Abstract
Increasing endogenous tear film production via pharmacological neuroactivation of the nasolacrimal reflex [NLR; also known as the trigeminal parasympathetic pathway (TPP)] is a novel therapeutic approach to treating dry eye disease (DED). An intranasal formulation of the water-soluble, small-molecule, nicotinic acetylcholine receptor (nAChR) agonist varenicline (Tyrvaya™) has been approved in the USA for the treatment of DED. Twice-daily administration of varenicline solution nasal spray resulted in rapid, statistically significant and clinically meaningful improvements in the signs and symptoms of DED over a period of 4 weeks in two pivotal studies (ONSET-1 and -2). The efficacy of varenicline solution was maintained over a longer-term period of 12 weeks in a third study (MYSTIC). Consistent with the nasal route of delivery, the most common adverse events reported by varenicline solution recipients were non-ocular in nature (mild and transient sneezing and cough). Thus, varenicline solution nasal spray is a rapidly-acting, effective and generally well tolerated treatment for DED that offers several potentially useful advantages over existing topical ocular therapies in terms of increasing endogenous tear secretion and reducing ophthalmic treatment burden.
Plain Language Summary
Dry eye disease (DED) is a common, often chronic, condition characterized by symptoms, such as irritation and blurred vision, that can negatively impact on quality of life. DED occurs due to the production of insufficient or unstable tear films and is typically treated with topically applied artificial tears and medications that reduce accompanying inflammation of the ocular surface. Using an intranasal formulation of the nicotinic acetylcholine receptor (nAChR) agonist varenicline (Tyrvaya™) to enhance natural tear production represents a novel approach to DED treatment. Varenicline solution nasal spray led to fast and sustained improvements in the signs and symptoms of DED in clinical trials of up to 12 weeks’ duration. Varenicline solution was also generally well tolerated, with the most common adverse events being mild and transient sneezing and cough. Varenicline solution nasal spray is a new type of treatment for DED that may increase natural tear production, have better ocular tolerability and, for some patients, be easier and/or more convenient to use compared with traditional topical therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.21070018. |
nAChR agonist administered via a preservative-free, low-volume (0.05 mL), aqueous nasal spray |
Increases endogenous tear film production by stimulating the NLR/TPP |
Improves signs and symptoms of DED in 4- and 12-week studies |
Most common adverse events are non-ocular (sneezing and cough) |
1 Introduction
Dry eye disease (DED; also known as keratitis sicca) is a multifactorial, often chronic, condition characterized by a persistently unstable and/or deficient tear film causing discomfort and/or visual impairment, accompanied by variable degrees of ocular surface epitheliopathy, inflammation, and neurosensory abnormalities [1, 2]. An estimated 20–40 million adults in the USA have DED; the prevalence is higher among women than men and increases with age [3, 4]. DED can have a detrimental impact on patients’ quality of life and confers a considerable economic burden, particularly in terms of indirect costs due to reduced work productivity [1, 4].
In the absence of curative therapies, the ongoing management of DED involves an individualized, step-wise approach, starting with education on dietary modifications, eyelid hygiene and avoidance of exacerbating factors, and progressing to the use of ophthalmic lubricants, and, eventually, pharmacological and nonpharmacological interventions, depending on disease severity [5,6,7]. Thus, over-the-counter artificial tears to replace/supplement natural tears—the mainstay of DED therapy—are recommended for mild disease, while topical anti-inflammatory drugs, such as corticosteroids, the calcineurin inhibitor immunosuppressant cyclosporine and the integrin antagonist lifitegrast, which aim to reduce the inflammation caused by unstable/deficient tear film, are also recommended as needed for moderate to severe disease [5,6,7].
Although topical eye drops are the most accepted and widely used formulation in ocular drug delivery, they are not without limitations, including a generally low bioavailability [8, 9]. Additionally, commercially available eye drops commonly contain excipients and/or preservatives that can potentially exhibit ocular toxicity and further exacerbate the signs and symptoms of DED [8, 10]. Moreover, artificial tears and anti-inflammatory agents only alleviate the symptoms of DED and do not address the underlying cause of the disease [6]. Available anti-inflammatory agents also have a slow onset of action and are associated with local adverse events (e.g. ocular burning, instillation-site pain/irritation and decreased visual acuity) [5], both of which may be barriers to patient adherence [11]. As such, there is a need for treatment options that are rapid-acting, effective and well tolerated and that can re-establish tear film homeostasis in DED.
Tear film production is mediated by the lacrimal functional unit (LFU) consisting of the ocular surface (cornea, conjunctiva, accessory lacrimal glands, and meibomian glands), the main lacrimal gland and the interconnecting innervation [12, 13]. Tear secretion is regulated by neural reflex arcs, including those that are triggered by activation of trigeminal afferent nerves in the cornea and conjunctiva (that lead to stimulation of efferent parasympathetic and sympathetic nerves in the facial nerve innervating the LFU) [12, 14] and the nasal cavity (that lead to stimulation of trigeminal efferent parasympathetic nerves innervating the LFU) [15, 16]. The latter pathway, known as the nasolacrimal reflex (NLR) or trigeminal parasympathetic pathway (TPP), accounts for approximately one-third of basal tear film production [17]. In recent years, intranasal neurostimulation of the NLR/TPP—a non-invasive approach to promoting tear film production that bypasses the ocular surface—has emerged as a novel therapeutic modality for DED [15, 16, 18].
Varenicline is a water-soluble, small-molecule, nicotinic acetylcholine receptor (nAChR) agonist that binds with high affinity and selectivity to various human neuronal nAChR subtypes, including α4β2, α4α6β2, α4β3 and α3α5β4 (where it displays partial agonist activity) and α7 (where it demonstrates full agonist activity) [19,20,21]. A preservative-free, intranasal formulation of varenicline (Tyrvaya™) has been developed and approved in the USA for the treatment of the signs and symptoms of DED [20]. This review briefly summarizes the pharmacological properties of varenicline administered via the nasal route and focuses on the efficacy and safety of varenicline solution nasal spray (hereafter also referred to as varenicline solution) in the treatment of DED. Varenicline was originally developed and approved in the USA as an oral smoking cessation aid [22]; however, discussion of this tablet formulation of the drug is beyond the scope of the current article.
2 Pharmacological Properties of Varenicline Solution
The exact mechanism of action underlying the efficacy of intranasally-administered varenicline in DED is unknown, but is believed to result from the drug binding to—and producing agonist activity at—nAChRs on trigeminal sensory nerve endings within the anterior nasal cavity. Activation of the NLR/TPP ultimately leads to the stimulation of endogenous tear film secretion from the LFU (Sect. 1) [11, 20, 23].
In IMPERIAL, a phase II study in 18 patients with DED and an Ocular Surface Disease Index (OSDI) score of ≥ 23, administration of a single intranasal dose of varenicline solution 0.06 mg [0.03 mg per 0.05 mL spray in each nostril, i.e. the approved dose strength (Sect. 5)] reduced conjunctival goblet cell area and perimeter relative to vehicle control ≈ 10 min after study drug administration [24]. This is suggestive of goblet cell degranulation and associated release of lubricating mucins into the tear film, which play an important role in re-establishing natural tear film homeostasis [24].
Varenicline was rapidly absorbed, being detected in plasma within 5 min, and generally reaching a peak plasma concentration (Cmax) within 2 h, after administration of a single intranasal dose of varenicline solution 0.12 mg [0.06 mg per 0.05 mL spray in each nostril, i.e. twice the approved dose strength (Sect. 5)] [11]. The mean Cmax was 0.34 ng/mL and the mean area under the plasma concentration-time curve from time zero to infinity (AUC0–∞) was 8.30 ng·h/mL. The relative bioavailability of varenicline after administration of varenicline solution 0.12 mg was lower than that following administration of oral varenicline at its highest approved single-dose strength (1 mg). Specifically, the mean Cmax and AUC0–∞ of varenicline after intranasal administration were, respectively, 7.0% and 7.5% of that following oral administration [11].
The mean elimination half-life of intranasally-administered varenicline was 18.9 h [11]. Circulating varenicline does not undergo significant hepatic metabolism (92% of the administered dose is excreted in the urine as unchanged drug [20]) and neither inhibits nor induces the major cytochrome P450 isoenzymes [11].
3 Therapeutic Efficacy of Varenicline Solution
The efficacy of varenicline solution nasal spray in the treatment of DED was evaluated in three randomized, double-masked, placebo (vehicle)-controlled trials: the short-term (4-week/28-day) ONSET-1 [25] and -2 [26] studies, which were conducted at multiple centres in the USA (Sect. 3.1; Fig. 1); and the longer-term (12-week/84-day) MYSTIC study [27], which was conducted at a single centre in Mexico (Sect. 3.2). This section focuses on findings for patients who received varenicline solution at the approved dosage of 0.03 mg in each nostril twice daily (Sect. 5) or vehicle in these studies.
Trial design of the randomized, double-masked, vehicle-controlled, multicentre, phase IIb ONSET-1 and phase III ONSET-2 trials in adults with dry eye disease [25, 26], with further information available in Table 1. Efficacy results for the intranasal varenicline solution dosage approved in the USA are reported in the animated figure (available online). BID twice daily, BL baseline, DED dry eye disease, LSM least squares mean, NA not reported, OR odds ratio, pts patients, STS Schirmer test score
Supplementary file2 (MP4 6190 KB)
Eligible patients were aged ≥ 22 years, had received a diagnosis of DED and had used (or expressed the desire to use) an artificial tear substitute for symptoms within 6 months of the study start [25,26,27]. The ocular inclusion criteria included: baseline Schirmer test score (STS) performed with topical anaesthesia (a measure of basal tear film production) of ≤ 10 mm/5 min and STS ≥ 7 mm greater in the same eye upon intranasal mechanical stimulation with a cotton swab; corneal fluorescein staining score of ≥ 2 in at least one corneal region or a sum of ≥ 4 for all corneal regions; OSDI score of ≥ 23, with up to three responses of “not applicable” (ONSET-1 and -2 only); and best-corrected visual acuity < 0.7 logarithm of the minimum angle of resolution [25,26,27]. If both eyes qualified, then the eye with the greatest increase in STS upon mechanical stimulation or, if no difference, the eye with the lower basal STS, was selected as the study eye [25, 26]. If neither measure differed, the right (as opposed to left) eye was chosen as the study eye [25, 26]. Of note, eligibility in the ONSET studies was not limited by the baseline severity of DED symptoms, as assessed in terms of the eye dryness score (EDS). All three studies excluded patients who used contact lenses within 7 days of screening or anticipated using lenses during the study period [25,26,27].
3.1 ONSET Studies
Participants in the phase IIb ONSET-1 study (n = 182) were randomized to receive varenicline solution 0.006 mg (n = 47), 0.03 mg (n = 48) or 0.06 mg (n = 44), or vehicle (n = 43) [25], while those in the phase III ONSET-2 study (n = 758) were randomized to receive varenicline solution 0.03 mg (n = 260) or 0.06 mg (n = 246), or vehicle (n = 252) [26] (Fig. 1). These doses of varenicline solution (and vehicle) were delivered with each actuation of an intranasal spray device; patients administered one 0.05 mL spray in each nostril twice daily for 4 weeks [25, 26]. Randomization was not stratified by baseline factors in ONSET-1 [25], but was stratified according to preprocedure anaesthetized STS (≤ 5 mm vs > 5 mm), preprocedure EDS (< 60 vs ≥ 60) and study site in ONSET-2 [26]. Use of artificial tears was permitted during the studies [20, 26].
The primary efficacy endpoint in ONSET-1 was the change in the anaesthetized STS in the study eye from baseline to day 28 [25], whereas that in ONSET-2 was the proportion of patients achieving a ≥ 10 mm improvement in anaesthetized STS in the study eye from baseline to week 4 [26]. Secondary endpoints included the change from baseline in EDS, as assessed in a controlled adverse environment (CAE) chamber (at day 21 in ONSET-1 and day 28 in ONSET-2) and in the clinic (at day 28 in both ONSET-1 and -2) [25, 26].
Baseline demographics and ocular assessments were generally similar between the varenicline solution 0.03 mg and vehicle groups in both trials [25, 26]. Across the four treatment groups, the mean age of patients ranged from ≈ 58–67 years; the majority were female (71–80%) and White (81–93%). Baseline anaesthetized STS and EDS score ranged from 4.5–5.1 and 58.1–65.2, respectively (Table 1) [25, 26].
Four weeks of treatment with varenicline solution 0.03 mg resulted in statistically significant and clinically meaningful improvements in signs and symptoms of DED (Table 1) [25, 26]. In terms of basal tear film production, the improvements in anesthetized STS from baseline to day 28 and the proportions of patients achieving a ≥ 10 mm improvement in anaesthetized STS at week 4 were significantly (p < 0.001) greater with varenicline solution than with vehicle in both studies (Table 1) [25, 26].
A post hoc exploratory analysis conducted on the primary endpoint of ONSET-1 using a last observation carried forward (LOCF) approach to account for missing data yielded comparable results to the primary analysis [25]. The least squares mean (LSM) improvements in anesthetized STS from baseline were 11.7 (95% CI 9.24–14.26) mm in the varenicline solution group (n = 48) versus 3.2 (95% CI 0.62–5.80) mm in the vehicle group (n = 43) [p < 0.0001] [25]. Similarly, post hoc analyses performed on the primary endpoint of ONSET-2 showed that significantly (p ≤ 0.03) greater proportions of varenicline solution than vehicle recipients achieved a ≥ 10 mm improvement in anaesthetized STS, irrespective of the baseline severity of basal tear production [odds ratios (ORs) of 3.46 (95% CI 1.99–6.04) and 1.83 (95% CI 1.03–3.24) for anaesthetized STS ≤ 5 and > 5 mm] or DED symptoms [ORs of 3.37 (95% CI 1.81–6.29) and 2.05 (95% CI 1.22–3.46) for EDS < 60 and ≥ 60]) [26].
In ONSET-1, varenicline solution significantly improved EDS from baseline relative to vehicle, as assessed in the CAE chamber (Table 1) and in the clinic [LSM treatment difference (varenicline solution vs vehicle) of − 13.3 mm; p = 0.021] [25]. In comparison, varenicline solution did not significantly improve EDS from baseline in the CAE chamber compared with vehicle in ONSET-2 (Table 1), potentially because of limitations imposed by social distancing requirements during the coronavirus disease 2019 pandemic, which restricted use of the CAE chamber and collection of symptom data in ≈ 30% of the study population [26]. In terms of EDS assessed in the clinic, the improvement from baseline to day 28 with varenicline solution was significantly greater than with vehicle by week 2 [LSM treatment difference (varenicline solution vs vehicle) of − 3.7 mm; nominal p = 0.049]; this trend continued at week 4 [LSM treatment difference of − 4.4 mm; nominal p = 0.038] [26].
3.1.1 Pooled Data
Varenicline solution was effective in increasing basal tear production, regardless of baseline severity of DED symptoms, according to an LOCF analysis of data pooled from ONSET-1 and -2 [28]. The mean improvement in anesthetized STS from baseline to week 4 was significantly (p ≤ 0.01) greater among varenicline solution 0.03 mg than vehicle recipients in both the baseline EDS < 40 subgroup (8.4 vs 4.1 mm) and the baseline EDS ≥ 40 subgroup (9.9 vs 3.9 mm). Similarly, significantly (p ≤ 0.03) more varenicline solution 0.03 mg than vehicle recipients achieved a ≥ 10 mm improvement in anaesthetized STS at week 4 in both the baseline EDS < 40 subgroup (30.9 vs 18.5%; OR of 3.02) and the baseline EDS ≥ 40 subgroup (38.8 vs 17.9%; OR of 3.17) [28].
Varenicline solution was administered bilaterally (i.e. to each nostril) in these studies and the pooled analysis also showed that basal tear production was increased in fellow eyes as well as in study eyes [28]. For study eyes, the LSM improvement in anesthetized STS from baseline to week 4 was 10.5 mm in varenicline solution 0.03 mg recipients versus 5.0 mm in vehicle recipients; the corresponding results for the fellow eyes were 8.8 versus 2.8 mm (values estimated from a graph). Similarly, more varenicline solution 0.03 mg than vehicle recipients achieved a ≥ 10 mm improvement in anaesthetized STS at week 4, based on outcomes in study eyes [OR of 2.86 (95% CI 1.98–4.14)] as well as in fellow eyes [OR of 3.53 (95% CI 2.34–5.31)] (values estimated from a graph) [28].
3.2 MYSTIC Study
Eligible patients enrolled in the phase II MYSTIC study (n = 123) were randomized to receive varenicline solution 0.03 mg (n = 41) or 0.06 mg (n = 41), or vehicle (n = 41) [27]. As in the ONSET studies, these doses of varenicline solution (and vehicle) were delivered with each actuation of an intranasal spray device; patients administered one spray (0.05 mL) in each nostril twice daily for 12 weeks. The primary efficacy endpoint was the change in the anesthetized STS in the study eye from baseline to day 84 [27].
Baseline characteristics were generally similar with respect to mean age (51 years in the varenicline solution group vs 56 years in the vehicle group), female sex (78% vs 80%) and anaesthetized STS (5.5 vs 5.3; Table 1); all patients were Hispanics/Latinos [27].
Varenicline solution 0.03 mg significantly improved tear film production in patients with DED over a 12-week period (Table 1) [27]. Notably, tear production increased as early as 5 min after administration of varenicline solution and was maintained throughout the 84-day study, with no evidence of drop-off in efficacy or development of tolerance with long-term use [27]. Just over one-third of varenicline solution recipients compared with one-quarter of vehicle recipients experienced a ≥ 10 mm improvement in anaesthetized STS at week 12, although the between-group difference was not statistically significant (Table 1) [27].
4 Tolerability of Varenicline Solution
Varenicline solution nasal spray was generally well tolerated in the ONSET-1 and -2 and MYSTIC studies (Sect. 3) [25,26,27]. Overall, a total of 349 patients in these three trials received at least one dose of varenicline solution at the approved strength (0.03 mg in each nostril twice daily; Sect. 5), with the majority having 31 days of treatment exposure (maximum treatment exposure was 105 days) [20]. The most common adverse reactions reported in varenicline solution recipients included sneezing (82%), cough (16%), throat irritation (13%), and instillation-site (nose) irritation (8%) [20].
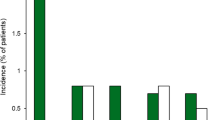
In ONSET-2, 16.5% and 97.3% of patients receiving varenicline solution (n = 260) reported at least one ocular treatment-emergent adverse event (TEAE) and at least one non-ocular TEAE, respectively, compared with 16.3% and 57.0% of patients receiving vehicle (n = 251) [26]. Conjunctival hyperaemia (4.6% with varenicline solution vs 2.8% with vehicle) and reduced visual acuity (3.5% vs 4.4%) were the most common ocular TEAEs, with most of these events not considered to be related to the study drug. Sneezing was the most common non-ocular TEAE reported by patients receiving varenicline solution (Fig. 2). Nearly all varenicline solution recipients sneezed at least once during treatment (93.8% vs 28.3% of vehicle recipients), with the majority (84.5%) of sneezing occurring within the first minute post administration. Other non-ocular TEAEs frequently reported byvarenicline solution recipients included cough, throat irritation and instillation site irritation (Fig. 2). Most non-ocular TEAEs were considered mild and were reported by more varenicline solution than vehicle recipients (Fig. 2). In ONSET-2, no serious TEAEs or deaths were considered to be related to the study drug. Five patients (1.9%) in the varenicline solution group and four (1.6%) in the vehicle group experienced TEAEs that led to treatment discontinuation [26].
Non-ocular treatment-emergent adverse events reported in ≥ 5% of patients treated with varenicline solution 0.03 mg or vehicle in each nostril twice daily in the ONSET-2 trial [26]. VAR varenicline solution, VEH vehicle
In MYSTIC, 9.8% and 14.6% of patients receiving varenicline solution (n = 41) reported at least one ocular TEAE and at least one non-ocular TEAE, respectively, compared with 9.8% and 22.0% of patients receiving vehicle (n = 41) [27]. The four varenicline solution recipients who experienced ocular TEAEs all had reduced visual acuity considered to be related to the study drug. In comparison, reduced visual acuity was not considered to be related to the study drug in any of the three vehicle recipients who reported this ocular TEAE. The most common non-ocular TEAE was transient sneezing (lasting 1–2 min), which was reported by two patients (4.9%) in each treatment group and in all cases was considered to be related to the study drug. Most TEAEs were mild in severity; no severe TEAEs, serious TEAEs or deaths occurred. TEAEs leading to treatment discontinuation were reported in one varenicline solution recipient (headache on day 1) versus three vehicle recipients (nausea, dizziness and nasal discomfort on day 1; chest pain and dyspnoea on day 1; depression on day 30). Notably, discontinuations generally occurred early in the study, suggesting that TEAEs did not increase over time [27].
5 Dosage and Administration of Varenicline Solution
Varenicline solution nasal spray is indicated for the treatment of the signs and symptoms of DED in the USA [20]. It is available in a device that delivers 0.03 mg of varenicline with each 0.05 mL spray; the recommended dosing regimen is one spray in each nostril twice daily (≈ 12 h apart) [20].
Consult local prescribing information for further details regarding priming instructions, contraindications, warnings and precautions and use in special populations.
6 Current Status of Varenicline Solution in the Management of Dry Eye Disease
Twice-daily, bilateral administration of varenicline solution nasal spray was an efficacious and generally well tolerated treatment for DED, as shown in the 4-week ONSET-1 and -2 studies (Sect. 3.1). Benefits were seen in both (study and fellow) eyes, and basal tear production was increased, regardless of the baseline severity of DED symptoms (Sect. 3.1.1). Moreover, the efficacy and safety of this novel therapeutic modality was maintained over the longer-term, as demonstrated in the 12-week MYSTIC study (Sect. 3.2). Across these trials, improvements in the signs and symptoms of DED resulting from pharmacological neuroactivation of the NLR/TPP with varenicline solution were statistically significant and clinically meaningful (Sect. 3). Of note, varenicline solution had a rapid onset of action, with an increase in tear film production seen as early as 5 min after the first administration; future studies will address how long the increase in tear production persists post treatment [27]
Consistent with the nasal route of delivery, varenicline solution recipients reported comparatively few ocular TEAEs; in particular, there were no cases of burning or stinging (Sect. 4). The most common TEAEs reported by varenicline solution recipients included sneezing (a well-documented reflex response to stimulation of the trigeminal nerve in the nasal cavity) and cough, which, in most cases, were mild and transient and occurred ≤ 1 min after administration (Sect. 4).
The generalizability of the ONSET and MYSTIC study results is variously aided by trial design features, such as enrolment not being dependent on DED symptom severity (EDS score), the lack of a placebo run-in period (often used in DED trials to exclude patients who respond to vehicle treatment) and the permitted use of artificial tears as needed [25,26,27]. Nonetheless, additional long-term studies are desirable as the MYSTIC study population was limited to Hispanic or Latino patients [27, 29]. Real-world studies designed to confirm the effectiveness of varenicline solution for the treatment of DED in clinical practice are also warranted.
Based on the results of the ONSET studies, varenicline solution has become the first pharmacological neuroactivator of the NLR/TPP to be approved for the treatment of DED in the USA (Sect. 1). As described hereafter in this section, the novel method of administration and mechanism of action of varenicline solution offers a number of potentially useful advantages over existing topical therapies for DED.
Normal tear film consists of aqueous fluid, electrolytes and an array of mucin glycoproteins, lipids and proteins (including trophic/wound healing, innate defence/antimicrobial and anti-inflammatory/antioxidant factors) that enable it to perform its basic functions of lubricating and protecting the ocular surface [30]. As such, there is an important distinction between intranasally administered varenicline solution and topically applied artificial tears in terms of how these differing treatment modalities fundamentally address DED symptoms: the former is designed to increase the secretion of endogenous tears (with all of their lubricant and protective properties), whereas the latter are intended to mimic the secretion of natural tears without replicating their complex composition (and hence all of their various functions) [31, 32]. Moreover, efficacy findings for varenicline solution compare favourably with those for topical anti-inflammatory drugs (cyclosporine and lifitegrast) in terms of the speed of onset and/or extent of increase in natural tear production, albeit based on indirect comparisons that are inherently limited in nature [27, 33, 34]. The outcomes of head-to-head studies are therefore awaited with interest. Notably, varenicline solution stimulates natural tear film production from the LFU through a physiologically relevant pathway that is independent of trigeminal afferent nerves in the cornea and conjunctiva, which can be damaged in patients with chronic DED [35].
By avoiding contact with the ocular surface, varenicline solution bypasses the challenge of ocular delivery in terms of limited bioavailability and, moreover, may reduce or possibly even eliminate some of the common administration-related ocular TEAEs associated with topical therapies, such as burning and instillation-site pain/irritation (Sect. 1). Intranasal delivery also spares the ocular surface from exposure to excipients and/or preservatives commonly used in eye drops that can potentially exhibit ocular toxicity and further exacerbate the signs and symptoms of DED (Sect. 1).
From the patient perspective, varenicline solution offers the option of a non-ophthalmic preparation for those who may prefer this mode of delivery over an ophthalmic one [29]. Such patients could include those wishing to reduce the ocular treatment burden if they are already using topical eye drops for other ocular conditions (e.g. glaucoma), those who have difficulty independently administering topical eye drops to treat their DED (e.g. due to tremors, dexterity issues or neck deformities) and contact lens users who must otherwise coordinate ophthalmic administration with the wearing of their lens [26, 27, 29]. As regards the latter, the efficacy of varenicline solution in treating the signs and symptoms of DED is currently being evaluated in 75 daily disposable contact lens wearers in an investigator-initiated, randomized, double-masked, placebo-controlled, single-centre study (NCT05161208).
In conclusion, therefore, varenicline solution nasal spray is a rapidly-acting, effective and generally well tolerated treatment for DED that, by virtue of its novel method of administration and mechanism of action, offers several potentially useful advantages over existing topical ocular therapies in terms of increasing endogenous tear secretion and reducing ophthalmic treatment burden.
Data Selection Varenicline Solution: 57 records identified
Duplicates removed | 10 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 0 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 12 |
Cited efficacy/tolerability articles | 6 |
Cited articles not efficacy/tolerability | 29 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were varenicline, OC-01, Tyrvaya, intranasal, dry eye disease, DED, keratitis sicca. Records were limited to those in English language. Searches last updated 19 Sep 2022. | |
References
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83.
Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15:438–510.
Farrand KF, Fridman M, Stillman IO, et al. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–8.
Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806.
Shen Lee B, Kabat AG, Bacharach J, et al. Managing dry eye disease and facilitating realistic patient expectations: a review and appraisal of current therapies. Clin Ophthalmol. 2020;14:119–26.
Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628.
Akpek EK, Amescua G, Farid M, et al. Dry eye syndrome preferred practice pattern®. Ophthalmology. 2019;126(1):286–334.
Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021;13(2):207.
Kumar Raj V, Mazumder R, Madhra M. Ocular drug delivery system: challenges and approaches. Int J Appl Pharm. 2020;12(5):49–57.
Walsh K, Jones L. The use of preservatives in dry eye drops. Clin Ophthalmol. 2019;13:1409–25.
Nau J, Wyatt DJ, Rollema H, et al. A phase I, open-label, randomized, 2-way crossover study to evaluate the relative bioavailability of intranasal and oral varenicline. Clin Ther. 2021;43(9):1595–607.
Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–77.
Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–16.
Labetoulle M, Baudouin C, Calonge M, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137–45.
Yu MD, Park JK, Kossler AL, et al. Stimulating tear production: spotlight on neurostimulation. Clin Ophthalmol. 2021;15:4219–26.
Dieckmann G, Fregni F, Hamrah P. Neurostimulation in dry eye disease: past, present, and future. Ocul Surf. 2019;17(1):20–7.
Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16(6):645–8.
Pflugfelder SC, Cao A, Galor A, et al. Nicotinic acetylcholine receptor stimulation: a new approach for stimulating tear secretion in dry eye disease. Ocul Surf. 2022;25:58–64.
Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–5.
Oyster Point Pharmaceuticals. TYRVAYA™ (varenicline solution) nasal spray: US prescribing information. 2021. https://www.tyrvaya.com. Accessed 18 Aug 2022.
Bordia T, Hrachova M, Chin M, et al. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012;342(2):327–34.
Pfizer Laboratories Div Pfizer Inc. CHANTIX® (varenicline tablets, for oral use): US prescribing information. 2019. https://www.chantix.com. Accessed 18 Aug 2022.
Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25(1):61–6.
Dieckmann GM, Cox SM, Lopez MJ, et al. A single administration of OC-01 (varenicline solution) nasal spray induces short-term alterations in conjunctival goblet cells in patients with dry eye disease. Ophthalmol Ther. 2022;11(4):1551–61.
Wirta D, Torkildsen GL, Boehmer B, et al. ONSET-1 phase 2b randomized trial to evaluate the safety and efficacy of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease. Cornea. 2022;41(10):1207–16.
Wirta D, Vollmer P, Paauw J, et al. Efficacy and safety of OC-01 (varenicline solution) nasal spray on signs and symptoms of dry eye disease: the ONSET-2 phase 3 randomized trial. Ophthalmology. 2022;129(4):379–87.
Quiroz-Mercado H, Hernandez-Quintela E, Chiu KH, et al. A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (varenicline solution) nasal spray for dry eye disease: the MYSTIC study. Ocul Surf. 2022;24:15–21.
Periman LM, Maiti S, Kabat AG, et al. Bilateral effect of OC-01 nasal spray for treatment of dry eye disease signs and symptoms in subjects with mild, moderate and severe dry eye disease, as determined by baseline eye dryness score [abstract no. A0253]. Investig Ophthalmol Vis Sci. 2022;63(7):1528.
Zitko KL, Ladd L, Dougherty TS. Intranasal varenicline: review of a novel formulation for the treatment of dry eye disease. J Pharm Pract. 2022. https://doi.org/10.1177/08971900221108725.
Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197: 108115.
Kim M, Lee Y, Mehra D, et al. Dry eye: why artificial tears are not always the answer. BMJ Open Ophthalmol. 2021;6: e000697.
Kathuria A, Shamloo K, Jhanji V, et al. Categorization of marketed artificial tear formulations based on their ingredients: a rational approach for their use. J Clin Med. 2021;10(6):1289.
Visco DM, Hendrix LH, Sun L, et al. Matching-adjusted indirect comparison of phase 3 clinical trial outcomes: OC-01 (varenicline solution) nasal spray and cyclosporine a 0.05% ophthalmic emulsion for the treatment of dry eye disease. J Manag Care Spec Pharm. 2022;28(8):892–902.
White DE, Hendrix LH, Sun L, et al. Matching-adjusted indirect comparison of phase 3 clinical trial outcomes of OC-01 (varenicline solution) nasal spray and lifitegrast 5% ophthalmic solution for the treatment of dry eye disease. J Manag Care Spec Pharm. 2022. https://doi.org/10.18553/jmcp.2022.22208.
Friedman NJ, Butron K, Robledo N, et al. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. 2016;10:795–804.
Acknowledgements
During the peer review process, the manufacturer of varenicline solution was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: M. Baiula, Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy; K. Tsubota, Department of Ophthalmology, Keio University School of Medicine, Tokyo, Japan.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frampton, J.E. Varenicline Solution Nasal Spray: A Review in Dry Eye Disease. Drugs 82, 1481–1488 (2022). https://doi.org/10.1007/s40265-022-01782-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01782-4