Abstract
Obinutuzumab (Gazyva®; Gazyvaro®) is an intravenously administered, glycoengineered, humanized, type II, anti-CD20 monoclonal antibody of the IgG1 subclass. It is available in the EU and the USA as combination therapy with oral chlorambucil in adults with previously untreated chronic lymphocytic leukaemia (CLL). In a multinational phase III study in this patient population, obinutuzumab plus chlorambucil significantly prolonged progression-free survival compared with oral chlorambucil alone and intravenous rituximab plus oral chlorambucil. Significant advantages with obinutuzumab plus chlorambucil over chlorambucil alone and rituximab plus chlorambucil were also observed in event-free survival, the time to a new anti-leukaemia treatment and overall response. The overall survival benefit with obinutuzumab plus chlorambucil is as yet unclear, although the most recent analysis suggests a benefit over chlorambucil alone. In the phase III study, obinutuzumab plus chlorambucil had a manageable tolerability profile in accordance with what would be expected for an anti-CD20 antibody. Neutropenia and infusion-related reactions were the most frequently reported grade 3 or higher treatment-emergent adverse events. In the majority of patients, infusion-related reactions were mild to moderate in severity and occurred predominantly during the first infusion and were managed by slowing or temporarily halting the infusion. Thus, current evidence suggests that obinutuzumab plus chlorambucil is a welcome addition to the treatment options currently available for adults with previously untreated CLL and is recommended by the National Comprehensive Cancer Network guidelines as the preferred first option for some, including those with comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glycoengineered, humanized, type II, anti-CD20 monoclonal antibody of the IgG1 subclass |
Prolongs progression-free and event-free survival, and improves time to new anti-leukaemia therapy and overall response compared with chlorambucil alone and rituximab plus chlorambucil |
The most recent analysis suggests an overall survival benefit over chlorambucil alone |
Manageable tolerability profile in accordance with what would be expected for an anti-CD20 antibody |
Most frequently reported grade 3 or higher treatment-emergent adverse events were neutropenia, infusion-related reactions, infections and thrombocytopenia |
1 Introduction
Chronic lymphocytic leukaemia (CLL) is characterized by the progressive accumulation of lymphocytes in the bone marrow, lymphoid tissues and peripheral blood [1]. Such cells co-express the B-cell surface antigens CD19, CD20 and CD23, and the CD5 antigen [2]; CD20 is expressed on over 95 % of B-cell lymphocytes throughout their development (but absent on haematopoietic stem cells, plasma cells and cells of other lineages) and on mature B-cell leukaemia and lymphoma cells [3, 4]. It has been identified as a therapeutic target for the treatment of B-cell malignancies [3], with the emergence of immunotherapies (e.g. monoclonal antibodies) that target cell surface antigens, and immunomodulatory agents [e.g. lenalidomide (an analogue of thalidomide)] resulting in the development of combination regimens encompassing agents with different mechanisms of action [1].
Obinutuzumab (Gazyva®; Gazyvaro®) is a glycoengineered, humanized, type II, anti-CD20 monoclonal antibody of the IgG1 subclass [5, 6]. This article reviews pharmacological, therapeutic efficacy and tolerability data relevant to the use of intravenous obinutuzumab in combination with oral chlorambucil in the treatment of adults with previously untreated CLL.
2 Pharmacodynamic Properties
Anti-CD20 monoclonal antibodies are categorized as type I (e.g. ocrelizumab, ofatumumab, rituximab and veltuzumab) or type II (e.g. obinutuzumab and tositumomab) based on their mode of CD20 binding and their primary cytolytic mechanism [3]. The majority of epitopes recognized by type I and II antibodies are located within the larger extracellular loop of the CD20 molecule, with some (e.g. those that are recognized by obinutuzumab and rituximab) overlapping. However, type II antibodies bind in a different orientation than type I antibodies. Type I antibodies are hypothesized to bind between two CD20 tetramers, resulting in the crosslinking of tetramers and their translocation into large lipid rafts within the plasma membrane, thereby enhancing complement conscription and activation and resulting in potent complement-dependent cytotoxicity. In contrast, type II antibodies are believed to bind within a tetramer (i.e. one antibody per tetramer), thus not provoking the localization of CD20 to lipid rafts, resulting in low complement-dependent cytotoxicity. Moreover, B-cell lymphocytes bind two-fold more type I than type II antibodies. Type II antibodies are more potent than type I antibodies in generating homotypic adhesion and direct, albeit non-apoptotic, cell death via actin-dependent enhancement of cell-to-cell contact, lysosome rupture and the generation of reactive oxygen species [3]. The interaction between the Fc region of anti-CD20 antibodies and FcγRIIIa (expressed on various immune effector cells) mediates the activity of antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis. The Fc region of obinutuzumab has been glycoengineered to enhance obinutuzumab’s affinity for FcγRIIIa compared with non-glycoengineered antibodies, thereby increasing its ability to bind and recruit immune effector cells [3, 5].
In two non-Hodgkin’s lymphoma (NHL) cell lines, the 50 % maximal concentration (EC50) values of obinutuzumab, rituximab and ofatumumab binding were generally similar (0.6–1.1 µg/mL) and independent of CD20 expression [7]. However, at antibody concentrations up to 20 µg/mL, the maximal binding intensity of obinutuzumab to tumour cells was approximately 50 % of that observed with the same concentrations of rituximab and ofatumumab [7].
2.1 Antitumour Effects
Consistent with the characteristics of type II anti-CD20 antibodies, obinutuzumab demonstrated less potent (10 to 1,000-fold [7]) complement-dependent cytotoxicity in two CD20-expressing tumour cell lines [7] and in cells from patients with CLL [8] than type I antibodies.
Obinutuzumab induced direct cell death (independent of mechanical manipulation required for cell aggregate disruption formed by antibody treatment) to a significantly (p < 0.05) greater extent than rituximab and/or ofatumumab in four CD20-expressing tumour cell lines [7] and in cells from patients with CLL [8]. Obinutuzumab also appeared to be more effective than rituximab and/or ofatumumab in ADCC in vitro, with EC50 target cell killing values of approximately 0.3–2 and 5–40 ng/mL, respectively [7]. Notably, non-glycoengineered obinutuzumab displayed comparable ADCC to rituximab and ofatumumab, suggesting that the augmented ADCC of obinutuzumab is conferred by the glycoengineering (and thus the enhanced affinity to FcγRIIIa) [7]. Natural killer cells are the major effector cell population implicated in the mediation of ADCC [8]. Obinutuzumab enhanced natural killer cell activation (p < 0.05) and mediated enhanced natural killer ADCC (p < 0.0001) to a significantly greater extent than rituximab and ofatumumab [8]. The phagocytic activity of obinutuzumab compared with that of other anti-CD20 antibodies is unclear, with results from in vitro studies variable [7–9].
Obinutuzumab displayed antitumour activity and appeared to be more effective than rituximab as either first- or second-line therapy in various murine xenograft models of lymphoma [7, 10]. The antitumour effects of obinutuzumab have also been observed in patients with CLL participating in phase II [11] and III [12] studies (see Sect. 4 for a discussion of the phase III data).
2.2 Other Effects
In phase I [13], I/II [14, 15] and III [5] studies in patients with CLL [5, 14, 15] or NHL [13], therapy with obinutuzumab was associated with B-cell depletion. For instance, among evaluable patients receiving obinutuzumab in the phase III study (discussed in Sect. 4), 91 % (40 of 44 patients) exhibited B-cell depletion (defined as CD19+ B-cell counts of <0.07 × 109/L) at the end of the treatment period and remained depleted during the first 6 months of follow-up [5]. B-cell recovery was observed within 12–18 months of follow-up in 35 and 13 % of patients without or with progressive disease [5].
Complement activation [13–15] and changes in IgG levels [13, 14] do not appear to be associated with obinutuzumab therapy, according to studies in patients with CLL [14, 15] or NHL [13]. Moreover, although elevated cytokine levels [e.g. interleukin (IL)-6 and IL-8] have been observed following the first infusion of obinutuzumab in studies in patients with B-cell malignancies [16], CLL [14, 15] or NHL [13], they were transient [14, 16] and became less common with subsequent infusions [13].
3 Pharmacokinetic Properties
Discussion in this section focuses on a population pharmacokinetic (PPK) analysis of data from four studies in patients with CLL or NHL (n = 678) [5, 6, 17].
Following the cycle 6 infusion in CLL patients, the estimated median maximum concentration and area under the concentration-time curve during a dosage interval values were 473.2 µg/mL and 9,516 µg·d/mL [5]. The volume of distribution of the central compartment was 2.76 L, suggesting that obinutuzumab is largely restricted to the plasma and interstitial fluid [5]; the geometric mean volume of distribution at steady state is approximately 3.8 L [6].
The metabolism of obinutuzumab has not been directly studied; however, antibodies are predominately cleared via catabolism [5].
The concentration-time course of obinutuzumab is best described by a two-compartment model with linear and time-dependent clearance components [17]. Specifically, the elimination of obinutuzumab is comprised of a linear clearance pathway and a time-dependent non-linear clearance pathway [5, 6]. During therapy initiation, the time-dependent non-linear clearance pathway is dominant; however, as therapy progresses, the impact of this pathway diminishes and the linear clearance pathway predominates, suggesting target mediated drug disposition (TMDD) [5, 6]. Of note, once the majority of CD20 cells are bound to obinutuzumab, TMDD has a reduced impact on the pharmacokinetics of obinutuzumab [5]. Following the cycle 6 infusion in CLL patients, the clearance of obinutuzumab and the median elimination half-life is approximately 0.083 L/day and 30.3 days [5].
Age and a creatinine clearance (CRCL) of ≥30 mL/min do not affect the pharmacokinetics of obinutuzumab, according to a population pharmacokinetic analysis [5, 6]. Data are limited in patients with severe renal impairment (CRCL <30 mL/min) and those with hepatic impairment [5, 6].
Although no formal drug interaction studies with obinutuzumab have been undertaken [5, 6], obinutuzumab is not an inducer, inhibitor or substrate of cytochrome P450, uridine diphosphate glucuronyltransferase enzymes and transporters such as P-glycoprotein; therefore, no pharmacokinetic interaction is expected with agents known to be metabolized by these systems [5].
4 Therapeutic Efficacy
The efficacy of intravenous obinutuzumab in combination with oral chlorambucil in adults with previously untreated CLL has been evaluated in a randomized, nonblind, multinational phase III study [12]. Some data are from the European Medicines Agency assessment report [18], the EU summary of product characteristics (SPC) [5], the US FDA medical review [19] and the US prescribing information [6].
Briefly, the study had a two-stage design [12, 19]. Stage 1 compared the efficacy of intravenous obinutuzumab plus oral chlorambucil (n = 238) and intravenous rituximab plus oral chlorambucil (n = 233) with that of oral chlorambucil alone (n = 118) [12]. Primary analyses were conducted at a data cut-off date of 11 July 2012 (for the comparison of obinutuzumab plus chlorambucil vs. chlorambucil alone) and 10 August 2012 (for the comparison of rituximab plus chlorambucil vs. chlorambucil alone) and updated analyses at a data cut-off date of 9 May 2013 [5]. Chlorambucil recipients in whom progressive disease developed during treatment or within 6 months following the end of treatment were permitted to crossover to the obinutuzumab plus chlorambucil group [12]. Discussion of the data from Stage 1 (Sect. 4.1) focuses on the comparison of obinutuzumab plus chlorambucil versus chlorambucil alone wherever possible; data pertaining to the comparison of rituximab plus chlorambucil versus chlorambucil alone are tabulated for completeness. An additional 192 patients were enrolled into the second stage of the study, which compared the efficacy of obinutuzumab plus chlorambucil (n = 333) with that of rituximab plus chlorambucil (n = 330); interim analyses were conducted at the data cut-off date of 9 May 2013 [12, 18]. Further key design details for this study are summarized in Table 1.
Eligible patients were stratified by Binet stage and geographical region and randomized to receive obinutuzumab plus chlorambucil, rituximab plus chlorambucil or chlorambucil alone for six 28-day cycles [12]. Obinutuzumab was administered intravenously at a dose of 1,000 mg on days 1, 8 and 15 of cycle 1, and day 1 of cycles 2–6 [12]. Of note, in order to reduce the rate of infusion reactions, the study protocol was amended so that the first infusion of obinutuzumab was administered over a period of 2 days (100 and 900 mg on days 1 and 2, respectively) (Sects. 5.1 and 6) [5]. Chlorambucil was administered orally at a dose of 0.5 mg/kg on days 1 and 15 of cycles 1–6 [12]. Rituximab was administered intravenously at a dose of 375 mg/m2 on day 1 of cycle 1 and 500 mg/m2 on day 1 of cycles 2–6. Owing to different dosing schedules, the median total dose of obinutuzumab was higher than that of rituximab in both Stage 1 (8,000 vs. 5,175 mg) [n = 240 and 225] and Stage 2 (8,000 vs. 5,106 mg) [n = 336 and 321] (Sect. 7). Prophylaxis (for infusion-related reactions and tumour lysis syndrome) included fluid intake and premedication with allopurinol, antihistamines, paracetamol (acetaminophen) and glucocorticoids [12].
At baseline, age and clinical characteristics were well balanced between the treatment groups. Overall, patients (n = 781) had a median age of 72–74 years, a median CRCL of 62.5–63.8 mL/min and a median Cumulative Illness Rating Scale (CIRS) score of 8 [12]. The majority (82 %) of patients had >3 coexisting conditions; 27 % had ≥1 coexisting condition that was not well controlled. The proportion of patients who were Binet Stage A, B or C was 22, 42 and 36 %, respectively [12].
No significant differences in the efficacy profile of obinutuzumab plus chlorambucil were observed between patients aged ≥75 years (n = 109) and those aged <75 years (n = 131) [6].
4.1 Comparison with Chlorambucil
First-line therapy with intravenous obinutuzumab plus oral chlorambucil significantly prolonged progression-free survival (PFS), as assessed by investigator review (primary endpoint), relative to chlorambucil alone at both the primary and updated analyses (Table 2) [12]. Consistent with the primary analysis, all prespecified sensitivity analyses demonstrated that the investigator-assessed PFS benefit obtained with obinutuzumab plus chlorambucil was robust to variations in event and population definitions [19].
Investigator-assessed PFS outcomes significantly favoured obinutuzumab plus chlorambucil over chlorambucil alone across most subgroups, including Binet stage (A, B and C), calculated CRCL (<70 and ≥70 mL/min), patient age (<65, ≥65, <75 and ≥75 years) and baseline total CIRS score (≤6 and >6) [12]. The reduction in the risk of disease progression or death associated with obinutuzumab plus chlorambucil relative to chlorambucil alone in patients with the 17p deletion was not statistically significant [12].
The majority of key secondary endpoints were also met. Obinutuzumab plus chlorambucil demonstrated significant advantages over chlorambucil alone in independent review committee-assessed PFS, event-free survival, time to new anti-leukaemia treatment and overall response at 3 months’ post-treatment at both the primary and updated analyses (Table 2) [12, 18, 19]. The estimated 1-year independent review committee-assessed PFS rate was 83 % in obinutuzumab plus chlorambucil recipients and 36 % in chlorambucil alone recipients [19]. At the primary and updated analyses, 12 and 21 % (29 and 51 of 238 patients) of obinutuzumab plus chlorambucil recipients and 35 and 55 % (41 and 65 of 118 patients) of chlorambucil recipients required new anti-leukaemia treatment [18].
At the time of the primary and updated analyses, the median overall survival duration with obinutuzumab plus chlorambucil, rituximab plus chlorambucil and chlorambucil alone had not yet been reached [12]. A significant difference in median overall survival in favour of obinutuzumab plus chlorambucil versus chlorambucil alone was evident at the time of the updated analysis (Table 2) [5, 12].
A negative minimal residual disease status (analysed by means of an allele-specific oligonucleotide polymerase chain-reaction assay) in either blood or bone marrow at 2–6 months' post-treatment was achieved by 20 % (28 of 142 patients) of obinutuzumab plus chlorambucil recipients and 0 % (0 of 80) of chlorambucil alone recipients at the primary analysis and in 27 % (45 of 168) and 0 % (0 of 90) of patients at the updated analysis [18].
Compared with chlorambucil alone, therapy with obinutuzumab plus chlorambucil did not appear to result in a deterioration in health-related quality of life (HRQOL; as assessed by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 global health scale) [12]. Moreover, HRQOL assessments specific to fatigue showed no statistically significant differences (comparison not reported), suggesting that the addition of obinutuzumab to a chlorambucil regimen does not increase the experience of fatigue [5].
4.2 Comparison with Rituximab Plus Chlorambucil
First-line obinutuzumab plus chlorambucil was significantly more effective than rituximab plus chlorambucil in prolonging PFS, as assessed by investigator review (primary endpoint), at the interim analysis (Table 3) [12]. Investigator-assessed PFS outcomes significantly favoured obinutuzumab plus chlorambucil over rituximab plus chlorambucil across most prespecified subgroups, including Binet stage (A, B and C), calculated CRCL (<70 and ≥70 mL/min), patient age (<65, ≥65, <75 and ≥75 years) and baseline total CIRS score (≤6 and >6) [12]. The reduction in the risk of disease progression or death associated with obinutuzumab plus chlorambucil relative to rituximab plus chlorambucil in patients with the 17p deletion or other karyotypes was not statistically significant [12].
The majority of key secondary endpoints were reached at the time of the interim analysis. Obinutuzumab plus chlorambucil demonstrated significant advantages over rituximab plus chlorambucil in independent review committee-assessed PFS, event-free survival, time to new anti-leukaemia treatment and overall response at 3 months’ post-treatment at the time of the interim analyses (Table 3) [5, 12]. Moreover, among patients with an end-of-treatment minimal residual disease result or who had progressive disease or had died before the end of therapy, a negative minimal residual disease status in blood or bone marrow was achieved in a significantly (p < 0.001) higher proportion of obinutuzumab plus chlorambucil recipients than rituximab plus chlorambucil recipients [blood: 37.7 % (87/231) vs. 3.3 % (8/243); bone marrow: 19.5 % (26/133) vs. 2.6 % (3/114)]. Of note, a negative minimal residual disease status in blood following therapy with obinutuzumab plus chlorambucil was associated with a favourable disease course during follow-up [12].
At the time of the interim analysis, the median overall survival duration with obinutuzumab plus chlorambucil and rituximab plus chlorambucil had not yet been reached, with the reduction in the risk of death associated with obinutuzumab plus chlorambucil relative to rituximab plus chlorambucil not statistically significant (Table 3) [12].
5 Tolerability
First-line therapy with intravenous obinutuzumab plus oral chlorambucil had a manageable tolerability profile in adults with previously untreated CLL [12] that was in accordance with what would be expected for an anti-CD20 antibody [18]. In the phase III study, treatment-emergent adverse events were managed by dose modification/interruption in 61 % (147 of 241 patients) of obinutuzumab plus chlorambucil recipients and 20 % (23 of 116) of chlorambucil alone recipients (primary analysis) and 63 % (211 of 336) of obinutuzumab plus chlorambucil recipients and 49 % (156 of 321) of rituximab plus chlorambucil recipients (interim analysis) [18]. Treatment-emergent adverse events led to treatment discontinuation in 20, 15, 20 and 15 % of patients in the respective groups [18].
At least one treatment-emergent adverse event was reported in 94 % of obinutuzumab plus chlorambucil recipients and 83 % of chlorambucil alone recipients (updated analysis) and 89 % of obinutuzumab plus chlorambucil recipients and 94 % of rituximab plus chlorambucil recipients (interim analysis) [12]. Infusion-related reactions were the most common adverse event in obinutuzumab plus chlorambucil recipients (occurring in at least two-thirds of patients) (Sect. 5.1) [12]. Treatment-related adverse events occurred in 86 and 54 % of patients in the obinutuzumab plus chlorambucil and chlorambucil alone groups (primary analysis) and 86 and 69 % of patients in the obinutuzumab plus chlorambucil and rituximab plus chlorambucil groups (interim analysis) [18].
Neutropenia (all grades) occurred in 41 % of obinutuzumab plus chlorambucil recipients and 18 % of chlorambucil alone recipients (updated analysis) and 38 % of obinutuzumab plus chlorambucil recipients and 32 % of rituximab plus chlorambucil recipients (interim analysis) [12]. It resolved spontaneously or following the use of granulocyte colony stimulating factors [5]. At the time of the interim analysis, late-onset (occurring ≥28 days following the completion of therapy [6]) and prolonged neutropenia occurred in 16 and 2 % of obinutuzumab plus chlorambucil recipients and 12 and 4 % of rituximab plus chlorambucil recipients [5]. Acute thrombocytopenia (occurring within 24 h following infusion) was experienced by 4 % of patients receiving obinutuzumab plus chlorambucil [5]. The incidence of tumour lysis syndrome (all grades) was low, occurring in 4 % of obinutuzumab plus chlorambucil recipients and 0 % of rituximab plus chlorambucil recipients (interim analysis), and resolved in all cases [12].
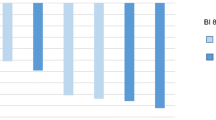
Figure 1 presents the incidence of adverse events of grade 3 or higher occurring at a ≥3 % incidence in either treatment group, with at least one reported in 73 % of obinutuzumab plus chlorambucil recipients and 50 % of chlorambucil alone recipients (updated analysis) and 70 % of obinutuzumab plus chlorambucil recipients and 55 % of rituximab plus chlorambucil recipients (interim analysis) [12]. The majority of reported infections were bacterial in origin, with the incidence of grade 3 or higher infection not significantly differing among the treatment groups. Grade 3 or higher pneumonia and febrile neutropenia occurred in ≤5 % of patients in each treatment group. Grade 5 adverse events considered related to the study medication (according to the investigator) were reported in two obinutuzumab plus chlorambucil recipients (one case each of haemorrhagic stroke and plasma cell myeloma), one rituximab plus chlorambucil recipient (cardiac arrest) and three chlorambucil recipients (one case each of intracranial haemorrhage, respiratory failure and respiratory tract infection). Whether the death of one further rituximab plus chlorambucil recipient was related to the study medication was unknown [12].
Treatment-emergent adverse events (grade ≥3) occurring at a ≥3 % incidence in any treatment group in a phase III study in adults with previously untreated chronic lymphocytic leukaemia [12]. CLB chlorambucil, NA not applicable, OBI obinutuzumab, RIT rituximab
At least one serious treatment-emergent adverse event occurred in 41 % of obinutuzumab plus chlorambucil recipients and 38 % of chlorambucil alone recipients (updated analysis) and 39 % of obinutuzumab plus chlorambucil recipients and 32 % of rituximab plus chlorambucil recipients (interim analysis) [12]. Infection was the most frequently reported serious treatment-emergent adverse event and, in general, was reported in a similar number of patients across the treatment groups (12–15 %) [12]. Serious neutropenia was reported in ≤1 % of patients receiving obinutuzumab plus chlorambucil or rituximab plus chlorambucil [12]. Death owing to an adverse event occurred in 4 % of obinutuzumab plus chlorambucil recipients, 6 % of rituximab plus chlorambucil recipients and 9 % of chlorambucil alone recipients [12]. Fatal infections occurred in <1 % of patients [5]. The number of fatal haemorrhagic events was generally similar between the obinutuzumab plus chlorambucil and rituximab plus chlorambucil groups (no further data reported) [5]. Of note, all fatal haemorrhagic events in the obinutuzumab plus chlorambucil group were reported in cycle 1. Moreover, a clear relationship between thrombocytopenia and haemorrhagic events has not been established [5].
According to a PPK analysis [17], there was no association between the occurrence of serious adverse events and serum obinutuzumab levels, with the occurrence and grade of serious adverse events and infusion-related reactions following the first infusion of obinutuzumab not affected by exposure. Moreover, no correlation between neutropenia (including the grade) and obinutuzumab exposure was observed [17].
Serious adverse events and adverse events leading to death were reported in 45 and 5 % of obinutuzumab plus chlorambucil recipients aged ≥75 years (n = 109) and in 30 and 2 % of obinutuzumab plus chlorambucil recipients aged <75 years (n = 131); similar rates were observed in the chlorambucil alone group (no data reported) [6]. Moreover, serious adverse events and adverse events leading to death were reported more frequently in obinutuzumab plus chlorambucil recipients with moderate renal impairment (CRCL <50 mL/min) than in those with mild renal impairment (CRCL ≥50 mL/min) [no further data reported] [5].
Among evaluable patients receiving obinutuzumab plus chlorambucil (n = 70), 13 % tested positive for anti-obinutuzumab antibodies at ≥1 timepoint during the 12-month follow-up period [6]. Of note, the neutralizing activity and clinical significance of anti-obinutuzumab antibodies is not yet known [6].
5.1 Infusion-Related Reactions
Infusion-related reactions, including severe reactions leading to the withdrawal of therapy, were identified as a particular risk with obinutuzumab plus chlorambucil therapy [12]. Indeed, 89 % of the first 53 patients in the phase III study experienced an infusion-related reaction [6]. The protocol was therefore amended during the study (Sect. 4), but appeared to have only a moderate effect on the frequency of infusion-related reactions [12], with a reduced incidence of all grade, but not Grade 3 or 4, infusion-related reactions observed in patients after versus prior to the implementation of the study protocol amendments [5]. Thus, mitigation measures to reduce infusion-related reactions should be followed (Sect. 6) [5].
In the phase III study, infusion-related reactions (all grades) occurred in 66 % of obinutuzumab plus chlorambucil recipients and 38 % of rituximab plus chlorambucil recipients (interim analysis) [12], with chills, diarrhoea, dyspnoea, flushing, headache, hypertension, hypotension, nausea, pyrexia, tachycardia and vomiting the most frequently reported symptoms associated with this adverse event [5]. In the majority of patients, infusion-related reactions were mild to moderate in severity and occurred during the first cycle, with the incidence in obinutuzumab plus chlorambucil recipients reducing from 65 % at the time of the first infusion to 3 % at the time of the second infusion and 1 % at the time of the third and subsequent infusions [5]. All of the grade 3 or 4 infusion-related reactions reported (Fig. 1) occurred during the first infusion of obinutuzumab; there were no grade 5 infusion-related reactions or deaths associated with this adverse event [12]. Infusion-related reactions were managed in the majority of patients by the slowing or temporary halting of the first infusion [5]. Dose interruptions or delays, hospitalization or treatment discontinuation due to infusion-related reactions were required by 36, 8 and 7 % of obinutuzumab plus chlorambucil recipients, respectively, and 21, 2 and <1 % of rituximab plus chlorambucil recipients, respectively [12].
An exploratory analysis determined that the higher rates of infusion-related reactions observed with obinutuzumab (vs. rituximab) therapy may be due to stronger activation upon binding to CD20, which enhances crosslinking between CD20 expressed on leukaemia cells and FcγRIIIa on effector cells (abstract presentation) [20] (Sect. 2). Although neither lymphocyte counts nor the tumour burden at baseline were strong predictors of obinutuzumab-related infusion reactions [12], the EU SPC advises that patients with a high tumour burden [i.e. high peripheral lymphocyte count in CLL (>25 × 109/L)] may be at an increased risk of severe infusion-related reactions [5]. Patients with renal impairment (CRCL <50 mL/min) and patients with both a CIRS score of >6 and a CRCL level of <70 mL/min are more at risk of infusion-related reactions [5].
6 Dosage and Administration
Intravenous obinutuzumab is indicated in the EU as combination therapy with oral chlorambucil in adults with previously untreated CLL and comorbidities that make them unsuitable for full-dose fludarabine-based therapy [5]. In the USA, intravenous obinutuzumab is indicated as combination therapy with oral chlorambucil in patients with previously untreated CLL [6].
The recommended dosage of obinutuzumab is 1,000 mg administered intravenously over days 1 and 2 (as a split dose of 100 and 900 mg, respectively) and on days 8 and 15 of the first 28-day cycle, and on day 1 of a further five 28-day cycles [5, 6].
The US prescribing information carries a boxed warning regarding progressive multifocal leukoencephalopathy (PML) and hepatitis B virus (HBV) reactivation in patients receiving obinutuzumab [6]. Therapy should be discontinued in patients who develop PML, with consideration given to discontinuing or reducing concomitant chemotherapy or immunosuppressive therapy [5, 6]. Patients should be screened for HBV infection prior to the commencement of [5, 6] and monitored during and following obinutuzumab therapy [6]. Obinutuzumab and concomitant therapies should be discontinued in the event of HBV reactivation in the USA [6]; in the EU, patients with active hepatitis B disease should not be treated with obinutuzumab [5]. Other events for which obinutuzumab therapy should be interrupted are active infection, grade 2 or higher non-haematological toxicity, and/or grade 3 or 4 cytopenia [5, 6].
The risk of infusion-related reactions can be reduced by premedication with paracetamol (acetaminophen), an anti-histamine and a glucocorticoid (i.e. prednisone/prednisolone 100 mg, dexamethasone 20 mg and/or methylprednisolone 80 mg; hydrocortisone is not recommended as it has not been effective in reducing the rate of infusion-related reactions). Patients should be closely monitored throughout the infusion, with more frequent monitoring advised for those with pre-existing cardiac or pulmonary conditions. The management (according to the grade) of an infusion-related reaction may require a reduction in the rate of the infusion, a temporary interruption and/or the discontinuation of obinutuzumab, along with the treatment of symptoms (see the local prescribing information for further details). Patients considered to be at high risk of tumour lysis syndrome [i.e. those with a high tumour burden and/or a high circulating lymphocyte count (>25 × 109/L)] should be pretreated with uricostatics (e.g. allopurinol) and adequately hydrated commencing 12–24 h prior to the start of therapy [5, 6].
Combination therapy with obinutuzumab and chlorambucil may increase neutropenia [5]. Therefore, patients with neutropenia should be frequently monitored, with the premedication of such patients with antimicrobial prophylaxis throughout the treatment period strongly recommended [5, 6]. Antiviral and antifungal prophylaxis should be considered. Patients should be frequently monitored for thrombocytopenia and/or haemorrhagic events, especially during the first cycle. Withholding concomitant medications that may increase the risk of bleeding (e.g. platelet inhibitors, anticoagulants), especially during the first cycle, should be considered [5, 6].
The efficacy and tolerability of obinutuzumab in children and adolescents aged <18 years have not been established [5, 6].
Local prescribing information should be consulted for detailed information, including contraindications, events for which dosage interruptions and/or reductions are recommended, drug interactions, missed doses, precautions, premedication, preparation and administration procedures and use in special patient populations.
7 Place of Obinutuzumab in the Management of Chronic Lymphocytic Leukaemia
The advent of the anti-CD20 monoclonal antibody rituximab has resulted in important advances in the treatment of CLL, particularly with regard to combination therapy [1]. Indeed, to date, targeting the CD20 antigen is the only therapeutic approach shown to prolong the survival of patients with previously untreated CLL [12]. Obinutuzumab is a humanized, type II, anti-CD20 monoclonal antibody of the IgG1 subclass [5, 6]. Its approval in patients with previously untreated CLL was primarily based on a multinational phase III study. In this study, obinutuzumab plus chlorambucil was effective in prolonging PFS, and was also associated with improvements in event-free survival, the time to a new anti-leukaemia treatment and overall response, versus both chlorambucil alone (Sect. 4.1) and rituximab plus chlorambucil (Sect. 4.2). As expected, overall survival data were not mature by the cut-off dates. Mature overall survival data are awaited with interest, particularly as a significant difference in median overall survival was evident between the obinutuzumab plus chlorambucil and chlorambucil alone groups at the time of the updated analysis (Sect. 4.1). Moreover, the rate of induction of negative minimal residual disease status was greater than tenfold higher with obinutuzumab plus chlorambucil than chlorambucil alone (Sect. 4.1) or rituximab plus chlorambucil (Sect. 4.2). An association between the capacity of a therapy to induce low levels of minimal residual disease in blood or bone marrow and improved overall survival, irrespective of clinically assessed response status, was recently identified. Thus, longer follow-up data from the phase III study are eagerly anticipated as the high rate of minimal residual disease eradication observed with obinutuzumab plus chlorambucil compared with rituximab plus chlorambucil may result in an overall survival benefit. Compared with chlorambucil alone, therapy with obinutuzumab plus chlorambucil did not appear to result in a deterioration in HRQOL (Sect. 4.1).
On the basis of findings from previous pharmacokinetic and modelling studies [21], a 1,000 mg dose of obinutuzumab was selected for phase III studies, with obinutuzumab administration occurring on days 1, 8 and 15 of cycle 1 to rapidly achieve and maintain adequate exposure levels. It is worthy of note that due to different dosing schedules used in the phase III study (Sect. 4), the median total dose of obinutuzumab was higher than that of rituximab and it is unclear to what extent this higher dose contributed to the greater activity of obinutuzumab plus chlorambucil over rituximab plus chlorambucil [12]. Indeed, concerns regarding the higher dose of obinutuzumab and its effect on efficacy have been previously raised [22]. However, according to current evidence, no additional benefit has been observed when high-dose rituximab is combined with chemotherapy [23], although high-dose rituximab monotherapy has demonstrated a dose-response relationship in patients with CLL [24]. Data from a planned phase II study (NCT01370772) investigating whether intensifying the dose of rituximab may improve the results in patients with CLL are awaited with interest.
Obinutuzumab in combination with chlorambucil is recommended by the US National Comprehensive Cancer Network (NCCN) [1] as the preferred first-line option for the treatment of CLL in patients without the 11q or 17p deletions who are aged ≥70 years, or <70 years with comorbidities (including frail patients with significant comorbidity who are not able to tolerate purine analogues), and in those with the 11q deletion who are aged ≥70 years, or <70 years with comorbidities. It is also recommended as a first-line option for the treatment of CLL in patients without the 11q or 17p deletions who are aged <70 years, or ≥70 years without significant comorbidities, in those with the 11q deletion who are aged <70 years, or ≥70 years without significant comorbidities and in those with the 17p deletion [1]. At the time of publication of the European Society for Medical Oncology guidelines [2], the role of obinutuzumab was yet to be determined.
CLL predominately affects the elderly, with almost three-quarters of patients diagnosed with CLL aged ≥65 years [1]. Older patients frequently present with comorbidities, which may reduce the patient’s ability to tolerate certain treatment regimens. In light of this, the tolerability of a treatment regimen relative to a patient’s physical fitness is an important consideration in the management of CLL [1]. The tolerability profile of first-line obinutuzumab plus chlorambucil is manageable and in accordance with what would be expected for an anti-CD20 antibody in adults with previously untreated CLL (Sect. 5). The most frequently reported grade 3 or higher treatment-emergent adverse events were neutropenia, infusion-related reactions, infections and thrombocytopenia. Whether the neutropenia associated with obinutuzumab therapy may be problematic with more intensive chemotherapy (as bone marrow reserves are usually impaired) remains to be determined.
Infusion-related reactions, including severe reactions leading to the withdrawal of therapy, have been identified as a particular risk with obinutuzumab plus chlorambucil therapy (Sect. 5.1). They occurred in two-thirds of obinutuzumab plus chlorambucil recipients in the phase III study (compared with over one-third of rituximab plus chlorambucil recipients) and, in the majority of these patients, were experienced during the first cycle and managed by the slowing or temporary halting of the first infusion. The impact of these reactions on HRQOL would be of interest. Although the risk of infusion-related reactions can be reduced by premedication, it is recommended that patients be closely monitored throughout the obinutuzumab infusion (Sect. 6). Of note, the authors of the phase III study suggested that the rapid and profound B-cell depletion induced by obinutuzumab (Sect. 2.2) may be associated with the greater frequency and intensity of infusion-related reactions observed during the first infusion of obinutuzumab compared with rituximab [12]. However, lymphocyte counts and lymphadenopathy were not strong predictors of infusion-related reactions [12].
Obinutuzumab plus chlorambucil was predicted to be cost effective relative to other currently approved agents in patients with previously untreated CLL, based on limited results of cost-utility analyses with a 10-year [25] or lifetime [26–28] horizon from a healthcare payer perspective that used Markov models to incorporate direct [25–27] and indirect [28] clinical data (available as abstracts; year of values 2014 [25] or not reported [26–28]). In the US analysis, obinutuzumab plus chlorambucil had similar total costs to rituximab plus chlorambucil ($US88,577 vs. $US88,595), but was more effective [3.36 vs. 2.80 per quality-adjusted life-year (QALY) gained], leading to an 89 % probability of obinutuzumab plus chlorambucil being cost effective relative to this comparator at a willingness-to-pay (WTP) threshold of $US100,000 per QALY gained [27]. A Canadian analysis [25] suggested that obinutuzumab plus chlorambucil was more effective than chlorambucil alone (total QALYs gained 3.521 vs. 2.546), but had higher total costs ($CAN57,747 vs. $CAN22,417), resulting in 94 and 100 % probabilities of combination therapy being cost effective relative to chlorambucil alone at WTP thresholds of $CAN50,000 and $CAN100,000 per QALY gained. In a UK analysis that assumed a range of plausible acquisition costs for obinutuzumab, obinutuzumab plus chlorambucil was cost effective relative to rituximab plus chlorambucil and chlorambucil alone (incremental costs per QALY gained £29,000–32,000 and £18,000–19,000, respectively) [26]. Moreover, in an analysis that incorporated clinical data from non-head-to-head studies, obinutuzumab plus chlorambucil was more effective (total QALYS gained 3.95 vs. 3.16) and more costly (total costs $US95,713 vs. $US92,132) than ofatumumab plus chlorambucil, resulting in a 99 % probability of obinutuzumab plus chlorambucil being cost effective relative to this comparator at a WTP threshold of $US100,000 per QALY gained [28]. The results of these preliminary cost-utility analyses are limited and cannot be generalized to other geographical locations. Further well-designed pharmacoeconomic studies are needed to clarify the relative cost effectiveness of obinutuzumab in this patient population.
A recent draft guidance from the UK National Institute for Health and Care Excellence (NICE) [29] recommends obinutuzumab combination therapy as an option for adults with previously untreated CLL and comorbidities that make them unsuitable for full-dose fludarabine-based therapy only if bendamustine-based therapy is not suitable and obinutuzumab is provided by the pharmaceutical company at the discount agreed in the patient access scheme. The final NICE guidance will be issued following consultation with the pharmaceutical company, healthcare professionals and the general public [29] and is awaited with interest. An application for the use of obinutuzumab combination therapy for the first-line treatment of patients with CLL within the National Health Service has been declined by the UK Cancer Drug Fund [30]. Specifically, concerns were raised regarding the dosage of chlorambucil used in the phase III study (Sect. 4), which is not recommended (by the British Committee for Standards in Haematology) as standard (and thus not in general use) in the UK and was therefore potentially considered inferior [30].
Although beyond the scope of this review, it is worth noting that obinutuzumab, alone or in combination with other anticancer agents, including bendamustine, chlorambucil, fludarabine plus cyclophosphamide, ibrutinib, idelalisib and lenalidomide, is being evaluated in ongoing and recently completed clinical studies in B-cell lymphoma, CLL, indolent NHL and small lymphocytic lymphoma. Findings are awaited with interest. Currently, head-to-head comparisons of obinutuzumab plus chlorambucil with bendamustine monotherapy and ofatumumab plus chlorambucil in patients with CLL are lacking. However, preliminary data from a network meta-analysis (currently available as an abstract) suggest that obinutuzumab plus chlorambucil is expected to prolong PFS over bendamustine [HR 0.53 (95 % CI 0.35–0.77)] and ofatumumab plus chlorambucil [HR 0.33 (95 % CI 0.22–0.47)] [31].
In conclusion, current evidence suggests that intravenous obinutuzumab in combination with oral chlorambucil is a welcome addition to the treatment options currently available for adults with previously untreated CLL and is recommended by the NCCN guidelines as the preferred first option for some, including those with comorbidities.
Data selection sources:
Relevant medical literature (including published and unpublished data) on obinutuzumab was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) (searches last updated 22 December 2014), bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Obinutuzumab, afutuzumab, GA101, R7159.
Study selection: Studies in patients with chronic lymphocytic leukaemia who received obinutuzumab. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): non-Hodgkin’s lymphoma (version 5.2014). 2014. http://www.nccn.org/. Accessed 24 Nov 2014.
Eichhorst B, Dreyling M, Robak T, et al. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi50–4.
Klein C, Lammens A, Schäfer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. mAbs. 2013;5(1):22–33.
Tam CS, Otero-Palacios J, Abruzzo LV, et al. Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent in-situ hybridization in 510 patients. Br J Haematol. 2008;141(1):36–40.
European Medicines Agency. Gazyvaro 1,000 mg concentrate for solution for infusion: summary of product characteristics. 2014. http://www.ema.europa.eu/ema/. Accessed 24 Nov 2014.
Genentech Inc. GAZYVA™ (obinutuzumab) injection, for intravenous infusion: prescribing information. 2014. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed 24 Nov 2014.
Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12(10):2031–42.
Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190(6):2702–11.
Herter S, Birk MC, Klein C, et al. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol. 2014;192(5):2252–60.
Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–402.
Flynn JM, Byrd JC, Kipps TJ, et al. Obinutuzumab (GA101) 1,000 versus 2,000 mg in patients with chronic lymphocytic leukemia (CLL): results of the phase II GAGE (GAO4768g) trial [abstract no. 7083]. J Clin Oncol. 2014;32(15 Suppl. 1).
Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10.
Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–32.
Morschhauser F, Cartron G, Lamy T, et al. Phase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemia [abstract no. 884]. Blood. 2009;114.
Cartron G, de Guibert S, Dilhuydy M-S, et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124(14):2196–202.
Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–25.
Gibiansky E, Gibiansky L, Carlile DJ, et al. Population pharmacokinetics of obinutuzumab (GA101) in chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma and exposure-response in CLL. CPT Pharmacomet Syst Pharmacol. 2014;3:e144.
European Medicines Agency. Gazyvaro: CHMP assessment report. 2014. http://www.ema.europa.eu/ema/. Accessed 24 Nov 2014.
US FDA Center for Drug Evaluation and Research. Medical review: GAZYA (obinutuzumab). 2013. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed 24 Nov 2014.
Freeman CL, Dixon M, Houghton R, et al. Risk factors associated with the development of infusion-related reactions in patients with chronic lymphocytic leukaemia treated with anti-CD20 monoclonal antibodies: analysis of the CLL11 study dataset [abstract no. 3339]. In: 56th American Society of Hematology Annual Meeting and Exposition; 2014.
Morschhauser A, Salles G, Cartron G, et al. Dose selection for phase III studies of the monoclonal anti-CD20 antibody obinutuzumab (GA101): a rational approach [abstract no. 0935]. Haematologica. 2011;96(Suppl 2):390.
Chang C-H, Rossi EA, Goldenberg DM. Monoclonal antibodies targeting CD20. mAbs. 2013;5(3):335–6.
O’Brien S, Wierda WG, Faderl S, et al. FCR-3 as frontline therapy for patients with chronic lymphocytic leukemia [abstract no. 2117]. Blood. 2005;106.
O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19(8):2165–70.
Cameron H, Thompson M, Marino J-P, et al. Obinutuzumab and chlorambucil versus chlorambucil monotherapy for treatment of previously untreated chronic lymphocytic leukemia where fludarabine-based chemotherapy is considered inappropriate: a Canadian cost-utility analysis [abstract no. 1288]. In: 56th American Society of Hematology Annual Meeting and Exposition; 2014.
Walzer S, Becker U, Samanta K, et al. The potential cost-effectiveness of obinutuzumab (GA101) in combination with chlorambucil in chronic lymphocytic leukemia [abstract no. PCN112]. Value Health. 2013;16(7):A412.
Veenstra DL, Reyes CM, Ramsey SD. Is obinutuzumab cost-effective in the first-line treatment of CLL? [abstract no. 7052]. J Clin Oncol. 2014;32(15 Suppl. 1).
Reyes C, Gazauskas G, Becker U, et al. Cost-effectiveness analysis of obinutuzumab versus ofatumumab for previously untreated chronic lymphocytic leukemia (CLL) [abstract no. 1324]. In: 56th American Society of Hematology Annual Meeting and Exposition; 2014.
National Institute for Health and Care Excellence. NICE set to recommend another leukaemia drug. 2014. https://www.nice.org.uk/. Accessed 3 Dec 2014.
National Health Service. National Cancer Drug Fund prioritization scores: obinutuzumab. 2014. http://www.england.nhs.uk/. Accessed 19 Nov 2014.
Waterboer T, Moreno SG, Shang A, et al. Indirect treatment comparisons of obinutuzumab (GA101) plus chlorambucil (Clb) versus bendamustine and versus ofatumumab plus Clb in patients with chronic lymphocytic Leukemia [abstract no. PSY9]. Value Health. 2014;17(3):A225.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. Sheridan Hoy is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: B. D. Cheson, Lombardi Comprehensive Cancer Center, Georgetown University Hospital, Washington, DC, USA; M. J. S. Dyer, The Ernest and Helen Scott Haematological Research Institute, University of Leicester, Leicester, UK; E. Montserrat, Department of Hematology, Institute of Hematology and Oncology, Hospital Clinic, University of Barcelona, Barcelona, Spain; T. Robak, Department of Haematology, Medical University of Lodz, Copernicus Hospital, Lodz, Poland.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Obinutuzumab: A Review of Its Use in Patients with Chronic Lymphocytic Leukaemia. Drugs 75, 285–296 (2015). https://doi.org/10.1007/s40265-014-0340-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0340-3