Abstract
Assisted reproduction technologies have substantially advanced since the birth of the first in vitro fertilization baby, and innovations within in vitro fertilization laboratories have been of paramount importance for the overall assisted reproduction technology success rates. However, one of the milestones in the history of in vitro fertilization is irrefutably the introduction of conventional ovarian stimulation. The objective of the present review is to provide an update on conventional ovarian stimulation, by giving an overview of treatment milestones, together with the latest innovations currently being investigated. The realization of an assisted reproduction technology treatment depends on many steps that can be medically manipulated and must be harmoniously combined, starting from the follicular phase and ending with luteal phase support. New technologies in the pharmaceutical sector are fundamental to optimize efficiency and tailor treatment approaches to individual needs. The present review aims to offer physicians a useful summary of the more recent publications and to facilitate the translation of research findings into daily clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gonadotropins, gonadotropin-releasing hormone agonists, and gonadotropin-releasing hormone antagonists are gold-standard drugs in ovarian stimulation to increase the number of follicles and to prevent spontaneous ovulation. |
The field remains in a continuous evolution towards personalized medicine. |
The goals of current research aim to develop safer, more cost-effective, and more patient-friendly interventions. |
1 Introduction
Ovarian stimulation (OS) is a complex longitudinal process inducing a super-physiological cycle with the intention of obtaining multiple oocytes. The number of oocytes is a pivotal prognostic indicator of cumulative live birth rate (LBR) [1], which is the final outcome measure of assisted reproductive technology (ART) treatments [2].
To maximize treatment efficacy, well-established algorithms should be adopted but also adapted to individual patients’ characteristics, taking into account clinical history, age, and ovarian reserve. The process of in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) consists of five sequential steps, which are the actual OS, to induce multi-follicular development, the ovulation trigger, aiming to induce final oocyte maturation, the oocyte retrieval, aiming to harvest the matured oocytes, the laboratory procedure, aiming to create embryos, and the embryo transfer, which aims to transfer the available embryo(s) inside the uterus. Specific pharmacological treatments are required at each stage, and the success of the former is a mandatory premise to the later phases. Despite a consistent body of evidence, the rate of success (cumulative LBR) is around 30%, indicating that there is still a need for improvement and research in the field [3].
The objective of the present review is to provide an update on OS, via an overview of treatment milestones together with the latest innovations currently being investigated. The structure of the review follows the timeline of a typical ART protocol, with the first four chapters covering interventions within the follicular phase, from initiation of treatment to ovulation triggers, along with potential modifications of the conventional OS protocol, whereas the last section will be dedicated to the management of the luteal phase following the embryo transfer (Fig. 1).
2 Ovarian Stimulation for In Vitro Fertilization/Intracytoplasmic Sperm Injection (IVF/ICSI)
Assisted reproduction technologies have substantially advanced since the birth of the first IVF baby [4], and innovations within IVF laboratories [5] have been of utmost importance for the overall ART success rates. However, one of the milestones in the history of IVF is irrefutably the introduction of OS [6], which resulted in a considerable increase in pregnancy rates as compared with the very low success rates following the first unstimulated IVF cycles [7].
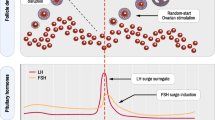
The aim of OS is to induce multi-follicular development after the administration of exogenous gonadotropins. This results in an extension of the follicle-stimulating hormone (FSH) window in the follicular phase and maintains FSH levels above the threshold, allowing multi-follicular instead of mono-follicular development [8] (Fig. 2).
Although initially IVF was developed for the management of couples with tubal disease, currently, IVF is used for several other causes of infertility, including male and unexplained infertility, patients with low ovarian reserve, endometriosis, and ovulatory disorders. Finally, in the case of severe male factor infertility, an ICSI could be applied, which involves the injection of a single live sperm directly into the middle of a human oocyte [5].
2.1 Aim of IVF/ICSI
The primary objective of OS is to increase the number of follicles and consequently the number of oocytes retrieved and the number of available embryos, enabling the selection of the best-quality embryo for transfer. Preferably one (or in some cases multiple) embryo(s) can be transferred following the oocyte retrieval, and supplementary embryos can be frozen to increase future chances of pregnancy without the need to repeat OS. Furthermore, it should be highlighted that over recent year, live births after the replacement of frozen-thawed embryos have substantially increased and frozen embryo transfers managed to reach the success rates observed in fresh embryo transfer cycles [9,10,11]. In this regard, the reporting of an IVF treatment should not only incorporate outcomes associated with fresh embryo transfers but also those resulting from the replacement of supplementary frozen/thawed embryos to provide an all-inclusive success rate, which is comprehensive, relevant, and meaningful for the infertile couple [2, 12]. A cumulative LBR could be defined as the first liveborn baby in the fresh or one of the subsequent frozen cycles, following a single OS cycle [13].
2.2 Gonadotropins
Despite the notion that the first IVF baby, Louise Brown, was born in 1978 following IVF performed in a natural cycle without exogenous stimulation [4], soon thereafter OS became the standard of care in clinical IVF. In this regard, exogenous gonadotropin administration is the major component of OS (Table 1). The concept of stimulating ovarian function by exogenous administration of gonadotropin preparations has intrigued investigators for many decades (Fig. 3).
Historical milestones in drug administrations for assisted reproductive technology [185]. FbM, FSH follicle-stimulating hormone, hCG human chorionic gonadotrophin, HP highly purified, LH luteinizing hormone, PMSG pregnant mare serum gonadotropin, rec recombinant, u-FSH urinary FSH, uHMG urinary human menopausal gonadotropin
Although those promising results were reported by gonadotropins extracted from the blood of pregnant mares (pregnant mare serum gonadotropin), the fact that animal gonadotropins may induce anti-hormone antibodies, which could neutralize both the preparation administered and endogenous gonadotropins, was the mainspring behind gonadotropin extraction and purification from human sources [14]. Clinical experiments in the late 1950s demonstrated that extracts derived from the human pituitary gland could be used to stimulate gonadal function [15]. Subsequently, experiments involving the extraction of both gonadotropic hormones (luteinizing hormone (LH) and FSH) from the urine of postmenopausal women resulted in the development of human menopausal gonadotropin (hMG) preparations. Numerous different OS regimens have been developed using exogenous gonadotropins, combining several preparations, different starting days, and doses [16]. The current products can be injected subcutaneously rather than intramuscularly and pen injection devices are available, enabling self-administration, which is more convenient and less time consuming. Currently, the most widely used gonadotropins are highly purified (hp) hMG and recombinant FSH (rFSH), showing similar pregnancy and LBRs [17, 18]; albeit rFSH may lead to more oocytes [16,17,18].
2.2.1 Urinary Gonadotropins
Currently, three different types of urinary-derived gonadotropins are available: urinary FSH, hMG, and hphMG. Human menopausal gonadotropin is the most recent urinary gonadotropin developed and is a mixture of FSH, LH, and human chorionic gonadotrophin (hCG) (with LH-like properties) that is collected, extracted, and purified from the urine of post-menopausal women. The usual preparation contains a 1:1 ratio of FSH to LH.
The most recently developed urinary gonadotropin is hphMG, which contains a higher concentration of hCG and a lower dose of LH compared to other urinary gonadotropins [18]. The higher hCG concentration provides most of the LH activity required for oocyte development.
There are few randomized controlled trials (RCTs) comparing the different urinary gonadotropins and they have been unable to demonstrate any significant difference in LBRs [19]; although rFSH was associated with a higher yield of oocytes [20]. The latest European Society of Human Reproductive Endocrinology guidelines suggest that the use of rFSH and hphMG for OS is equally recommended [21].
2.2.2 Recombinant Follicle-Stimulating Hormone
Although initial preparations were not pure, purification technology resulted in the development of molecules with a smaller amount of contaminating non-active proteins [22]. In more recent years, next to purified urinary products, recombinant DNA technology allowed for the mass production of human rFSH and recombinant LH, while the introduction of the filled-by-mass formulation, improved batch-to-batch consistency compared with products quantified by the standard rat in vivo bioassay [23, 24].
2.2.2.1 Follitropin Alfa and Follitropin Beta
Follitropin alfa and follitropin beta were the first gonadotropins developed with recombinant technology. They are both produced in Chinese hamster ovary cell lines and they both have an amino acid sequence identical to that of endogenous human FSH. The half-life is about 24 h, thus a daily administration is required. The major breakthrough was the easier administration through pre-filled pens, the purity, and the lack of batch-to-batch variation [25]. Despite some differences between follitropin alfa and beta, previous studies showed no significant differences between the preparations in terms of efficacy and safety [26]. In terms of pregnancy outcomes and safety, previous evidence demonstrated equivalent pregnancy rates with urinary or rFSH; furthermore, a large recent meta-analysis comparing rFSH with hphMG demonstrated similar biopotency between the diverse type of stimulations, suggesting that the clinician should decide the type of gonadotropin based on convenience, availability, costs, and patient preferences [27].
2.2.2.2 Recombinant Luteinizing Hormone
Following initial studies with rFSH, recombinant LH has been developed mainly for the treatment of hypogonadotropic hypogonadism, which is characterized by the lack or reduced function of the ovaries due to GnRH deficiency, which results in inadequately low serum levels of FSH and LH [28]. Subsequent studies have investigated the role of LH in women undergoing OS for IVF/ICSI, owing to suggestions of a potential deep suppression of LH during OS protocols. Several randomized trials and meta-analyses have been published to date. Initial meta-analyses supported a potentially beneficial effect in poor responders [29]. A more recent RCT failed to identify the difference of the addition in poor responders [30]. However, other studies and systematic reviews suggest that women with a suboptimal response [31, 32] or women of advanced age [33] may experience benefits from the addition of LH.
2.2.2.3 Corifollitropin Alfa
At the end of the twentieth century, a long-acting FSH (corifollitropin alfa) was created by the fusion of the carboxy-terminal arm of the beta-subunit of hCG in the FSH molecule through recombinant technology [34]. Because of its pharmacokinetic profile, corifollitropin alfa can function as a sustained follicle stimulant with a similar pharmacodynamic profile as rFSH, but with the ability to initiate and sustain multiple follicular growth for an entire week. Furthermore, previous large randomized trials in women with normal ovarian responses have demonstrated that corifollitropin alfa as compared with daily rFSH may result in comparable pregnancy rates with a potential increase in the number of oocytes retrieved [35,36,37,38], while studies in poor responders have shown that corifollitropin alfa seems to be an alternative for daily rFSH or hMG injections in poor responder patients [39,40,41].
2.2.2.4 Follitropin Delta
Follitropin delta, an rFSH derived from a human fetal retinal cell line, was recently introduced in OS. The major difference of follitropin delta as compared with previously developed gonadotropins is that for the first-time gonadotropin dosing was linked to patients’ ovarian reserve and weight. Although ovarian reserve parameters are used in clinical practice to tailor stimulation medication dose and a downregulation protocol in OS [42], in all gonadotropins, except follitropin delta, actual dosing is based on clinicians’ discretion.
A follitropin delta dosing algorithm is predefined and considers the serum level of the anti-Müllerian hormone, one of the best serum ovarian reserve markers [43], and the bodyweight to tailor a dose individualized to each woman [44]. The efficacy and safety of the individualized follitropin delta dosing regimen were evaluated in a large RCT that demonstrated the non-inferiority of individualized follitropin delta compared with conventional follitropin alfa regarding pregnancy rates, while the individualized follitropin delta stimulation resulted in in a more targeted response and an improved safety profile [44, 45].
2.3 Pituitary Suppression
The hypothalamus begins the process of ovulation by releasing GnRH in a pulsatile manner. This pulsatile release causes the anterior pituitary gland to secrete LH and FSH, which then act on the ovary. Luteinizing hormone stimulates the theca cells to produce androstenedione, which is then converted to oestradiol. Upon achieving a critical level of oestradiol at the end of the follicular phase of the menstrual cycle, the negative feedback on LH that normally occurs by oestrogen is shut off, and it begins to have a positive feedback on LH release, which results in an “LH surge” that initiates ovulation [46] (Fig. 4). The first stimulated IVF cycles in the early 1980s were characterized by a premature late follicular rise in LH, resulting in compromised pregnancy rates [47].
2.3.1 Gonadotropin-Releasing Hormone (GnRH) Analogs
Therefore, a crucial part of OS involves co-medication to prevent premature luteinization. The two approaches used in clinical practice are pituitary desensitization with extended daily administration of a GnRH agonist in the luteal phase or an immediate suppression of the pituitary LH release with a GnRH antagonist [48, 49] (Fig. 5).
Mechanism of action of the gonadotropin-releasing hormone (GnRH) analog: left-hand side: explanation of how to induce a negative feedback with a GnRH analog; right-hand side: different timing to start the analogs depending on which analog is used and the respective mechanism of action. FSH follicle-stimulating hormone, LH luteinizing hormone
Both methods are effective in preventing premature luteinization, while providing similar pregnancy and fresh LBRs [50]. However, the advantages of antagonists are the shorter duration of stimulation with gonadotropins and the lower risk of developing ovarian hyperstimulation syndrome (OHSS) [50, 51]. Moreover, apart from the significantly lower OHSS rate, GnRH antagonist administration offers the additional benefit of inducing final oocyte maturation with a GnRH agonist instead of hCG, which further decreases and practically eliminates the risk of OHSS [52, 53]. However, the drastic luteolysis following GnRH agonist triggering is associated with an important luteal phase defect, which is due to either pituitary desensitization with central LH deficiency or a direct effect of the GnRH agonist on luteal steroidogenesis [54, 55]. The first RCTs reported poor clinical outcomes with a high early pregnancy loss rate when a GnRH agonist was used to trigger final ovulation in GnRH antagonist cycles [56]. Following these disappointing results, further studies were performed to explore the possibility of correcting the luteal phase, which was finally achieved by implementing a modified luteal support in the case of fresh embryo transfer [57] or by applying a “freeze all” strategy and transfer of the embryo(s) in a subsequent frozen/thawed cycle [58].
Last, although previous RCTs and meta-analyses pertained to the fresh cycle outcome when comparing the two downregulation protocols, a recent well-designed RCT including 1050 women compared the GnRH agonist and antagonist protocol in terms of cumulative LBRs (including the first live birth generated during the complete first IVF cycle as the numerator and censoring additional live births) [59]. Based on the results of this study, cumulative LBRs were found to be similar between the two groups, although more oocytes were retrieved in the GnRH agonist group (10.1 vs 8.4, p < 0.01). However, the time to pregnancy and a live birth were significantly shorter in the GnRH antagonist group. In conclusion, it could be stated that the GnRH antagonist is as effective as the GnRH agonist protocol in terms of fresh and cumulative LBRs, with the advantage of being more patient friendly and safer [21, 50].
2.3.2 Novel Pituitary Suppression Protocols
Studies originally focused on contraception have shown that progesterone can block the LH surge and therefore ovulation. In fact, experiments in monkeys have demonstrated that the administration of a progestin (levonorgestrel) in the beginning of the cycle may prevent the occurrence of the LH surge, while this inhibition is completely reversible by discontinuing the progestin [60].
Furthermore, over the last decade, new OS regimens have emerged with gonadotropin administration starting in the luteal phase of the cycle in the case of urgent fertility preservation (in patients with cancer) and, more recently, these strategies have been also applied in infertile patients [61]. Oocyte competence does not seem to be compromised, while aneuploidy rates are similar between follicular and luteal phase stimulation [62]. In this regard and given that endogenous progesterone in the luteal phase is sufficient to block the LH surge and does not impair oocyte competence in cycles followed by oocyte or embryo cryopreservation, a crucial question is whether exogenous progesterone could replace the use of an agonist or antagonist in the follicular phase, with the advantage of oral administration and potential cost reduction. Kuang et al. [63] reported the first RCT with progestin-primed OS: the authors added medroxyprogesterone acetate (MPA) to gonadotropins in a follicular phase stimulation, which is progestin that does not interfere with endogenous progesterone. The investigators showed that although the duration of stimulation was significantly longer and the total dose of gonadotropins was higher with MPA, the number of mature oocytes and the main reproductive outcomes were not significantly different between the two groups.
The same results were reported by other investigators, showing comparable pregnancy rates in progestin-primed OS cycles [64]. However, caution is needed, given some inconsistencies that have been recently reported regarding the use of MPA. In particular, a large trial conducted in oocyte donors found worse pregnancy rates in recipients of oocytes from the MPA group [65]. In this regard, further studies are warranted, especially on reproductive and long-term neonatal outcomes, before these protocols can be introduced in clinical practice.
3 Modifications of Ovarian Stimulation for IVF/ICSI
3.1 Mild Ovarian Stimulation
Although conventional stimulation has been considered the standard of care treatment for the management of women undergoing OS for IVF/ICSI, consisting of the use of a reasonable dose of gonadotropins in a GnRH agonist or antagonist protocol to allow multi-follicular development and retrieval of multiple oocytes, several investigators supported the use of a milder protocol that can be equally efficacious in certain cases. To this extent, the International Society for Mild Approaches in Assisted Reproduction introduced the term “mild-stimulation” to define the approach when FSH is administered at a lower dose and/or for a shorter duration in a GnRH antagonist co-treated cycle, or when oral compounds, such as anti-oestrogens or aromatase inhibitors, are used either alone or in combination with gonadotropins with the aim of collecting fewer oocytes [66] (Fig. 6).
Mild-stimulation IVF (MS-IVF) has gained recognition as a safer, less expensive, and patient-friendly IVF option. However, there is resistance among providers of ART in incorporating this approach into their practice, mainly owing to doubt as to its clinical effectiveness. The basic concept underpinning the success of MS-IVF is that, because of gentle stimulation, only the healthier follicles with more competent eggs are encouraged to develop [67]. In favor of MS-IVF, several RCTs on a population of normal/high responders found a trend toward a higher proportion of good-quality embryos/blastocysts with MS-IVF; however, there is growing laboratory evidence to support the concept that MS-IVF creates a physiologic milieu consistent with a normal menstrual cycle and optimizes endometrial receptivity [68, 69]. Mild-stimulation IVF has gained acceptance in the treatment of poor responders by virtue of its cost saving and avoidance of unnecessarily high-stimulation drugs. Mild-stimulation IVF incorporating tamoxifen or aromatase inhibitors has secured a place in the treatment of women with oestrogen-sensitive malignancies (breast or endometrial) [70]. “Mild OS stimulation for IVF” involves multiple strategies using the following agents as monotherapy or in combination: clomiphene citrate (CC), aromatase inhibitors, low-dose exogenous gonadotropins, GnRH antagonists, and late follicular phase hCG/LH.
3.1.1 Oral Compounds (Anti-oestrogens and Aromatase Inhibitors)
Different hormonal manipulations have been examined to augment follicular recruitment and to coordinate subsequent antral follicle growth during MS-IVF. Specifically, two oral drugs are frequently used for OS: CC and letrozole (LTZ). Clomiphene citrate is a selective oestrogen-receptor modulator that acts primarily by competing for the oestrogen receptor in the hypothalamus, stimulating natural FSH secretion. Clomiphene citrate has two isomers that act antagonistically to the oestradiol receptor at the hypothalamus level, inhibiting both negative and positive feedback, resulting in OS and suppression of ovulation: enclomifene and zuclomifene (Fig. 3). Enclomifene has a short half-life of 24 h and may affect the hypothalamus levels as an oestrogen antagonist. Zuclomiphene has a far longer half-life of 3–5 days and it may weakly affect the pituitary level as an oestrogen agonist inducing increased sensitivity of the pituitary gland to GnRH [71] (Fig. 7). Clomiphene citrate is administered orally, typically starting on the third to seventh day after the onset of spontaneous or progestin-induced menses. Treatment begins with a single 50-mg tablet daily for 5 consecutive days. The effective dose range of CC is 50–250 mg/day; doses in excess of 100 mg/day are not approved by the US Food and Drug Administration [72].
Letrozole is an aromatase inhibitor that decreases negative feedback to the pituitary gland and increases endogenous FSH secretion [73] (Fig. 7). Letrozole was at first given to postmenopausal women with breast cancer to suppress oestrogen production [74]. Precisely, LTZ inhibits the aromatase enzyme by competitively binding to the heme of the cytochrome P450 subunit of the enzyme resulting in a blockage of androgens conversion into oestrogens with a subsequent increase in intraovarian androgens [75]. Letrozole has a half-life of 48 h and, at doses of 1–5 mg/day, inhibits aromatase activity by 97–99% [76]. The benefit of such treatments in replacing the injectable and expensive FSH is possibly to reduce the costs of the therapy, without hindering the pregnancy outcomes [67, 77, 78].
Several retrospective and prospective studies have compared outcomes between mild OS with these oral superovulation agents with low-dose gonadotropins and normal- or high-stimulation protocols [78,79,80]. In one of the largest of these trials, Revelli et al. randomized 695 patients with diminished ovarian reserve to mild stimulation (100 mg of CC on cycle days 2–6, 150 IU of rFSH per day started on cycle day 5, GnRH antagonist started on cycle day 8) or a long GnRH-agonist protocol (300–450 IU of rFSH per day) [78]. The mild stimulation led to a significantly shorter follicular phase, lower consumption of exogenous gonadotropins, and a lower peak oestradiol level than the long regimen. With the long protocol, significantly fewer cycles were cancelled because of the lack of ovarian response; further, it obtained significantly more oocytes, more mature oocytes, more embryos, and a thicker endometrium. Despite the study being powered on the number of oocytes, the clinical pregnancy rate (CPR) per oocyte retrieval did not vary significantly between the two groups (15.2% and 15.7%, respectively, for a mild vs a long protocol).
Regarding LTZ supplementation, the study of Bastu et al. analyzed in a poor prognosis population three different strategies of stimulation: group 1 was treated with 450 IU of gonadotropins, group 2 with 300 IU of gonadotropins, and group 3 with 150 IU of gonadotropins in combinations with letrozole 5 mg/day for the first 5 days of stimulation [80]. The results indicate that differences in doses of FSH in poor responder patients result in a similar number of retrieved MII, fertilized oocytes, fertilization rates, number of transferred embryos, and implantation, cancellation, chemical, clinical, and ongoing pregnancy rates. Furthermore, using a mild stimulation with the addition of LTZ was as effective as stimulation with higher doses of gonadotropins alone.
A recent systematic review and meta‐analysis of the literature, including 22 studies, was performed to assess the available evidence comparing the effectiveness of OS using CC and/or LZ to reduce FSH consumption compared with standard OS [73]. The main conclusion of the review was that in women with expected poor responders, the risk ratios for a live birth and clinical pregnancy were comparable between CC stimulation and OS (0.9, 95% confidence interval [CI] 0.6–1.2 and 1.0, 95% CI 0.8–1.4, respectively). In the CC group, a significant reduction in FSH consumption was demonstrated (MD, − 18, 95% CI − 21 to − 15). When comparing LTZ to OS in women with expected poor responders, there was no difference in terms of the number of oocytes retrieved (MD, − 0.4, 95% CI − 0 0.9 to 0.1) and a considerable reduction in FSH consumption (MD, − 35, 95% CI − 47 to − 23). However, in the normal responder population, CC compared with OS showed a decreased risk of OHSS together with a decreased consumption of gonadotropins; at the expense of a significant reduction in the number of oocytes retrieved in the CC group. Nonetheless, the available evidence comparing LTZ with OS is limited for the normal responder population.
Regarding the safety of the treatment, at the 2005 Annual Meeting of the American Society for Reproductive Medicine, an abstract presentation examined a relatively small number of LTZ pregnancies compared with a large control group of spontaneous conceptions. The investigators reported that the incidence of cardiac anomalies was higher in the LTZ group than in the control group [81]. Recent data [82] showed that the efficacy and safety of letrozole are at minimum comparable to those of clomiphene in women with unexplained infertility. More specifically, no difference in the number of fetal malformations was observed.
3.1.2 Modified Natural Cycle
Although the use of high doses of gonadotropins is effective in many patients, this may not be that effective in women with a low ovarian reserve (low number of follicles in their ovaries) and/or advanced maternal age, a population that is increasing worldwide. Despite the remarkable advances made, and the numerous therapeutic agents developed for OS during recent years, those patients do not gain the benefits related to ART, simply because they to do not respond to treatment [83]. It is of outmost importance when talking about the poor ovarian response women to carefully define the population we are referring and, possibly, use one of the standardized definitions [84, 85]. Different protocols of OS have been proposed for optimizing IVF results in poor responders [86]; however, good response to stimulation still remains a challenge. The most common method of obtaining oocytes in a poor prognosis patient is well known to be OS by the use of high doses of gonadotrophins. However, previous RCTs [87] and retrospective studies [88, 89] have evaluated the effectiveness of high-dose FSH, and the results of these studies have shown that there is little or no benefit, at the expense of very high costs. In more detail, in the previous RCT by Morgia et al., 129 consecutive patients were randomized to either a managed natural cycle or a microdose flare cycle; pregnancy rates per cycle were low and similar for both groups: 6.1% in the natural-cycle group and 6.9% in the traditional-stimulation group (p value not significant) [87]. Similar results were obtained in the above-mentioned retrospective studies, providing fair evidence that CPRs after IVF are comparable between a managed natural cycle and OS in women with diminished ovarian reserve.
3.2 Pre-treatment Strategies
Although the major concept of OS is to start gonadotropin administration in the early follicular phase (2–3 days following menstruation) in an antagonist protocol, or 2 weeks after downregulation in a long-agonist protocol, over the years clinicians have adopted several methods of pre-treatment prior to OS. The rationale behind the use of these methods is mainly to plan treatment (to allow oocyte retrieval and embryo transfer on a specific day), or to synchronize the follicle cohort. Four major pre-treatment strategies have been developed: combined oral contraceptive pills, isolated progestogens, isolated oestrogens, and GnRH antagonists.
3.2.1 Combined Oral Contraceptive Pills Pre-treatment
Combined oral contraceptive pills (COCP) were initially developed in 1950 based on the concept that ovulation was suppressed during pregnancy under the action of progesterone [90]. However, natural progesterone is relatively impotent when given orally. It was a long time before the first synthetic progestogen was developed and it was not until 1960 that the first COCP was approved by the Food and Drug Administration. Synthetic progestins are very active when given orally, producing reliable effects with small doses [91]. Progestins are classically classified according to their structure (Table 2) and present wide inter-individual and inter-compound variability regarding their pharmacokinetic parameters [92].
Oestradiol is the most potent natural oestrogen and is the main oestrogen produced by the ovaries. However, it has low activity when given orally. In 1938, the discovery that the addition of an ethinyl group at the 17 position increased its oral activity was a turning point in the history of oral contraception [91]. Ethinylestradiol is a very potent oestrogen and is present in most COCPs, while estradiol valerate is an esterified form of oestradiol, with significant potency when administered orally. Ethinylestradiol, like oestradiol, undergoes hepatic bypass and enterohepatic recirculation [92]. As for progestins, the metabolism of oestradiol has wide inter- and intra-individual variation. Oral ethinylestradiol is rapidly absorbed in the stomach and upper intestine (typically 90% in the first hour, but it can take up to 2 h); the peak blood concentration is usually reached within 1–2 h but it can take as long as 6 h; the bioavailability of ethinylestradiol has a range of 25–65% and its half-life has a range of 6–27 h [92].
The primary effect of COCPs is the prevention of ovulation. By inhibiting the pituitary secretion LH, the progestin compound inhibits ovulation, while estrogenic agents inhibit FSH secretion thus preventing follicular development. Furthermore, oestrogen also stabilizes the endometrium, therefore preventing breakthrough bleeding, and potentiates the action of progestogens [91].
In the context of controlled OS for IVF, the resulting pituitary suppression with COCP aims to provide a more homogenous follicular development, as well as the prevention of spontaneous LH surges [93, 94]. However, disadvantages attributed to the COCP pre-treatment include a slower follicular growth, with lower oestradiol levels, longer stimulation, and higher rFSH consumption [95,96,97].
When analyzing the effect of COCP pre-treatment in IVF, both GnRH agonist and antagonist protocols must be considered. Regarding GnRH agonist protocols, only one RCT has been published. A significant reduction in cyst formation (0 vs 52.9%, p < 0.001), a shorter interval until confirmation of downregulation (median difference: − 7 days), a shorter duration of OS (median difference: − 1 day), and a decreased consumption of gonadotropins (median difference: − 10 ampoules) were reported, without a significant difference in CPRs (37.2% in the COCP group vs 33.3% in the control group) [98].
With regard to GnRH antagonist protocols, as OS depends on the occurrence of menstruation, COCPs have been advocated as a means of programming IVF cycles. The efficacy of COCP pre-treatment on cycle scheduling has been proven in different studies, providing a more homogenous patient distribution during the week, with all the convenience for patients, physicians, and embryologists [94,95,96, 99]. However, evidence regarding the effect of this approach on pregnancy outcomes is far from consensual. Rombauts et al. conducted the first RCT on the effects of COCP pre-treatment in patients undergoing a GnRH antagonist protocol. Gonadotropin stimulation was started 2 days after discontinuation of COCP. The authors reported no significant difference regarding the number of oocytes (13.1 vs 11.5), number of good-quality embryos (5.1 vs 5.0), and the ongoing pregnancy rate (16.2% vs 20%) in patients with and without COCP pre-treatment. However, a longer duration of stimulation and higher gonadotropin consumption were observed in the COCP pre-treatment group [95]. Kolibianakis et al. reported the effect of COCP pre-treatment on OPR in 425 patients. Ovarian stimulation was started on day 5 after COCP discontinuation and a similar OPR was observed in the control and COCP groups (27.5% vs 22.9%) [97]. Other authors compared the COCP pre-treatment in patients undergoing an antagonist protocol with the long-agonist protocol and concluded that both strategies were comparable in terms of clinical outcomes [99, 100]. However, a recent meta-analysis concluded that COCP pre-treatment in women undergoing OS in an antagonist protocol resulted in lower OPR and LBRs [101].
Few studies have analyzed the effect of this approach in poor responders. Kim et al. evaluated 120 patients and concluded that the GnRH antagonist protocol with COCP pre-treatment was at least as effective as the GnRH antagonist protocol or GnRH agonist protocol without COCP pre-treatment in terms of CPRs (37.5% vs 22.5% vs 32.5%, p-value not significant) and provided a shortened time for follicular maturation and diminished rFSH consumption [102]. Bakas et al. prospectively studied the effect of COCP dose on the cycle outcome in 123 women over 35 years [103]. The authors observed that lower dose COCPs were associated with a faster FSH recovery with a similar number of oocytes retrieved and CPR when compared to higher dose COCPs and controls, and concluded that lower dose COCPs can be used to schedule cycle initiation in patients undergoing an antagonist protocol [103].
The effect of COCP pre-treatment in women with polycystic ovarian syndrome (PCOS) has also been analyzed. Although Pan et al. reported improved pregnancy outcomes with successive pre-treatment of patients with PCOS with OCP for three cycles or more [104], the accumulated body of evidence does not seem to corroborate these findings. Hwang et al. and Ozmen et al. reported no beneficial effect of COCP pre-treatment in patients with PCOS [105, 106], while Decanter et al. reported lower pregnancy rates in this group [107]. Finally, a recent prospective analysis of 1508 patients with PCOS revealed a lower CPR and LBR after a fresh embryo transfer in patients with COCP-induced menses when compared with women with spontaneous menses (respectively, 48.8% vs 63.6%, and 36.1% vs 48.1%) [108]. The effect of COCP pre-treatment for endometrial preparation before FET was also analyzed. Although a thinner endometrium was observed in patients with COCP pre-treatment, no differences were reported in terms of pregnancy outcomes [108].
A possible effect of COCP pre-treatment in early pregnancy loss has been a matter of debate. While some report no impact on the miscarriage rate [99, 102, 109], Kolibianakis et al. found a higher miscarriage rate in the COCP pre-treatment group (36.4% vs 21.6%, 95% CI − 28.4 to − 2.3) and Wei et al. described a higher rate of pregnancy loss in patients with PCOS undergoing FET after COCP pre-treatment (27.7% vs 13.0%, p = 0.004) [97, 108]. Farquhar et al., in a recent systematic review, concluded that there was no evidence of a higher rate of pregnancy loss when COCP in antagonist cycles were compared with no pre-treatment antagonist cycles, but fewer pregnancy losses were observed in COCP antagonist cycles when compared with no pre-treatment agonist cycles [101].
Finally, another controversy regarding COCP pre-treatment is the ideal washout period. In fact, the rebound effect on gonadotropin secretion that follows COCP discontinuation should guide the beginning of OS. Rombauts et al. reported strongly suppressed FSH levels on washout day 2, while other authors observed normal baseline FSH after a 5-day washout period [94, 95, 97, 110], suggesting that this should be the optimal interval.
This is further supported by the results of a recently published retrospective analysis including over 4000 patients with all causes of infertility that evaluated the effect of COCP not only on the LBR but also on the cumulative LBR. This study analyzed the effect of COCP pre-treatment (containing either desogestrel or drospirenone) when administered for 12–30 days with a 5-day pill-free interval, and failed to find any detrimental effect of the use of COCP pre-treatment, reassuring clinicians about the safety of this approach prior to OS [111].
3.2.2 Progestogen Pre-treatment
Synthetic progestogens, mainly norethisterone 10 mg daily, have also been used as a pre-treatment strategy in IVF cycles for programming oocyte retrieval on working days [94]. Progestins act by inhibiting the basal secretion of pituitary LH, as well as spontaneous LH surges during GnRH agonist cycles [94]. A wide variety of progestogen pre-treatment strategies have been used in different studies [101]. Cédrin-Durnerin et al. observed that progestogen pre-treatment in an antagonist cycle, with norethisterone 10 mg daily started on cycle day 15 for 10–15 days, was associated with a more homogenous follicular cohort, although no differences were reported regarding CPRs and LBRs [94]. The authors also suggested an optimal 5-day washout period for patients pre-treated with progestogen [94].
Wei et al. analyzed the effect of progestogen pre-treatment in patients with PCOS [108]. The authors found a similar CPR and LBR after fresh embryo transfer in the progestogen pre-treatment group when compared with controls (66.9% vs 63.6% and 51.8% vs 48.1%, respectively). Additionally, no difference was found regarding pregnancy loss. In patients undergoing FET, the CPR, LBR, and pregnancy loss rate were also similar between patients undergoing progestin-induced menses and spontaneous menses before endometrial preparation [108]. A recent meta-analysis also concluded that there was insufficient evidence to determine whether there was a difference regarding the dose of gonadotropins administered, number of oocytes retrieved, OPR, LBR, or miscarriage rate in progestogen pre-treatment cycles for both agonist and antagonist protocols. However, a lower incidence of ovarian cyst formation was noticed for patients pre-treated with progestogens undergoing an agonist protocol [101].
3.2.3 Estrogen Pre-treatment
Taking into account the results reported with COCP pre-treatment, interest has been drawn to whether oestrogen (E2) pre-treatment might represent a better alternative. When given in the luteal phase, E2 inhibits follicle growth through its negative feed- back on the FSH increase during the luteal follicular transition and this effect stops as soon as E2 is discontinued [94, 112]. Both estradiol valerate 4 mg daily and 17β-estradiol 4 mg daily starting on cycle days 15–21 for 10–17 days have been used as pre-treatment approaches [101].
Earlier studies showed an improved homogeneity of follicle growth with E2 pre-treatment [113, 114]. However, these studies did not correlate this finding with clinical outcomes. Cédrin-Durnerin et al. observed a more heterogeneous follicular cohort after E2 pre-treatment when compared with COCP or progestogen pre-treatment but reported no difference regarding pregnancy outcomes [94]. Moreover, an abrupt FSH rebound was observed after a 1- or 2-day washout period, suggesting that OS should be started earlier in E2-pretreated patients [94]. A recent RCT showed a longer duration of stimulation and higher FSH consumption after E2 pre-treatment in an antagonist protocol [115]. However, no difference was found regarding IVF outcomes. In the absence of any deleterious effect, the authors concluded that E2 pre-treatment could be used for cycle scheduling [115]. Accordingly, a RCT conducted by Blockeel et al. also demonstrated that the proportion of patients undergoing an oocyte retrieval during the weekend was significantly lower in the pre-treatment group without compromising neither the number of oocytes retrieved nor the CPR [116].
Regarding poor responders, Dragisic et al. described improved pregnancy outcomes with a protocol of E2 pre-treatment undergoing an antagonist protocol [117]. These results were confirmed in a recent retrospective analysis of 155 patients [118]. The authors observed a higher number of oocytes retrieved and higher pregnancy rates in the pre-treatment group and hypothesized that in this population a more homogenous follicular growth and the stimulatory effect of E2 on granulosa cell FSH receptors might have contributed to these findings [118]. A trend towards better pregnancy outcomes was also observed when E2 pre-treatment in an antagonist protocol was compared to the long-agonist protocol [119].
3.2.4 GnRH Antagonist Pre-treatment
Gonadotropin-releasing hormone antagonist pre-treatment has emerged as another strategy aiming at sparing early antral follicles from the dis-coordinating effects of luteal phase FSH. Gonadotropin-releasing hormone antagonists bind to the pituitary GnRH receptor exerting an antagonistic effect by competing with endogenous GnRH for pituitary binding. Because of the lack of intrinsic effect, a rapid suppression of gonadotropin is achieved upon administration [120]. Fanchin et al. conducted a small prospective study reporting reduced size disparities among early antral follicles with cetrorelix acetate 3-mg administration on cycle day 25 and hypothesized that this could improve the OS outcome [121]. A subsequent RCT evaluated the effect of a 3-day GnRH antagonist pre-treatment on OS. The authors reported a trend towards a higher number of oocytes retrieved (12.8 ± 7.8 vs 9.9 ± 4.9, p = 0.067) and higher CPRs (42% vs 33%, p = 0.596) in the pre-treatment group [122]. More recently, the efficacy of GnRH antagonist pre-treatment in cycle scheduling was evaluated [123]. The authors observed a five-fold reduction in the number of retrieval procedures on Saturdays without significant differences in OS or pregnancy outcomes.
4 Regimens for Final Oocyte Maturation
Luteinizing hormone surge is essential for the final stages of oocyte maturation, which is followed by the expulsion of the oocyte from the follicle (ovulation) and luteinization. In fact, LH exposure induces the resumption of meiosis and the maturation of the oocyte from “metaphase I” stage to the mature “metaphase II” stage of development, while the remainder of the follicle forms the corpus luteum, which produces sex steroids, mainly progesterone, to prepare the endometrium for implantation [124]. Ovarian hyperstimulation syndrome is a serious and potentially life-threatening physiologic complication of ART treatment, characterized by the growth of multiple follicles with a massive extravascular protein-rich fluid. The main complications of this pathology are related to hypovolemia, hemoconcentration, electrolyte disturbance, and oliguria [125]. Different options for inducing final oocyte maturation have been developed as a part of the OS IVF procedures, such as the use of hCG or a GnRH agonist, with a clearly different effect on the LH surge (Fig. 7).
4.1 Urinary/Recombinant Human Chorionic Gonadotrophin and Recombinant Luteinizing Hormone
Traditionally, in ART, hCG has been used to trigger final oocyte maturation, given the significant structural similarities between hCG and human LH, and the fact that both hormones stimulate the same receptor [126]. However, although hCG activates the LH receptor, it does not appear to do so in the same manner as LH, based on the fact that the intracellular signaling following activation of the LH receptor differs between the two molecules depending on the ligand site [127].
For decades, the only formulation of hCG was derived from the urine of pregnant women. However, urinary hCG may contain significant batch-to-batch variation in activity, and it has the potential for impurities and immunological reactions [128]. The advent of recombinant DNA technology facilitated the synthesis of recombinant hCG and recombinant LH in Chinese hamster ovary cells and the high purity of these products made the drugs suitable for subcutaneous injection and self-administration [129]. Although recombinant products may appear more convenient to use, the results of a recent meta-analysis found no difference between recombinant hCG or recombinant LH and urinary hCG for live birth or ongoing pregnancy rates or rates of OHSS in women undergoing IVF [130].
4.2 GnRH Agonist Triggering
More than 30 decades ago, the possibility of triggering an endogenous LH surge sufficient for induction of ovulation with a single injection of a GnRH agonist was first reported [131]. However, its potential to induce oocyte maturation occurred with the introduction of the competitive reversible GnRH antagonist protocol for OS in the 1990s [132]. In contrast to hCG, which has a long duration of action with peak serum levels at ~ 18 h following administration, the GnRH agonist induces a peak of serum LH at ~ 4 h following administration and the duration of endogenous LH surge is ~ 48 h [133]. Of note, a GnRH agonist activates pituitary GnRH receptors to release both endogenous LH and FSH, while hCG has only LH-like activity. Although the mid-cycle FSH surge is not essential for oocyte maturation to occur, FSH is known to increase LH receptor expression in granulosa cells and may have a role in oocyte maturation [134]. The shorter duration of the endogenous LH surge induced by GnRH agonist triggering seems to play a fundamental role for the reduced risk of OHSS when a GnRH agonist is used [135]. However, the luteal phase is altered in the case of GnRH agonist triggering, presumably owing to the excessive negative steroid feedback resulting in suppressed pituitary LH release [54].
The introduction of oocyte/embryo vitrification as a method of cryopreservation associated with high post-thawing survival rates resolved this issue with the implementation of the “freeze-all” strategy [136]. Specifically, OS is segmented with the use of an antagonist protocol followed by GnRH agonist triggering and freezing of all embryos (which will be then transferred in a subsequent frozen-thawed cycle). This approach appears promising, resulting in high cumulative LBRs, and remains the first-line treatment in patients with a high risk for OHSS [12]. Several analogs are used in clinical practice, with no evidence of superiority of one over another, and when compared to hCG [137].
4.3 Dual Trigger or Double Trigger
After ovulation induction with human chorionic gonadotropin (hCG), or an agonist trigger, about 70–85% of the oocytes should reach the meiosis I and stop at the metaphase II stadium until fertilization, when meiosis II is completed [138]. However, a group of patients experienced a higher percentage of germinal vesicles or metaphase despite the ovum pick-up performed 36 h after the ovulation induction [139]. Consequently, a high percentage of immature oocytes is associated with an increased risk of fewer embryos available, which reduces the chance of conceiving with IVF. To reduce the incidence of immature oocytes, some authors proposed to perform the ovum pick-up 38 h after the ovulation trigger [140], while others investigated new medical approaches.
In light of the above, recent studies demonstrated an increase in the number of mature oocytes retrieved when final oocyte maturation was induced by dual triggering in patients who had a high immature oocyte rate in a previous IVF cycle. The explanation given by the authors to justify this phenomenon is that in natural cycles final oocyte maturation is induced by a dual increase of LH and FSH, where the LH activity lasts for around 48 h. Conversely, hCG triggering simulates only the LH peak binding with the LH receptors but lacks FSH activity. Thus, the role of hCG is to maintain the LH activity in the luteal phase; while the agonist trigger induces a more physiological peak by displacing the GnRH antagonist from the GnRH receptors in the pituitary gland and inducing both LH and FSH surges (flare-ups) [141, 142].
However, in the attempt to rescue the luteal phase and to further optimize pregnancy outcomes in cycles with GnRH antagonist protocols, some authors proposed the double trigger technique, which consists of adding a reduced or standard dosage of hCG to the single bolus of the GnRH agonist trigger of the ovulation [143, 144]. Specifically, 1500 IU of hCG can be administered 12–35 h after the GnRH agonist administration [145] without increasing the OHSS in normal ovarian responders; however, a recent RCT showed that in high ovarian responders the extra-administration of hCG was significantly associated with the incidence of OHSS [146]. Specifically, the dual/double triggers have, respectively, the primary scoops of implementing the number of mature oocytes in patients with maturation issues and eventually ameliorating the luteal phase without affecting the incidence of OHSS in low/normal ovarian responders.
4.4 Kisspeptin
The hormonal stimulation of ovulation is very important to induce a good maturation of the oocytes and to avoid the risk of OHSS. In recent years, new molecules able to induce ovulation were introduced into the market, such as the neuropeptide kisspeptin. Kisspeptin is a novel neuromodulator that acts upstream of GnRH, coded by the KiSS1 gene, and it is sensitive to sex steroid feedback and metabolic cues. Kisspeptin has been described as a crucial regulator of the onset of puberty, the regulation of sex hormone-mediated secretion of gonadotrophins, and the control of fertility [147]. In humans, exogenously administered subcutaneous kisspeptin (1.6–12.8 nmol/kg) [148] induces a dose-dependent secretion of endogenous LH and to a lower extent FSH. A previous study showed that kisspeptin is associated with good results in terms of oocyte maturity rates [149]; however, the luteal phase after a kisspeptin trigger of the ovulation is even more hindered than when using GnRH agonists because of a shorter and smaller amplitude LH surge [150]. To conclude, the future role of kisspeptin in OT needs to be further investigated to enter into clinical practice.
5 Luteal Phase Support Regimens
Despite the effectiveness of the ovaries to induce multi-follicular development and the retrieval of many oocytes, the embryo selection, and the extensive embryo culture, a major shortcoming of an ART treatment is still the detrimental effect that former steps have on the luteal phase. Some possible reasons to explain why there is a luteal phase deficit in patients undergoing OS are the removal of granulosa cells at the oocyte retrieval or the hCG or oestrogen suppression of the LH activity. Specifically, Fatemi et al. described the phenomena of iatrogenic luteal phase weakness due to the supra-physiological steroids level in a stimulated cycle [151] and consequently, the higher risk of pregnancy loss. Currently, luteal phase support is considered mandatory if a fresh ET is planned and different options have been proposed, namely progesterone, hCG, GnRH agonists, and oestrogen supplementation [21].
5.1 Progesterone
The role of progesterone in the luteal phase has been established by earlier studies [152] where the function of the corpus luteum was analyzed. The corpus luteum is a transient ovarian gland active in the luteal phase and early pregnancy producing significant amounts of progesterone, oestrogens (E2), androgens, growth factors, and non-steroid hormones. In fact, as demonstrated by Csapo et al., in 1972, the removal of the corpus luteum is associated with a steep decrease in the progesterone level and this is associated with increased pregnancy loss [152].
As earlier described by Smitz et al. and Beckers et al., the use of GnRH agonists during OS impairs the corpus luteum function, leading to a suboptimal luteal phase [153, 154]. Furthermore, the ovulation trigger with GnRH agonists is associated with a time-limited LH surge, resulting in a premature luteolysis and thus a high rate of pregnancy loss, as described by Humaidan et al. [145]. The before-mentioned studies explained that the possible reason for the hindered progesterone production of the corpus luteum after a prolonged blockage of pituitary gonadotropin release depends on the longer effect of the medications that can last after discontinuation of the treatment. Considering that the endogenous production of progesterone in the luteal phase after IVF treatment is insufficient, progesterone supplementation becomes particularly essential in women undergoing ART treatments [155]. Furthermore, advances in the vitrification process currently allow the freezing of the supernumerary embryos and, in most clinics, the frozen embryo transfers take place in an artificial cycle, where progesterone supplementation is mandatory.
5.1.1 Type of Progesterone and Route of Administration
Progesterone can be administered in different methods: vaginally, intramuscularly, rectally, orally, or subcutaneously. An important aspect to consider is that the pharmacokinetics of progesterone is dependent on the route of administration, consequently, as described by Miles et al., the serum concentration of progesterone is significantly higher when progesterone is administered intramuscularly compared with when administered vaginally. However, micronized progesterone capsules formulated for oral use can be administered vaginally and this offers an effective alternative to intramuscular injections, given a lower serum concentration but higher endometrial concentration of progesterone [156]. Despite the practical benefit of the oral administration of micronized progesterone, it should be emphasized that when taken per os, it is associated with higher side effects such as somnolence. The major advantages of intravaginal progesterone over oral application include higher bioavailability, rapid absorption and avoidance of first-pass metabolism, higher plasma concentrations, and higher endometrial concentrations, owing to the first uterine-pass effect [157, 158]. However, vaginal administration advantages over intramuscular administration are that it is more patient friendly and has a reduced risk of site pain and infections. However, vaginal administration is associated with vaginal irritation, discharge, and bleeding [159]. Eventually, when looking at the CPR, the odds ratio between vaginal and intramusclar is comparable [160].
As suggested by the European Society of Human Reproduction and Embryology guidelines (2019), the dosing of natural progesterone has evolved empirically within recent years. According to the latest evidence, the following dosages of different routes of administration of progesterone are equally efficient: 50 mg of intramuscular progesterone in a daily administration is comparable to a dose of 25 mg of subcutaneous progesterone, which is comparable to 200 mg of micronized vaginal progesterone administered trice a day [21].
To deepen the concept of oral progesterone, dydrogesterone has been introduced and extensively used for a variety of indications worldwide. Dydrogesterone is a stereoisomer of progesterone, with an additional double bond between carbons 6 and 7, with a greater affinity with the progesterone receptors and that can be used at a lower dose (10 mg trice a day) to promote endometrial proliferation [161, 162].
In Lotus I, an international, phase III, non-inferiority RCT of 1034 patients, dydrogesterone was shown to provide similar ongoing pregnancy rates compared to vaginal micronized progesterone [162]. On the same dataset, another paper was published in 2018 where the LBR was shown to be comparable between the study groups [163]. Recently, a further study called the Lotus II trial was published in 2019; in this study, a prespecified subgroup analysis was performed on 239 Chinese mainland subjects from the overall study population, who were randomized to oral dydrogesterone 30 mg or 8% micronized vaginal progesterone gel 90 mg daily from the day of oocyte retrieval until 12 weeks of gestation. Dydrogesterone had similar efficacy and safety to vaginal micronized progesterone gel [164]. The main conclusion of this series of papers is that with convenient oral administration, dydrogesterone is safe, well tolerated, and has potential to transform luteal support treatment.
With regard to the duration of progesterone administration, according to a meta-analysis including six RCTs investigating the duration of progesterone administration, no significant difference in LBRs was found between patients who discontinued progesterone at the time of the pregnancy test and those who continued progesterone administration until week 6/7, which indicates that generalized progesterone supplementation beyond the first positive pregnancy may not be necessary [165]. In view of this, the European Society of Human Reproductive Endocrinology developed a recommendation for clinical practice suggesting that progesterone administration for luteal phase support should be continued at least until the day of the pregnancy test [21].
5.2 Other Alternatives: Human Chorionic Gonadotrophin and GnRH Agonists
Luteal phase supplementation in stimulated IVF cycles has been investigated for a long time. The first meta-analysis of a RCT was conducted by Soliman et al. and was published in 1994. The results of data extrapolated by the 18 studies included in the meta-analysis showed that luteal phase supplementation using both progesterone or hCG improved pregnancy rates in stimulated IVF cycles with superior efficacy for hCG but a higher risk of OHSS [166]. There are two rationales to explain the superiority of hCG to progesterone. First, intramuscular hCG administration in the luteal phase of IVF cycles will rescue a corpus luteum and allow continuation of secretion of both oestrogen and progesterone. Second, other unknown products secreted from the corpus luteum affecting implantation may be stimulated by hCG. Enhancement of corpus luteum function, therefore, might be more beneficial than replacing just oestrogen and progesterone in the luteal phase [167].
In fact, to this extent, Pritts and Atwood showed that hCG use was superior to oral progesterone use in the luteal phase. When hCG was compared with either vaginal or intramuscular progesterone, there were no differences in outcomes [168]. Despite the promising results of the previous paper, a systematic review including 59 studies about LPS reported that the risk of OHSS was more than three-fold higher using hCG administration, compared with progesterone alone [169]; and the same results were confirmed by the last updated version of the review including 94 RCTs (26,198 women), highlighting the increased risk of OHSS when hCG was given in the luteal phase, either on its own or combined with progesterone [159]. These data led to the establishment of a consensus for the use of progesterone as a preferential product for hormonal support in the luteal phase of assisted reproduction cycles.
However, despite the potential advantage of preventing OHSS, the LBR after a GnRH agonist (as mentioned in Sect. 4.2) trigger decreased by more than 50% compared with an HCG trigger [52, 170, 171]. This poor outcome after GnRH agonist triggering may be explained by a luteal phase defect: a luteal defect in hCG-triggered cycles is usually corrected by progesterone supplements, but in GnRH agonist-triggered cycles this seems to be insufficient. This defect is likely to be due to a single GnRh agonist dose, which induces a time-limited surge in LH, resulting in premature luteolysis and thus a high rate of early pregnancy loss.
An alternative approach of luteal phase support is administrating a GnRH agonist, while enabling a fresh embryo transfer within the same cycle. This approach includes daily continuous administration of a GnRH agonist for luteal phase support. The pilot study of Pirard et al. demonstrates that repeated administration of buserelin during the luteal phase of GnRH antagonist-treated ART cycles is able to support the luteal phase and is compatible with an ongoing viable pregnancy [172]. This study also suggests that a regimen of three intranasal administrations per day could be at least as effective as 10,000 IU of hCG administered subcutaneously followed by 3 × 200 mg/day micronized progesterone administered vaginally. These beneficial effects of LH on the endometrium may be mediated through classic stimulation of the corpus luteum, but also through LH receptors expressed in the endometrium [173]. The recent study of Bar-Hava and colleagues showed that an intranasal GnRH agonist is as effective in achieving luteal phase support in high responder patients triggered with GnRH agonists as avoiding OHSS [174]. Furthermore, Wiser et al. showed recently that repeated doses of GnRH agonists every other day (from day 3 after ovum pick-up until day 11) provided safe and effective luteal support for women who underwent GnRH agonist triggering and had oestradiol levels of less than 4500 pg/mL on triggering and/or fewer than 25 oocytes retrieved [175].
5.3 Oestradiol Co-administration
The effect of luteal phase oestrogen supplementation on pregnancy rates is still a controversial issue, despite the publication of various studies seeking to answer the question [176]. Most of the studies compared progesterone alone with progesterone with an oestradiol addition for luteal support in long-agonist protocols [177,178,179] while few studies used the same comparison in antagonist protocols [180,181,182].
Interestingly, trials that used the antagonist protocol did not show a beneficial effect of adding E2 to P4 on the pregnancy outcomes; while those conducted used a long-agonist protocol provided conflicting results with some favoring the use of E2 and progesterone in combination while others did not. A possible explanation of this finding might be the profound prolonged hormonal suppression that occurs with agonist compared to antagonist protocols.
Drakakis et al. studied the effect of E2 in the luteal phase in patients undergoing ICSI with GnRH analog cycles and found a beneficial effect on the pregnancy outcome without having any adverse effect [178]. However, the meta-analysis by Huang et al. stated that oral oestrogen supplementation for the luteal phase in agonist IVF cycles did not improve IVF/ICSI results [183]. Consistently, the Cochrane review comparing 693 cases with only progesterone and 490 cases with E2 and progesterone supplementation in the luteal phase found no difference between the two groups in terms of ongoing pregnancy, clinical pregnancy, and miscarriage rates. In conclusion, the addition of oestrogen 4 mg daily to progesterone for luteal support in the ART cycle utilizing the antagonist protocol does not improve pregnancy outcomes [184].
6 Conclusions
The realization of an ART treatment depends on many steps that can be medically manipulated and must be harmoniously combined. Despite increased knowledge on the phenomena that result in a pregnancy, the development of new technologies in the pharmaceutical sector is fundamental to optimize the efficiency and tailor treatment approaches to individual needs. Future research should continue to focus on how to improve the success rates in ART treatments and how to develop cheaper and more patient-friendly strategies.
References
Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril. 2018;110(661–70):e1.
Maheshwari A, McLernon D, Bhattacharya S. Cumulative live birth rate: time for a consensus? Hum Reprod. 2015;30(12):2703–7.
Castelló D, Motato Y, Basile N, Remohí J, Espejo-Catena M, Meseguer M. How much have we learned from time-lapse in clinical IVF? Mol Hum Reprod. 2016;22(10):719–27.
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340(8810):17–8.
Trounson AO, Leeton JF, Wood C, Webb J, Wood J. Pregnancies in humans by fertilization in vitro and embryo transfer in the controlled ovulatory cycle. Science. 1981;212(4495):681–2.
Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87(9):737–56.
Macklon NS, Stouffer RL, Giudice LC, Fauser BCJM. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207.
Groenewoud ER, Macklon NS, Cohlen BJ. Cryo-thawed embryo transfer: natural versus artificial cycle: a non-inferiority trial (ANTARCTICA trial). BMC Womens Health. 2012;12:27.
Groenewoud ER, Cantineau AEP, Kollen BJ, Macklon NS, Cohlen BJ. What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update. 2013;19(5):458–70.
Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19–26.
Drakopoulos P, Blockeel C, Stoop D, Camus M, De Vos M, Tournaye H, et al. Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod. 2016;31(2):370–6.
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406.
Maddock WO, Leach RB, Tokuyama I, Paulsen CA, Roy WR. Effects of hog pituitary follicle-stimulating hormone in women: antihormone formation and inhibition of ovarian function. J Clin Endocrinol Metab. 1956;16(4):433–48.
Gemzell CA. Induction of ovulation with human pituitary gonadotrophins. Fertil Steril. 1962;13:153–68.
Santi D, Casarini L, Alviggi C, Simoni M. Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the “personalized” medicine era: a meta-analysis. Front Endocrinol. 2017;8:114.
Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2006;21(12):3217–27.
Devroey P, Pellicer A, Nyboe Andersen A, Arce JC. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil Steril. 2012;97(3):561–71.
Al-Inany HG, Abou-Setta AM, Aboulghar MA, Mansour RT, Serour GI. Efficacy and safety of human menopausal gonadotrophins versus recombinant FSH: a meta-analysis. Reprod Biomed Online. 2008;16(1):81–8.
van Wely M, Kwan I, Burt AL, Thomas J, Vail A, Van der Veen F, et al. Recombinant versus urinary gonadotrophin for ovarian stimulation in assisted reproductive technology cycles: a Cochrane review. Hum Reprod Update. 2012;18(2):111.
ESHRE Reproductive Endocrinology Guidelines Group. Ovarian stimulation for IVF/ICSI: guideline of the European Society of Human Reproduction and Embryology. Belgium: ESHRE; 2019.
Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. 2004;10(6):453–67.
Howles CM. Genetic engineering of human FSH (Gonal-F). Hum Reprod Update. 1996;2(2):172–91.
Martinez G, Sanguineti F, Sepulveda J, Dorey J, Arici A, Patrizio P. A comparison between follitropin α filled by mass and follitropin a filled by bioassay in the same egg donors. Reprod Biomed Online. 2011;22(S1):S20–S2222.
Lunenfeld B, Bilger W, Longobardi S, Alam V, D’Hooghe T, Sunkara S. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol. 2019;10:429.
Tulppala M, Milla A, Tuuri T, Vilska S, Foudila T, Hakala-Ala-Pietilä T, et al. Comparison of two recombinant follicle-stimulating hormone preparations in in-vitro fertilization: a randomized clinical study. Hum Reprod. 1999;14(11):2709–15.
Bordewijk EM, Mol F, van der Veen F, Van Wely M. Required amount of rFSH, HP-hMG and HP-FSH to reach a live birth: a systematic review and meta-analysis. Hum Reprod Open. 2019;2019(3):1–12.
The European Recombinant Human LH Study Group. Recombinant human luteinizing hormone (LH) to hormone (FSH)-induced follicular development in LH- and FSH-deficient anovulatory women: a dose-finding study. J Clin Endocrinol Metab. 1998;83(5):1507–14.
Lehert P, Kolibianakis EM, Venetis CA, Schertz J, Saunders H, Arriagada P, et al. Recombinant human follicle-stimulating hormone (r-hFSH) plus recombinant luteinizing hormone versus r-hFSH alone for ovarian stimulation during assisted reproductive technology: systematic review and meta-analysis. Reprod Biol Endocrinol. 2014;12:17.
Humaidan P, Chin W, Rogoff D, D’Hooghe T, Longobardi S, Hubbard J, et al. Efficacy and safety of follitropin alfa/lutropin alfa in ART: a randomized controlled trial in poor ovarian responders. Hum Reprod. 2017;32(7):1537–8.
Polyzos NP, Sunkara SK. Sub-optimal responders following controlled ovarian stimulation: an overlooked group? Hum Reprod. 2015;30(9):2005–8.
Polyzos NP, Drakopoulos P. Management strategies for POSEIDON’s Group 1. Front Endocrinol. 2019;10:679.
Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Buhler K, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril. 2018;109(4):644–64.
Fares FA, Suganuma N, Nishimori K, LaPolt PS, Hsueh AJ, Boime I. Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin beta subunit to the follitropin beta subunit. Proc Natl Acad Sci USA. 1992;89(10):4304–8.
Boostanfar R, Shapiro B, Levy M, Rosenwaks Z, Witjes H, Stegmann BJ, et al. Large, comparative, randomized double-blind trial confirming noninferiority of pregnancy rates for corifollitropin alfa compared with recombinant follicle-stimulating hormone in a gonadotropin-releasing hormone antagonist controlled ovarian stimulation pr. Fertil Steril. 2015;104(1):94–103.e1.
Devroey P, Boostanfar R, Koper NP, Mannaerts BMJL, Ijzerman-Boon PC, Fauser BCJM. A double-blind, non-inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Hum Reprod. 2009;24(12):3063–72.
Griesinger G, Boostanfar R, Gordon K, Gates D, McCrary Sisk C, Stegmann BJ. Corifollitropin alfa versus recombinant follicle-stimulating hormone: an individual patient data meta-analysis. Reprod Biomed Online. 2016;33(1):56–60.
Corifollitropin alfa Ensure Study Group. Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower-body-weight women. Reprod Biomed Online. 2010;21(1):66–76.
Drakopoulos P, Vuong TNL, Ho NAV, Vaiarelli A, Ho MT, Blockeel C, et al. Corifollitropin alfa followed by highly purified HMG versus recombinant FSH in young poor ovarian responders: a multicentre randomized controlled clinical trial. Hum Reprod. 2017;32(11):2225–33.
Polyzos NP, Corona R, Van De Vijver A, Blockeel C, Drakopoulos P, Vloeberghs V, et al. Corifollitropin alfa followed by hpHMG in GnRH agonist protocols: two prospective feasibility studies in poor ovarian responders. Gynecol Endocrinol. 2015;31(11):885–90.
Errazuriz J, Romito A, Drakopoulos P, Frederix B, Racca A, De Munck N, et al. Cumulative live birth rates following stimulation with corifollitropin alfa compared with hp-hMG in a GnRH antagonist protocol in poor ovarian responders. Front Endocrinol. 2019;10:175.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update. 2014;20(1):124–40.
Iliodromiti S, Anderson RA, Nelson SM. Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update. 2015;21(6):698–710.
Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce JC, et al. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107(2):387–96.e4.
Bosch E, Havelock J, Martin FS, Rasmussen BB, Klein BM, Mannaerts B, et al. Follitropin delta in repeated ovarian stimulation for IVF: a controlled, assessor-blind phase 3 safety trial. Reprod Biomed Online. 2019;38(2):195–205.
Barbieri RL. The endocrinology of the menstrual cycle. Methods Mol Biol. 2014;1154:145–69.
Fleming R, Adam AH, Barlow DH, Black WP, MacNaughton MC, Coutts JR. A new systematic treatment for infertile women with abnormal hormone profiles. Br J Obstet Gynaecol. 1982;89(1):80–3.
Huirne JAF, Lambalk CB. New drug classes gonadotropin-releasing-hormone-receptor antagonists. Lancet. 2001;358(9295):1793–803.
Porter RN, Smith W, Craft IL, Abdulwahid NA, Jacobs HS. Induction of ovulation for in-vitro fertilisation using buserelin and gonadotropins. Lancet. 1984;324(8414):1284–5.
Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. 2017;23(5):560–79.
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:CD001750.
Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12(2):159–68.
Youssef M, Van Der Veen F, Van Wely M. GnRHa to trigger final oocyte maturation: a time to reconsider. Hum Reprod. 2010;25(2):559.
Kol S, Humaidan P, Alsbjerg B, Engmann L, Benadiva C, Garcia-Velasco JA, et al. The updated Cochrane review 2014 on GnRH agonist trigger: repeating the same errors. Reprod Biomed Online. 2015;30(6):563–5.
Casper RF. Introduction: gonadotropin-releasing hormone agonist triggering of final follicular maturation for in vitro fertilization. Fertil Steril. 2015;103(4):865–6.
Kolibianakis EM, Schultze-Mosgau A, Schroer A, van Steirteghem A, Devroey P, Diedrich K, et al. A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod. 2005;20(10):2887–922.
Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod. 2013;28(9):2511–21.
Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593–7.
Toftager M, Bogstad J, Løssl K, Prætorius L, Zedeler A, Bryndorf T, et al. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod. 2017;32(3):556–67.
Wildt L, Hutchison S, Marshall G, Pohl R, Knobil E. On the site of action of progesterone in the blockade of the estradiol-induced gonadotropin discharge in the rhesus monkey. Endocrinology. 1981;109(4):1293–4.
Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23(2):211–20.
Ubaldi FM, Capalbo A, Vaiarelli A, Cimadomo D, Colamaria S, Alviggi C, et al. Follicular versus luteal phase ovarian stimulation during the same menstrual cycle (DuoStim) in a reduced ovarian reserve population results in a similar euploid blastocyst formation rate: new insight in ovarian reserve exploitation. Fertil Steril. 2016;105(6):1488–95.e1.
Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(1):62–70.e3.
Chen Q, Wang Y, Sun L, Zhang S, Chai W, Hong Q, et al. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod Biol Endocrinol. 2017;15(1):1–9.
Beguería R, García D, Vassena R, Rodríguez A. Medroxyprogesterone acetate versus ganirelix in oocyte donation: a randomized controlled trial. Hum Reprod. 2019;34(5):872–80.
Nargund G, Chian RC. ISMAAR: leading the global agenda for a more physiological, patient-centred, accessible and safer approaches in ART. J Assist Reprod Genet. 2013;30(2):155–6.
Nargund G, Datta AK, Fauser BCJM. Mild stimulation for in vitro fertilization. Fertil Steril. 2017;108(4):558–67.
Labarta E, MartÍnez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813–25.
Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Implantation: clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10(9):2432–7.
Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23(19):4347–53.
Teramoto S, Kato O. Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod Biomed Online. 2007;15(2):134–48.
Yilmaz S, Yilmaz Sezer N, Gönenç IM, Ilhan SE, Yilmaz E. Safety of clomiphene citrate: a literature review. Cytotechnology. 2018;70(2):489–95.
Bechtejew TN, Nadai MN, Nastri CO, Martins WP. Clomiphene citrate and letrozole to reduce follicle-stimulating hormone consumption during ovarian stimulation: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):315–23.
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–29.
Akhtar M, Njar VCO, Neville WJ. Mechanistic studies on aromatase and related C–C bond cleaving P-450 enzymes. J Steroid Biochem Mol Biol. 1993;44(4–6):375–87.
Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22(1):61–78.
Fauser B, Devroey P, Yen SSC, Gosden R, Crowley WF, Baird DT, et al. Minimal ovarian stimulation for IVF: appraisal of potential benefits and drawbacks. Hum Reprod. 1999;14(11):2681–6.
Revelli A, Chiadò A, Dalmasso P, Stabile V, Evangelista F, Basso G, et al. “Mild” vs. “long” protocol for controlled ovarian hyperstimulation in patients with expected poor ovarian responsiveness undergoing in vitro fertilization (IVF): a large prospective randomized trial. J Assist Reprod Genet. 2014;31(7):809–15.
Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19(9):2031–5.
Bastu E, Buyru F, Ozsurmeli M, Demiral I, Dogan M, Yeh J. A randomized, single-blind, prospective trial comparing three different gonadotropin doses with or without addition of letrozole during ovulation stimulation in patients with poor ovarian response. Eur J Obstet Gynecol Reprod Biol. 2016;203:30–4.
Biljan M, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins. Fertil Steril. 2005;84(Suppl. 1):S95.
Shao Y-H, Tulandi T. Letrozole and unexplained infertility: a contemporary meta-analysis. J Obstet Gynaecol Can. 2019;41(6):832–4.
Lainas TG, Sfontouris IA, Venetis CA, Lainas GT, Zorzovilis IZ, Tarlatzis BC, et al. Live birth rates after modified natural cycle compared with high-dose FSH stimulation using GnRH antagonists in poor responders. Hum Reprod. 2015;30(10):2321–30.
Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–24.
Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. 2016;105(6):1452–3.
Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update. 2003;9(1):61–766.
Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, et al. A controlled trial of natural cycle versus microdose gonadotropin-releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertil Steril. 2004;81(6):1542–7.
Polyzos NP, Blockeel C, Verpoest W, De Vos M, Stoop D, Vloeberghs V, et al. Live birth rates following natural cycle IVF in women with poor ovarian response according to the Bologna criteria. Hum Reprod. 2012;27(12):3481–6.
Busnelli A, Papaleo E, Del Prato D, La Vecchia I, Iachini E, Paffoni A, et al. A retrospective evaluation of prognosis and cost-effectiveness of IVF in poor responders according to the Bologna criteria. Hum Reprod. 2015;30(2):315–22.
Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181(5 Pt 1):1263–9.
Taylor H, Pal L, Seli E. Speroff’s clinical gynecologic endocrinology and infertility. 9th ed. Philadelphia: Wolters Kluwer Health; 2019.
Goldzieher JW, Stanczyk FZ. Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception. 2008;78(1):4–9.
Huirne JAF, van Loenen ACD, Donnez J, Pirard C, Homburg R, Schats R, et al. Effect of an oral contraceptive pill on follicular development in IVF/ICSI patients receiving a GnRH antagonist: a randomized study. Reprod Biomed Online. 2006;13(2):235–45.
Cédrin-Durnerin I, Bständig B, Parneix I, Bied-Damon V, Avril C, Decanter C, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum Reprod. 2007;22(1):109–16.
Rombauts L, Healy D, Norman RJ, Speirs A, Watkins B, Yovich J, et al. A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. Hum Reprod. 2006;21(1):95–103.
Barmat LI, Chantilis SJ, Hurst BS, Dickey RP. A randomized prospective trial comparing gonadotropin-releasing hormone (GnRH) antagonist/recombinant follicle-stimulating hormone (rFSH) versus GnRH-agonist/rFSH in women pretreated with oral contraceptives before in vitro fertilization. Fertil Steril. 2005;83(2):321–30.
Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Hum Reprod. 2006;21(2):352–7.
Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F, Tan SL. Effects of pretreatment with an oral contraceptive on the time required to achieve pituitary suppression with gonadotropin-releasing hormone analogues and on subsequent implantation and pregnancy rates. Fertil Steril. 1998;70(6):1063–9.
Garcia-Velasco JA, Bermejo A, Ruiz F, Martinez-Salazar J, Requena A, Pellicer A. Cycle scheduling with oral contraceptive pills in the GnRH antagonist protocol vs the long protocol: a randomized, controlled trial. Fertil Steril. 2011;96(3):590–3.
Huirne JA, Hugues JN, Pirard C, Fischl F, Sage JC, Pouly JL, et al. Cetrorelix in an oral contraceptive-pretreated stimulation cycle compared with buserelin in IVF/ICSI patients treated with r-hFSH: a randomized, multicentre, phase IIIb study. Hum Reprod. 2006;21(6):1408–15.
Farquhar C, Rombauts L, Kremer JAM, Lethaby A, Ayeleke RO. Oral contraceptive pill, progestogen or oestrogen pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database Syst Rev. 2017;5:CD006109.
Kim CH, You RM, Kang HJ, Ahn JW, Jeon I, Lee JW, et al. GnRH antagonist multiple dose protocol with oral contraceptive pill pretreatment in poor responders undergoing IVF/ICSI. Clin Exp Reprod Med. 2011;38(4):228–33.
Bakas P, Hassiakos D, Grigoriadis C, Vlahos NF, Liapis A, Creatsas G. Effect of a low dose combined oral contraceptive pill on the hormonal profile and cycle outcome following COS with a GnRH antagonist protocol in women over 35 years old. Gynecol Endocrinol. 2014;30(11):825–9.
Pan JX, Liu Y, Ke ZH, Zhou CL, Meng Q, Ding GL, et al. Successive and cyclic oral contraceptive pill pretreatment improves IVF/ICSI outcomes of PCOS patients and ameliorates hyperandrogenism and antral follicle excess. Gynecol Endocrinol. 2015;31(4):332–6.
Özmen B, Şükür YE, Seval MM, Ateş C, Atabekoʇlu CS, Sönmezer M, et al. Dual suppression with oral contraceptive pills in GnRH antagonist cycles for patients with polycystic ovary syndrome undergoing intracytoplasmic sperm injection. Eur J Obstet Gynecol Reprod Biol. 2014;183:137–40.
Hwang JL, Seow KM, Lin YH, Huang LW, Hsieh BC, Tsai YL, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane-35 pre-treatment for patients with polycystic ovary syndrome: a prospective randomized study. Hum Reprod. 2004;19(9):1993–2000.
Decanter C, Robin G, Thomas P, Leroy M, Lefebvre C, Soudan B, et al. First intention IVF protocol for polycystic ovaries: does oral contraceptive pill pretreatment influence COH outcome? Reprod Biol Endocrinol. 2013;11(1):1–9.
Wei D, Shi Y, Li J, Wang Z, Zhang L, Sun Y, et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Hum Reprod. 2017;32(2):354–61.
Bellver J, Albert C, Labarta E, Pellicer A. Early pregnancy loss in women stimulated with gonadotropin-releasing hormone antagonist protocols according to oral contraceptive pill pretreatment. Fertil Steril. 2007;87(5):1098–101.
Andersen AN, Witjes H, Gordon K, Mannaerts B. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pre-treatment. Hum Reprod. 2011;26(12):3413–23.
Montoya-Botero P, Martinez F, Rodríguez-Purata J, Rodríguez I, Coroleu B, Polyzos N. The effect of type of oral contraceptive pill and duration of use on fresh and cumulative live birth rates in IVF/ICSI cycles. Hum Reprod. 2020;35(4):826–36.
Guivarc’h-Levêque A, Homer L, Arvis P, Broux PL, Moy L, Priou G, et al. Programming in vitro fertilization retrievals during working days after a gonadotropin-releasing hormone antagonist protocol with estrogen pretreatment: does the length of exposure to estradiol impact on controlled ovarian hyperstimulation outcomes? Fertil Steril. 2011;96(4):872–6.
Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Hum Reprod. 2003;18(12):2698–703.
Fanchin R, Cunha-Filho JS, Schonäuer LM, Kadoch IJ, Cohen-Bacri P, Frydman R. Coordination of early antral follicles by luteal estradiol administration provides a basis for alternative controlled ovarian hyperstimulation regimens. Fertil Steril. 2003;79(2):316–21.
Cédrin-Durnerin I, Guivarc’h-Levêque A, Hugues JN. Pretreatment with estrogen does not affect IVF-ICSI cycle outcome compared with no pretreatment in GnRH antagonist protocol: a prospective randomized trial. Fertil Steril. 2012;97(6):1359–64.e1.
Blockeel C, Engels S, De Vos M, Haentjens P, Polyzos NP, Stoop D, et al. Oestradiol valerate pretreatment in GnRH-antagonist cycles: a randomized controlled trial. Reprod Biomed Online. 2012;24(3):272–80.
Dragisic KG, Davis OK, Fasouliotis SJ, Rosenwaks Z. Use of a luteal estradiol patch and a gonadotropin-releasing hormone antagonist suppression protocol before gonadotropin stimulation for in vitro fertilization in poor responders. Fertil Steril. 2005;84(4):1023–6.
Chang EM, Han JE, Won HJ, Kim YS, Yoon TK, Lee WS. Effect of estrogen priming through luteal phase and stimulation phase in poor responders in in-vitro fertilization. J Assist Reprod Genet. 2012;29(3):225–30.
Shastri SM, Barbieri E, Kligman I, Schoyer KD, Davis OK, Rosenwaks Z. Stimulation of the young poor responder: comparison of the luteal estradiol/gonadotropin-releasing hormone antagonist priming protocol versus oral contraceptive microdose leuprolide. Fertil Steril. 2011;95(2):592–5.
Griesinger G, Felberbaum R, Diedrich K. GnRH-antagonists in reproductive medicine. Arch Gynecol Obstet. 2005;273(2):71–8.
Fanchin R, Branco AC, Kadoch IJ, Hosny G, Bagirova M, Frydman R. Premenstrual administration of gonadotropin-releasing hormone antagonist coordinates early antral follicle sizes and sets up the basis for an innovative concept of controlled ovarian hyperstimulation. Fertil Steril. 2004;81(6):1554–9.
Blockeel C, Riva A, De Vos M, Haentjens P, Devroey P. Administration of a gonadotropin-releasing hormone antagonist during the 3 days before the initiation of the in vitro fertilization/intracytoplasmic sperm injection treatment cycle: impact on ovarian stimulation: a pilot study. Fertil Steril. 2011;95(5):1714–9.e2.
Viardot-Foucault V, Nadarajah S, Lye WK, Tan HH. GnRH antagonist pre-treatment: one centre’s experience for IVF-ICSI cycle scheduling. Reprod Biomed Online. 2015;30(4):366–72.
Castillo JC, Humaidan P, Bernabeu R. Pharmaceutical options for triggering of final oocyte maturation in ART. Biomed Res Int. 2014;2014:580171.
Humaidan P, Nelson SM, Devroey P, Coddington CC, Schwartz LB, Gordon K, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004.
Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One. 2012;7(10):e46682.
Abbara A, Clarke SA, Dhillo WS. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev. 2018;39(5):593–628.
Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment: recombinant HCG versus urinary HCG. The European Recombinant Human Chorionic Gonadotrophin Study Group. Hum Reprod. 2000;15(7):1446–51.
Chang P, Kenley S, Burns T, Denton G, Currie K, DeVane G, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of a clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (Profasi) for induction of final follicular maturation in in vitro fertilization-embryo t. Fertil Steril. 2001;76(1):67–74.
Youssef MA, Abou-Setta AM, Lam WS. Recombinant versus urinary human chorionic gonadotrophin for final oocyte maturation triggering in IVF and ICSI cycles. Cochrane Database Syst Rev. 2016;4:CD003719.
Nakano R, Mizuno T, Kotsuji F, Katayama K, Wshio M, Tojo S. “Triggering” of ovulation after infusion of synthetic luteinizing hormone releasing factor (LRF). Acta Obstet Gynecol Scand. 1973;52(3):269–72.
Gonen Y, Balakier H, Powell W, Casper RF. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab. 1990;71(4):918–22.
Itskovitz J, Boldes R, Levron J, Erlik Y, Kahana L, Brandes JM. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril. 1991;56(2):213–20.
Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71(3):358–67.
Kol S. Luteolysis induced by a gonadotropin-releasing hormone agonist is the key to prevention of ovarian hyperstimulation syndrome. Fertil Steril. 2004;81(1):1–5.
Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–7.
Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online. 2010;20(6):783–8.
Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87(2):709–15.
Griffin D, Feinn R, Engmann L, Nulsen J, Budinetz T, Benadiva C. Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril. 2014;102(2):405–9.
Son WY, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23(9):2010–6.
Fabris AM, Cruz M, Legidos V, Iglesias C, Muñoz M, García-Velasco JA. Dual triggering with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin in patients with a high immature oocyte rate. Reprod Sci. 2017;24(8):1221–5.
Ben-Haroush A, Sapir O, Salman L, Altman E, Garor R, Margalit T, et al. Does “dual trigger” increase oocyte maturation rate? J Obstet Gynaecol. 2019;71:1–3.
Kasum M, Kurdija K, Orešković S, Čehić E, Pavičić-Baldani D, Škrgatić L. Combined ovulation triggering with GnRH agonist and hCG in IVF patients. Gynecol Endocrinol. 2016;32(11):861–5.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90(1):231–3.
Humaidan P, Bungum L, Bungum M, Andersen CY. Rescue of corpus luteum function with peri-ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reprod Biomed Online. 2006;13(2):173–8.
Santos-Ribeiro S, Mackens S, Popovic B, Polyzos N, Racca A, Drakopoulos P, et al. Implantation enhancement by elective cryopreservation of all viable embryos (ICE): randomized controlled trial (RCT) comparing the two safest approaches in IVF/ICSI for high-responders. Hum Reprod. 2018;33:110.
Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–316.
Jayasena CN, Comninos AN, Narayanaswamy S, Bhalla S, Abbara A, Ganiyu-Dada Z, et al. Acute and chronic effects of kisspeptin-54 administration on GH, prolactin and TSH secretion in healthy women. Clin Endocrinol (Oxf). 2014;81(6):891–8.
Jayasena CN, Abbara A, Comninos AN, Nijher GMK, Christopoulos G, Narayanaswamy S, et al. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Investig. 2014;124(8):3667–77.
Dosouto C, Haahr T, Humaidan P. Advances in ovulation trigger strategies. Panminerva Med. 2019;61(1):42–51.
Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13(6):581–90.
Csapo AI, Pulkkinen MO, Ruttner B, Sauvage J, Wiest W. The significance of the human corpus luteum in pregnancy maintenance: I. Preliminary studies. Am J Obs Gynecol. 1972;112(8):1061–7.
Smitz J, Devroey P, Camus M, Deschacht J, Khan I, Staessen C, et al. The luteal phase and early pregnancy after combined GnRH-agonist/HMG treatment for superovulation in IVF or GIFT. Hum Reprod. 1988;3(5):585–90.
Beckers NG, Laven JS, Eijkemans MJ, Fauser BC. Follicular and luteal phase characteristics following early cessation of gonadotrophin-releasing hormone agonist during ovarian stimulation for in-vitro fertilization. Hum Reprod. 2000;15(1):43–9.
Delcour C, Robin G, Delesalle A-S, Drumez E, Plouvier P, Dewailly D, et al. Weekly intramuscular progesterone for luteal phase support in women receiving oocyte donation is associated with a decreased miscarriage rate. Reprod Biomed Online. 2019;39(3):446–51.
Miles R, Paulson R, Lobo R, Press M, Dahmoush L, Sauer M. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: a comparative study. Fertil Steril. 1994;62(3):485–90.
Bulletti C, De Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12(5):1073–9.
Cicinelli E, De Ziegler D. New hypotheses. Transvaginal progesterone: evidence for a new functional 'portal system' flowing from the vagina to the uterus. Hum Reprod Update. 1999;5(4):365–72.
van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;7:CD009154.
Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP. Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod. 2014;29(8):1706–11.
Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. 2009;65(Suppl. 1):S3–11.
Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A Phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017;32(5):1019–27.
Griesinger G, Blockeel C, Sukhikh G, Patki A, Dhorepatil B, Yang D-Z, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum Reprod. 2018;123:10–87.
Yang D-Z, Griesinger G, Wang W, Gong F, Liang X, Zhang H, et al. A phase III randomized controlled trial of oral dydrogesterone versus intravaginal progesterone gel for luteal phase support in in vitro fertilization (Lotus II): results from the Chinese mainland subpopulation. Gynecol Endocrinol. 2019;3:1–9.
Watters M, Noble M, Child T, Nelson S. Short versus extended progesterone supplementation for luteal phase support in fresh IVF cycles: a systematic review and meta-analysis. Reprod Biomed Online. 2019;40(1):143–50.
Soliman S, Daya M, Collins J, Hughes E. The role of luteal phase support in infertility treatment: a meta-analysis of randomized trials. Fertil Steril. 1994;61(6):1068–76.
Mochtar MH, Hogerzeil HV, Mol BW. Progesterone alone versus progesterone combined with HCG as luteal support in GnRHa/HMG induced IVF cycles: a randomized clinical trial. Hum Reprod. 1996;11(8):1602–5.
Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod. 2002;17(9):2287–99.
Daya S, Gunby J. Luteal phase support in assisted reproduction cycles. Cochrane Database Syst Rev. 2004;3:CD004830.
Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. 2014;101(4):1008–111.
Haahr T, Roque M, Esteves SC, Humaidan P. GnRH agonist trigger and LH activity luteal phase support versus hCG trigger and conventional luteal phase support in fresh embryo transfer IVF/ICSI cycles-a systematic PRISMA review and meta-analysis. Front Endocrinol (Lausanne). 2017;8:116.
Pirard C, Donnez J, Loumaye E. GnRH agonist as luteal phase support in assisted reproduction technique cycles: results of a pilot study. Hum Reprod. 2006;21(7):1894–900.
Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online. 2003;7(1):59–64.
Bar-Hava I, Mizrachi Y, Karfunkel-Doron D, Omer Y, Sheena L, Carmon N, et al. Intranasal gonadotropin-releasing hormone agonist (GnRHa) for luteal-phase support following GnRHa triggering, a novel approach to avoid ovarian hyperstimulation syndrome in high responders. Fertil Steril. 2016;106(2):330–3.
Wiser A, Klement AH, Shavit T, Berkovitz A, Koren RR, Gonen O, et al. Repeated GnRH agonist doses for luteal support: a proof of concept. Reprod Biomed Online. 2019;39(5):770–6.
Çakar E, Tasan HA, Kumru P, Cogendez E, Usal NT, Kutlu HT, et al. Combined use of oestradiol and progesterone to support luteal phase in antagonist intracytoplasmic sperm injection cycles of normoresponder women: a case-control study. J Obstet Gynaecol. 2019;104:1–6.
Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2000;73(4):761–6.
Drakakis P, Loutradis D, Vomvolaki E, Stefanidis K, Kiapekou E, Anagnostou E, et al. Luteal estrogen supplementation in stimulated cycles may improve the pregnancy rate in patients undergoing in vitro fertilization/intracytoplasmic sperm injection-embryo transfer. Gynecol Endocrinol. 2007;23(11):645–52.
Ghanem ME, Sadek EE, Elboghdady LA, Helal AS, Gamal A, Eldiasty A, et al. The effect of luteal phase support protocol on cycle outcome and luteal phase hormone profile in long agonist protocol intracytoplasmic sperm injection cycles: a randomized clinical trial. Fertil Steril. 2009;92(2):486–93.
Engmann L, DiLuigi A, Schmidt D, Benadiva C, Maier D, Nulsen J. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertil Steril. 2008;89(3):554–61.
Fatemi H, Kolibianakis E, Camus M, Tournaye H, Donoso P, Papanikolaou E, et al. Addition of estradiol to progesterone for luteal supplementation in patients stimulated with GnRH antagonist/rFSH for IVF: a randomized controlled trial. Hum Reprod. 2006;21(10):2628–32.
Kwon S-K, Kim C-H, Lee K-H, Jeon IK, Ahn J-W, Kim S-H, et al. Luteal estradiol supplementation in gonadotropin-releasing hormone antagonist cycles for infertile patients in vitro fertilization. Clin Exp Reprod Med. 2013;40(3):131–4.
Huang N, Situ B, Chen X, Liu J, Yan P, Kang X, et al. Meta-analysis of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2015;103(2):367–73.e5.
Ismail Madkour WA, Noah B, Abdel Hamid AMS, Zaheer H, Al-Bahr A, Shaeer M, et al. Luteal phase support with estradiol and progesterone versus progesterone alone in GnRH antagonist ICSI cycles: a randomized controlled study. Hum Fertil (Camb). 2016;19(2):142–9.
Leão Rogériode Barros F, Esteves Sandro C. Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech. Clinics. https://doi.org/10.6061/clinics/2014(04)10. Available from: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322014000400279&lng=en
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was used in the preparation of this review article.
Conflict of interest
Racca Annalisa, Drakopoulos Panagiotis, Neves Ana Raquel, and Polyzos P. Nikolaos have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Racca, A., Drakopoulos, P., Neves, A.R. et al. Current Therapeutic Options for Controlled Ovarian Stimulation in Assisted Reproductive Technology. Drugs 80, 973–994 (2020). https://doi.org/10.1007/s40265-020-01324-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01324-w