:99. https://doi.org/10.1186/1471-2474-7-99.
Johnson RE, Jones GT, Wiles NJ, Chaddock C, Potter RG, Roberts C, et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine. 2007;32(15):1578–85. https://doi.org/10.1097/BRS.0b013e318074f890.
McLean SM, Klaber Moffett JA, Sharp DM, Gardiner E. A randomised controlled trial comparing graded exercise treatment and usual physiotherapy for patients with non-specific neck pain (the GET UP neck pain trial). Man Ther. 2013;18(3):199–205. https://doi.org/10.1016/j.math.2012.09.005.
Author information
Authors and Affiliations
Contributions
QAL conceived the research and contributed to the development of the study design. Both authors screened a portion of abstracts and performed full-text review. DD oversaw quality appraisal tasks and drafted the manuscript. Both authors provided critical review in the draft of the final manuscript.
Corresponding author
Ethics declarations
Funding
Unrestricted funding was received through the PhRMA Foundation Value Assessment Research Award.
Conflict of Interest
Dimittri Delevry and Quang A. Le have no conflicts of interests that are directly relevant to the content of this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Electronic Supplementary Material files.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Delevry, D., Le, Q.A. Effect of Treatment Preference in Randomized Controlled Trials: Systematic Review of the Literature and Meta-Analysis. Patient 12, 593–609 (2019). https://doi.org/10.1007/s40271-019-00379-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-019-00379-6