Abstract
Introduction
Amyotrophic lateral sclerosis is a fatal progressive disease with a still unclear multi-factorial etiology. This study focused on the potential relationship between drug exposure and the development of amyotrophic lateral sclerosis by performing a detailed analysis of events reported in the FDA Adverse Event Reporting System database.
Methods
The FDA Adverse Event Reporting System quarterly data (January 2004–June 2020) were downloaded and deduplicated. The reporting odds ratios and their 95% confidence intervals were calculated as a disproportionality measure. The robustness of the disproportion was assessed accounting for major confounders (i.e., using a broader query, restricting to suspect drugs, and excluding reports with amyotrophic lateral sclerosis as an indication). Disproportionality signals were prioritized based on their consistency across analyses (reporting odds ratio stability).
Results
We retained 1188 amyotrophic lateral sclerosis cases. Sixty-two drugs showed significant disproportionality for amyotrophic lateral sclerosis onset in at least one analysis, and 31 had consistent reporting odds ratio stability, including tumor necrosis factor-alpha inhibitors and statins. Disproportionality signals from ustekinumab, an immunomodulator against interleukins 12–23 used in autoimmune diseases, and the anti-IgE omalizumab were consistent among analyses and unexpected.
Conclusions
For each drug emerging as possibly associated with amyotrophic lateral sclerosis onset, biological plausibility, underlying disease, and reverse causality could be argued. Our findings strengthened the plausibility of a precipitating role of drugs primarily through immunomodulation (e.g., tumor necrosis factor-alpha, ustekinumab, and omalizumab), but also by impacting metabolism and the musculoskeletal integrity (e.g., statins and bisphosphonates). Complement and NF-kB dysregulation could represent interesting topics for planning translational mechanistic studies on amyotrophic lateral sclerosis as an adverse drug effect.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Amyotrophic lateral sclerosis has a complex unclear etiology including genetic susceptibility and environmental factors. |
By analyzing reports from the FDA Adverse Event Reporting System, 31 drugs were found to be disproportionally reported with amyotrophic lateral sclerosis, including drugs potentially implicated in amyotrophic lateral sclerosis development (e.g., immunomodulators and statins). |
Future translational research should focus on the possible role of complement and NF-kB dysregulation in amyotrophic lateral sclerosis onset or worsening. |
1 Introduction
Amyotrophic lateral sclerosis (ALS) is a rare, subtle, and progressive disease beginning with focal weakness, then spreading to involve most muscles. Patients die because of respiratory paralysis when the diaphragm is involved, in 3–5 years from symptoms onset. Disease progression is highly variable among affected individuals, and clinical features encompass heterogeneous motor and non-motor signs and symptoms [1]. Therefore, late diagnosis is common. Medications to relieve symptoms are available, together with two specific agents, riluzole and edaravone, which demonstrated a survival benefit of months.
One of the reasons accounting for the failure of almost all previous attempts with clinical trials in ALS is the incomplete comprehension of the pathogenic processes that cause disease onset and progression. Although ALS is a clinically defined syndrome where upper and lower motor neurons degenerate, the pathogenesis is probably heterogeneous across individual cases. It has been suggested that multiple events need to befall, or multiple factors need to be present for the disease to manifest. Presumably, these would include genetic susceptibility and environmental factors. Nonetheless, no specific environmental factor has been proven to cause ALS, and there is a remarkable gap between the relative abundance of proposed genetic ALS determinants and the lack of findings on environmental risk. Of note, the two drugs used to prolong life in patients diagnosed with ALS are thought to act directly on the survival of the motor neurons: edaravone as a free radical scavenger that prevents oxidative stress damage [2] and riluzole inhibiting the glutamatergic excitotoxicity that results in high levels of intracellular calcium and free radical generation [3].
Drug exposure has been investigated as a potential risk/precipitating factor in the occurrence of ALS. Population-based observational studies (case-control design) found no association with proton pump inhibitors, an association with antibiotics, contradictory results for statins and tumor necrosis factor-alpha (TNF-α) inhibitors, and a potential inverse association with antidiabetic agents [4,5,6,7]. Of note, gut microbiota, altered by several of these drugs, was proposed as a working hypothesis [8].
Large-scale post-marketing databases are suitable sources to suggest potential associations between specific drug exposures and rare events, such as ALS, which may escape detection and/or reporting from randomized controlled trials and population-based studies [9]. Of note, real-world pharmacovigilance databases have been used to investigate the role of drugs in modulating complex and multi-factorial neuropsychiatric diseases such as multiple sclerosis, thus supporting clinicians in real-life risk-benefit evaluations [10]. The aim of this study is to characterize spontaneous reports of ALS using one of the largest publicly available repositories of adverse events, namely the FDA Adverse Event Reporting System (FAERS), in order to assess whether specific drug exposures could represent a potential risk or precipitating factor in ALS development.
2 Methods
2.1 Study Design and Data Source
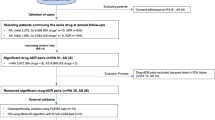
The study was conceived as a case/non-case analysis using the FAERS, a surveillance system collecting reports submitted on a voluntary basis by healthcare professionals and consumers. Reports may be submitted directly to the US Food and Drug Administration using MedWatch forms but are usually submitted first to the Marketing Authorization Holders, for which forwarding to the Food and Drug Administration is mandatory. It currently collects more than 20 million reports worldwide (including European reports potentially related to serious events and other non-US non-European data), with public data availability since 2004, thus offering an emerging opportunity for signal detection and characterization [9]. Adverse events and indications are coded using the Medical Dictionary for Regulatory Activities (MedDRA®) preferred terms. Drug names, instead, are recorded as a free text and are, therefore, extremely heterogenous (e.g., referring to the brand or the active ingredient, not uncommonly with misspellings).
We downloaded the FAERS quarterly data (January 2004–June 2020) and pre-processed them for drug name standardization. We used the WHO Drug Dictionary (accessed in March 2020) and manual integration to reach a coverage of more than 97% of the recorded drugs, which were translated into their active ingredients and referred to their class in the Anatomic Therapeutic Chemical classification. Further, a semiautomatic algorithm was used to keep only the last update of each report and to remove duplicates (i.e., reports with the same sex, age, weight, country, event date, list of drugs, and list of events) [11]. No ethics board was needed as this was a pharmacovigilance analysis using an existing database with anonymous public data availability.
2.2 Disproportionality Analyses
A pre-specified multi-step strategy was applied to assess the consistency of findings and minimize bias. To evaluate if, and to what extent, ALS was reported as a potential adverse drug reaction, we performed a disproportionality analysis, a consolidated approach in pharmacovigilance. Through a case/non-case approach, the reporting odds ratio (ROR) was calculated and deemed statistically significant when the lower limit of its 95% confidence interval (CI) exceeded 1 [11]. If the proportion of ALS reports was greater among patients exposed to a specific drug (cases) than among patients not exposed to it (non-cases), an association was hypothesized (disproportionality signal). Notably, the performance of the ROR (i.e., the capacity to discriminate true from false positive drug–event associations) is striking especially for adverse events with a low/rare background incidence such as ALS [12].
As for the main analysis, we calculated the ROR between the PT “amyotrophic lateral sclerosis” and individual active ingredients. In this analysis, we recognized a report as a case both whether the drug was recorded as the suspect therapy or whether it was recorded as concomitant therapy. This choice was made because it may be difficult to identify the culprit for the development of a plausibly delayed-onset reaction as ALS, and because, if we are considering concomitant therapy as non-case, then the same drug would appear both at the numerator and the denominator of the ROR formula.
To assess the robustness of the disproportionality signal, we performed three other disproportionalities. To increase the sensitivity of our study we considered (1) a broader ALS definition for case retrieval, including also “familial ALS,” “hereditary ALS,” “motor neuron disease,” “progressive bulbar palsy,” and “progressive muscular atrophy”. This way we could account for the redundancy in MedDRA® terms, for which ALS may be recorded using different terms depending on the reporter. To increase the specificity of the study, we restricted the disproportionality analysis to (2) drugs recorded as primary or secondary suspects, and (3) reports without ALS among the specified indications. This way we could account for the judgment of the reporter on the drug role, and for the expected association between ALS and drugs used in its management. In particular, we decided to exclude drugs used to treat ALS only in a specific analysis because the reverse causality bias (i.e., the fact that a disproportion may be the result of drugs being taken to treat ALS or its symptoms) cannot be completely foreseen. This is true, in particular, for drugs used to treat ALS prodromes, which are less specific and develop before the diagnosis.
To further reduce the likelihood of false positives, the ROR was calculated only for drugs with at least five cases. Disproportionality signals were prioritized based on their consistency across the different analyses (ROR stability). Finally, aiming for maximum specificity, we focused on the drugs with the top ROR stability: all the analyses detected a significant ROR.
3 Results
Overall, 14,526,399 reports were downloaded from the FAERS quarterly data and reduced to 10,760,574 with the de-duplication process. Among these, 1181 reports specified ALS as a reaction (1464 using the broader definition), 543 (44%) recording ALS as the only adverse event. The geographical areas contributing with more reports were North America (405, 34.1%), Europe (181, 15.2%), Asia (60, 5.1%), and South America (23, 1.9%).
Amyotrophic lateral sclerosis reports occurred more commonly in male individuals, peaked between 50 and 74 years of age, and were submitted after 2012. Sixty-two drugs were disproportionally reported with ALS using the broad definition. Of these, 31 drugs kept the signal in all more specific analyses, five drugs in three, and 17 drugs in two (see Table 1). For further information about case numbers and RORs calculated in the analyses, see the tables in the Electronic Supplementary Materials 1 and 2.
Some pharmacological classes included more drugs disproportionally reported with ALS. Concerning cardiovascular/metabolic drugs, all marketed statins showed a “top” ROR stability (four out of four analyses) and a high number of cases (atorvastatin, N = 154, median ROR of the main analysis = 4.97, 95% CI = [4.19–5.88]; simvastatin, N = 95, ROR = 4.01 [3.25–4.94]; rosuvastatin, N = 58, ROR = 4.34 [3.33–5.65]). Only a small decrease in cases was observed in the more specific analyses. As for other drugs in the cardiovascular area, atenolol, enalapril, and candesartan were recorded in a non-negligible number of cases (respectively, 27, 15, and 19), but with a lower ROR stability.
Concerning immunomodulating agents, TNF-α inhibitors showed the highest number of cases (adalimumab, N = 99, ROR = 2.03 [1.65–2.49]; etanercept, N= 89, ROR = 1.76 [1.42–2.19]; infliximab, N = 78, ROR = 5.46 [4.34–6.87]). Notable findings were also obtained for ustekinumab (top ROR stability, N = 10, ROR = 2.04 [1.10–3.81]) and omalizumab (top ROR stability, N = 8, ROR = 2.06 [1.03–4.12]).
Among antineoplastic agents, methotrexate (ROR = 2.46 [1.97–3.09]) and rituximab (ROR = 3.3 [2.37–4.59]) had the top ROR stability and the highest number of cases. However, the 99 broad cases of methotrexate decrease to 41 when restricting to suspect drugs and cases without ALS in the indications.
Regarding musculoskeletal system drugs, we observed top ROR stability for risedronic acid (N = 7, ROR = 2.57 [1.22–5.41]), baclofen (N = 28, ROR= 3.61 [2.48–5.26]), and celecoxib (N = 19, ROR = 1.91 [1.21–3]). Among nervous system drugs, only duloxetine showed a top ROR stability. Edaravone and riluzole, the only two drugs labeled for ALS treatment, showed a disproportionality signal only in the first three case definitions, losing it in the correction for the reverse causality bias. We also observed top ROR stability for dextromethorphan (N = 17, ROR = 7.51 [4.65–12.12]). Three agents belonging to different therapeutic classes, but with a common quinolinic chemical structure and antinicotinic activity, emerged with not negligible cases and disproportionality signal: quinidine (N = 16, ROR = 40.18 [24.51–65.88], top ROR stability), hydroxychloroquine (N = 19, ROR = 2.11 [1.34–3.32], top ROR stability), and quinine (N = 3, ROR = 3.21 [1.03–9.97], intermediate ROR stability).
4 Discussion
We investigated ALS from a pharmacovigilance perspective and found a higher-than-expected reporting (disproportionality signal) for 62 different drugs, especially TNF-α inhibitors and statins, thus supporting the hypothesis that drug exposure can have a role in ALS onset. For every single drug or drug class, the biological plausibility of the adverse drug effect was discussed, accounting for a potential reverse causality bias between indication for use and ALS.
4.1 Role of Immunomodulators
Immunomodulator agents had the highest number of signals, especially TNF-α inhibitors (adalimumab, infliximab, etanercept, abatacept). They are usually prescribed to treat chronic inflammatory/autoimmune diseases (e.g., rheumatoid arthritis and psoriasis), although some preliminary studies proposed them even as a potential treatment for ALS, owing to their ability in reducing microglial inflammation [13].
As a matter of fact, specific autoimmune mechanisms responsible for the loss of motoneurons have been advocated as factors promoting ALS. In particular, complement dysregulation and deposition have been identified at the neuromuscular junction prior to nerve cell death in patients with ALS [14]. The role of complement in ALS was confirmed in animal models [15, 16] and indeed, two randomized clinical trials on complement inhibitors (ravulizumab and pegcetacoplan) in patients with ALS are currently ongoing [17, 18]. As upregulation of TNF-α causes complement induction, TNF-α inhibitors could hamper ALS progression.
However, TNF-α inhibitors may also represent a risk factor for ALS onset and progression [13]. This “Janus effect” results from their ability to both activate the PI3K/Akt pathway and inhibit the NF-kB-dependent pathways, which are the major survival pathways in motor neurons [19]. Clinical evidence is also conflicting: some reports, indeed, describe ALS onset in patients with autoimmune diseases treated with TNF-α inhibitors (adalimumab) [20], but a population-based cohort study did not find an increased incidence of ALS in patients with rheumatoid arthritis, regardless of treatment with TNF-α inhibitors [7].
Other immunosuppressants also emerged. The signal of ustekinumab, a fully humanized monoclonal antibody that binds the p40 subunit of unbound interleukin-12 and interleukin-23, used in inflammatory bowel diseases and psoriasis, found support in a case report where its use anticipated ALS onset [21]. The signal of omalizumab, an IgE antagonist monoclonal antibody mainly used in severe asthma and allergic respiratory diseases, seems completely unexpected. The signal of hydroxychloroquine, frequently used for its immunosuppressive properties (e.g., in rheumatoid arthritis), is partly supported by evidence on other hydroxychloroquine-induced neuromuscular disorders (i.e., myasthenia gravis) [22].
Our findings from the FAERS suggest that a patient who starts receiving immunomodulating drugs to treat autoimmune diseases may have a greater susceptibility to ALS either for the drug or for the underlying autoimmunity. Going forward, translational medicine studies and larger cohort studies should be conducted, in order to further investigate this relationship between each specific agent (or the whole pharmacological class) and ALS development.
4.2 Role of Antineoplastic Agents
Concerning antineoplastic drugs, we observed statistically significant data for imatinib, rituximab, and methotrexate, the most commonly prescribed drugs of their respective class. Other agents with the same mechanisms could share this effect, but their less common use probably limited the number of reports and hampered the achievement of significant disproportionality.
The mechanism of action of rituximab includes direct effects with complement-mediated cytotoxicity together with antibody-dependent cell-mediated cytotoxicity [23]. Furthermore, treatment with methotrexate is associated with systemic complement activation [24]. The complex activities of these drugs leading to complement activation may be implicated in ALS pathogenesis.
Of interest is the case of imatinib, an oral tyrosine kinase inhibitor targeting c-KIT, which also inhibits TNF-α production and could reduce the survival of astrocytes, similarly to other TNF-α inhibitors [25]. Masitinib, an analog to imatinib with higher selectivity and a potentially better safety profile, was found to reduce neuroinflammation by targeting mast cells and neutrophils in pre-clinical models [26] and is now tested for ALS treatment in a phase III, multi-center, international, randomized controlled trial after a promising phase II study [27]. Overall, these data suggest a possible role of the drugs, rather than of the underlying diseases, as risk factors for ALS, and their multi-faceted mechanisms of action deserve to be better explored in relation to ALS with preclinical and clinical studies.
4.3 Role of Agents for Metabolic-Cardiovascular Disorders
Top ROR stability for all statins emerged from our analyses. Statins are widely prescribed drugs and are generally considered safe medications, except for rare side effects such as myositis. As a matter of fact, according to Golomb et al. [28], we found higher disproportionality for lipophilic statins, for which the incidence of rhabdomyolysis is four times higher than for monotherapy with pravastatin and fluvastatin [29]. It could be argued that rhabdomyolysis (as well as the rare peripheral neuropathy [30]), caused by lipophilic statins, may uncover the onset of ALS.
However, high plasma cholesterol levels have been suggested to be neuroprotective for ALS and to be associated with an increased survival time. Statins, by lowering cholesterol-inhibiting HMG-CoA reductase, may accelerate functional decline or clinical onset in patients inclined to ALS. Patients receiving statin therapy also reported a significant increase in muscle cramp frequency and severity [31].
By considering all these aspects, we can argue that statins may unmask pre-existing ALS or worsen the disease. Given the milder muscular effects of ezetimibe [32], our findings on this drug may be because of a previous or concomitant use of statins.
As for antihypertensive agents, we found disproportionality for only a few drugs, with a strong decrease in cases in the more sensitive analyses. Published evidence on patients with ALS from clinical registries provides controversial findings on the impact of cardiovascular disorders on the ALS course: Moglia et al. observed that arterial hypertension, type 2 diabetes mellitus, and cardiovascular risk factors do not influence ALS phenotype and prognosis [33]; while Mandrioli et al., by performing a multi-center retrospective study including patients in 13 referral centers for ALS located in ten Italian regions, showed that patients with ALS affected by hypertension at diagnosis had a median survival of 37 months as opposed to 49 months for those who were not affected, by concluding that hypertension and heart diseases, as well as hematological diseases, are independently associated with shorter survival [34]. Evidence on the possible impact of cardiovascular drugs on ALS is even more scarce. It is of interest that the mechanism of action of riluzole also encompasses sodium and calcium channel modulation; some drugs (e.g., verapamil) identified in our analysis could act oppositely. Beta-blockers could instead worsen respiratory symptoms, by acting as antagonists on bronchial beta-2 receptors.
4.4 Role of Bisphosphonates
Bisphosphonates are used to treat patients with osteoporosis, to prevent hip and vertebral fractures, and among known side effects, paradoxical bone damage is included: osteonecrosis of the jaw and atypical femoral fracture. Our findings on bisphosphonates as possible risk factors for ALS onset may be because of different reasons. First, patients taking bisphosphonates to treat osteoporosis might already have non-diagnosed ALS. Consequently, reporters could have inappropriately identified bisphosphonates as a suspected cause for ALS onset. Bone mineral loss has been noted in ALS and aberrant calcium metabolism and vertebral anomalies have been detected in some patients with ALS [35], and according to the study by Peters et al. [36], poor bone health may be related to ALS. There are in fact some common features that may link ALS and osteoporosis, including the influence of several neurotoxic metals, such as lead and the age at onset [37].
Again, ALS worsening might depend on the paradoxical side effects of bisphosphonates on bone growth, already affected in ALS. In fact, it is likely that skeletal muscle experiences double pathological insults, both intrinsic and extrinsic, during ALS progression, leading to severe atrophy in a very short period of time (3–5 years) after the onset of the ALS symptoms [38].
4.5 Signals for Drugs Used in Patients with ALS
Among other signals that we detected, several drugs belong to the symptomatic treatment offered to patients with ALS in order to control and delay the disease process. We could therefore exclude the following drugs from hypotheses on the biological plausibility of adverse drug effects:
-
riluzole and edaravone (both used as treatments of choice for ALS [39]);
-
dextromethorphan and quinidine combination (authorized for pseudobulbar syndrome in patients with ALS in the USA [40]);
-
quinine (used to treat cramps [41]);
-
muscle relaxant agents (tizanidine, baclofen, and botulinum toxin used to reduce spasticity in patients with ALS [42]);
-
benzodiazepines (lorazepam, to treat both spasticity and anxiety symptoms [43]);
-
immunomodulatory agents (interferon beta-1b, tested in ALS because it might interfere with immune mechanisms involved in the pathogenesis of the disease [44, 45]);
-
cholecalciferol (offered to contrast neuroinflammation and also because the impact of vitamin D deficiency is considered a favoring factor in various central or peripheral neurological diseases such as ALS [46]);
-
antidepressant agents (paroxetine, escitalopram, and duloxetine, used in ALS to treat depression, excessive salivation for their anticholinergic side effects, and emotional outbursts [40, 47]). Moreover, as for other neurodegenerative conditions, depression may be a prodromal symptom of ALS. Although motor symptoms are traditionally perceived as the first and main symptoms of ALS, the involvement of non-motor symptoms both at the onset and after the debut of motor symptoms is likely [47]. This case represents an additional reason for reporting ALS after antidepressant use;
-
non-steroidal anti-inflammatory drugs (used to treat pain, which commonly affects the limbs, trunk, and neck in all stages of ALS and becomes more common as the disease progresses [40]).
Nevertheless, it has to be said we cannot totally exclude a role in ALS development for some of the agents listed above. As an example, quinine and quinidine, as well as hydroxychloroquine, might contribute to the worsening of ALS by antagonizing acetylcholine on its nicotinic receptors [48]. At the same time, we cannot exclude that some other promising drug is affected by reverse causality bias. In fact, the ALS pre-symptomatic stage could last also for years [49], and early non-motor symptoms have been increasingly recognized in the disease [50], increasing the possibility of confounders in this type of retrospective study. Thus, we emphasize that the results generated in the present study should be interpreted with caution. Further prospective population-based cohort studies may provide more reliable evidence with a lower interference of reverse causation than retrospective studies.
4.6 Role of Gut Microbiota
Finally, ALS development seems to be connected with gut dysbiosis and altered intestinal permeability, although the precise biological mechanisms underlying such a relationship remain to be clarified [8]. It is noteworthy that several classes of drugs with an ALS signal in FAERS are known to alter intestinal microbiota: statins, by interacting with bile acids and impacting upon the expression of inflammatory markers that influence microbial community structure; antineoplastic agents, for which a mechanistic framework of interaction with a microbiome was recently described (TIMER, Translocation, Immunomodulation, Metabolism, Enzymatic degradation, and Reduced diversity); non-steroidal anti-inflammatory drugs, by altering the function of the small intestine; and antihypertensive drugs, for which animal data suggested an interaction with gut microbiota [51, 52].
4.7 Limitations and Strengths
The analysis of spontaneous reporting data allows for an inexpensive and timely detection of unexpected drug–event disproportionalities. It collects a considerable number of reports from a large heterogeneity of environments and accounts for a complexity that is usually excluded from randomized clinical trials (e.g., comorbidity, polytherapy, misuse, extreme ages). Nonetheless, it does not allow a formal risk assessment and requires considering multiple biases and limitations. Under-reporting is an important problem, particularly for multi-factorial and delayed-onset events such as ALS is thought to be, therefore spontaneous reporting systems cannot be used to calculate incidence. Furthermore, reports are submitted on a spontaneous basis, both by doctors and consumers, and are often unverified. To account for inter-reporter heterogeneity in the choice of terms, partly owing to the redundancy of MedDRA®, we extended the case codification for the broader analysis. We also discussed the reverse causality bias and prioritized disproportionality signals with ROR stability on multiple sensitivity analyses.
5 Conclusions
With this pharmacovigilance analysis, we detected drugs disproportionally reported with ALS and discussed their potential role. Our findings strengthened the plausibility of a precipitating role of drugs primarily through immunomodulation (e.g., TNF-α, ustekinumab, and omalizumab), but also by impacting metabolism and the musculoskeletal integrity (e.g., statins and bisphosphonates). Our findings provide a useful background for planning large cohort studies to reinforce or weaken the plausibility of a causal role for specific drugs in the development of ALS. They also represent an input for translational research on mechanisms of ALS and on relevant possible therapeutic strategies.
References
Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc. 2018;93:1617–28. https://doi.org/10.1016/j.mayocp.2018.04.007.
Schultz J. Disease-modifying treatment of amyotrophic lateral sclerosis. Am J Manag Care. 2018;24:S327–35.
Jaiswal MK. Calcium, mitochondria and the pathogenesis of ALS: the good, the bad and the ugly. Front Cell Neurosci. 2013;7:199. https://doi.org/10.3389/fncel.2013.00199.
Mariosa D, Kamel F, Bellocco R, Ronnevi LO, Almqvist C, Larsson H, et al. Antidiabetics, statins and the risk of amyotrophic lateral sclerosis. Eur J Neurol. 2020;27:1010–6. https://doi.org/10.1111/ene.14190.
Cetin H, Sun J, Almqvist C, Reichardt B, Tomschik M, Zimprich F, et al. No association between proton pump inhibitor use and ALS risk: a nationwide nested case–control study. Sci Rep. 2020;10:13371. https://doi.org/10.1038/s41598-020-70373-8.
Freedman DM, Kuncl RW, Cahoon EK, Rivera DR, Pfeiffer RM. Relationship of statins and other cholesterol-lowering medications and risk of amyotrophic lateral sclerosis in the US elderly. Amyotroph Lateral Scler Front Degener. 2018;19:538–46. https://doi.org/10.1080/21678421.2018.1511731.
Arkema EV, Feltelius N, Olsson T, Askling J. No association between rheumatoid arthritis, amyotrophic lateral sclerosis, and tumour necrosis factor inhibitor treatment. Ann Rheum Dis. 2014;73:2061–2. https://doi.org/10.1136/annrheumdis-2014-205622.
Sun J, Zhan Y, Mariosa D, Larsson H, Almqvist C, Ingre C, et al. Antibiotics use and risk of amyotrophic lateral sclerosis in Sweden. Eur J Neurol. 2019;26:1355–61. https://doi.org/10.1111/ene.13986.
Raschi E, Moretti U, Salvo F, Pariente A, Antonazzo IC, De Ponti F, et al. Evolving roles of spontaneous reporting systems to assess and monitor drug safety. IntechOpen. 2018. https://doi.org/10.5772/intechopen.79986.
Antonazzo IC, Raschi E, Forcesi E, Riise T, Bjornevik K, Baldin E, et al. Multiple sclerosis as an adverse drug reaction: clues from the FDA Adverse Event Reporting System. Expert Opin Drug Saf. 2018;17:869–74. https://doi.org/10.1080/14740338.2018.1506763.
Poluzzi E, Raschi E, Piccinni C, Ponti FD. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (FAERS). IntechOpen. 2012. https://doi.org/10.5772/50095.
Harpaz R, DuMouchel W, LePendu P, Bauer-Mehren A, Ryan P, Shah NH. Performance of pharmacovigilance signal-detection algorithms for the FDA Adverse Event Reporting System. Clin Pharmacol Ther. 2013;93:539–46. https://doi.org/10.1038/clpt.2013.24.
Petitpain N, Devos D, Bagheri H, Rocher F, Gouraud A, Masmoudi K, et al. Is TNF inhibitor exposure a risk factor for amyotrophic lateral sclerosis? Fundam Clin Pharmacol. 2019;33:689–94. https://doi.org/10.1111/fcp.12480.
Bahia El Idrissi N, Bosch S, Ramaglia V, Aronica E, Baas F, Troost D. Complement activation at the motor end-plates in amyotrophic lateral sclerosis. J Neuroinflammation. 2016;13:72. https://doi.org/10.1186/s12974-016-0538-2.
Woodruff TM, Lee JD, Noakes PG. Role for terminal complement activation in amyotrophic lateral sclerosis disease progression. Proc Natl Acad Sci USA. 2014;111:E3-4. https://doi.org/10.1073/pnas.1321248111.
Parker SE, Hanton AM, Stefanou SN, Noakes PG, Woodruff TM, Lee JD. Revisiting the role of the innate immune complement system in ALS. Neurobiol Dis. 2019;127:223–32. https://doi.org/10.1016/j.nbd.2019.03.003.
Alexion Pharmaceuticals. A phase 3, double-blind, randomized, placebo-controlled, parallel group, multicenter study with an open-label extension to evaluate the efficacy and safety of ravulizumab in patients with amyotrophic lateral sclerosis (ALS). 2021. https://clinicaltrials.gov/ct2/show/NCT04248465. Accessed 18 May 2021.
Apellis Pharmaceuticals, Inc. A phase 2, randomized, double-blind, placebo-controlled, multicenter study to evaluate the efficacy and safety of pegcetacoplan in subjects with amyotrophic lateral sclerosis (ALS). 2021. https://clinicaltrials.gov/ct2/show/NCT04579666. Accessed 18 May 2021.
Ouali Alami N, Schurr C, Olde Heuvel F, Tang L, Li Q, Tasdogan A, et al. NF-κB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018;37: e98697. https://doi.org/10.15252/embj.201798697.
Bougea A, Anagnostou E, Stamboulis E, Kararizou E. Amyotrophic lateral sclerosis developing during adalimumab therapy for psoriatic arthritis. Rev Neurol (Paris). 2014;170:228–9. https://doi.org/10.1016/j.neurol.2013.11.004.
Aj GA. Development of amyotrophic lateral sclerosis during ustekinumab use for refractory Crohn’s disease. Ann Clin Case Rep. 2017;2:2.
Varan O, Kucuk H, Tufan A. Myasthenia gravis due to hydroxychloroquine. Reumatismo. 2016;67:125. https://doi.org/10.4081/reumatismo.2015.849.
Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2018;18:5–18. https://doi.org/10.1038/nri.2017.97.
Marchi LF, Paoliello-Paschoalato AB, Oliveira RDR, Azzolini AECS, Kabeya LM, Donadi EA, et al. Activation status of peripheral blood neutrophils and the complement system in adult rheumatoid arthritis patients undergoing combined therapy with infliximab and methotrexate. Rheumatol Int. 2018;38:1043–52. https://doi.org/10.1007/s00296-018-3997-1.
Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, et al. The kinase inhibitor imatinib mesylate inhibits TNF-α production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci USA. 2005;102:13622–7. https://doi.org/10.1073/pnas.0501758102.
Palomo V, Nozal V, Rojas-Prats E, Gil C, Martinez A. Protein kinase inhibitors for amyotrophic lateral sclerosis therapy. Br J Pharmacol. 2021;178:1316–35. https://doi.org/10.1111/bph.15221.
Mora JS, Genge A, Chio A, Estol CJ, Chaverri D, Hernández M, et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph Lateral Scler Front Degener. 2020;21:5–14. https://doi.org/10.1080/21678421.2019.1632346.
Golomb BA, Verden A, Messner AK, Koslik HJ, Hoffman KB. Amyotrophic lateral sclerosis associated with statin use: a disproportionality analysis of the FDA’s Adverse Event Reporting System. Drug Saf. 2018;41:403–13. https://doi.org/10.1007/s40264-017-0620-4.
Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:S52–60. https://doi.org/10.1016/j.amjcard.2005.12.010.
Jones MR, Urits I, Wolf J, Corrigan D, Colburn L, Peterson E, et al. Drug-induced peripheral neuropathy: a narrative review. Curr Clin Pharmacol. 2020;15:38–48. https://doi.org/10.2174/1574884714666190121154813.
Zinman L, Sadeghi R, Gawel M, Patton D, Kiss A. Are statin medications safe in patients with ALS? Amyotroph Lateral Scler. 2008;9:223–8. https://doi.org/10.1080/17482960802031092.
Florentin M, Liberopoulos EN, Elisaf MS. Ezetimibe-associated adverse effects: what the clinician needs to know. Int J Clin Pract. 2008;62:88–96. https://doi.org/10.1111/j.1742-1241.2007.01592.x.
Moglia C, Calvo A, Canosa A, Bertuzzo D, Cugnasco P, Solero L, et al. Influence of arterial hypertension, type 2 diabetes and cardiovascular risk factors on ALS outcome: a population-based study. Amyotroph Lateral Scler Front Degener. 2017;18:590–7. https://doi.org/10.1080/21678421.2017.1336560.
Mandrioli J, Ferri L, Fasano A, Zucchi E, Fini N, Moglia C, et al. Cardiovascular diseases may play a negative role in the prognosis of amyotrophic lateral sclerosis. Eur J Neurol. 2018;25:861–8. https://doi.org/10.1111/ene.13620.
Mallette LE, Patten B, Cook JD, Engel WK. Calcium metabolism in amyotrophic lateral sclerosis. Dis Nerv Syst. 1977;38:457–61.
Peters TL, Weibull CE, Fang F, Sandler DP, Lambert PC, Ye W, et al. Association of fractures with the incidence of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener. 2017;18:419–25. https://doi.org/10.1080/21678421.2017.1300287.
Chen X, Wang K, Wang Z, Gan C, He P, Liang Y, et al. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone. 2014;63:76–80. https://doi.org/10.1016/j.bone.2014.02.017.
Zhou J, Yi J, Bonewald L. Muscle-bone crosstalk in amyotrophic lateral sclerosis. Curr Osteoporos Rep. 2015;13:274–9. https://doi.org/10.1007/s11914-015-0281-0.
Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 2019;39:733–48. https://doi.org/10.1002/med.21528.
Hobson EV, McDermott CJ. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2016;12:526–38. https://doi.org/10.1038/nrneurol.2016.111.
Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2012;4: CD004157. https://doi.org/10.1002/14651858.CD004157.pub2.
Park J, Park HJ. Botulinum toxin for the treatment of neuropathic pain. Toxins. 2017;9:260. https://doi.org/10.3390/toxins9090260.
Beeldman E, Raaphorst J, Twennaar MK, de Visser M, Schmand BA, de Haan RJ. The cognitive profile of ALS: a systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry. 2016;87:611–9. https://doi.org/10.1136/jnnp-2015-310734.
Dumitrescu L, Constantinescu CS, Tanasescu R. Recent developments in interferon-based therapies for multiple sclerosis. Expert Opin Biol Ther. 2018;18:665–80. https://doi.org/10.1080/14712598.2018.1462793.
Beghi E, Chiò A, Inghilleri M, Mazzini L, Micheli A, Mora G, et al. A randomized controlled trial of recombinant interferon beta-1a in ALS. Italian Amyotrophic Lateral Sclerosis Group. Neurology. 2000;54:469–74. https://doi.org/10.1212/WNL.54.2.469.
Mpandzou G, Aït Ben Haddou E, Regragui W, Benomar A, Yahyaoui M. Vitamin D deficiency and its role in neurological conditions: a review. Rev Neurol (Paris). 2016;172:109–22. https://doi.org/10.1016/j.neurol.2015.11.005.
Roos E, Mariosa D, Ingre C, Lundholm C, Wirdefeldt K, Roos PM, et al. Depression in amyotrophic lateral sclerosis. Neurology. 2016;86:2271–7. https://doi.org/10.1212/WNL.000000000000267.1.
Gisselmann G, Alisch D, Welbers-Joop B, Hatt H. Effects of quinine, quinidine and chloroquine on human muscle nicotinic acetylcholine receptors. Front Pharmacol. 2018;9:1339. https://doi.org/10.3389/fphar.2018.01339.
Benatar M, Turner MR, Wuu J. Defining pre-symptomatic amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener. 2019;20:303–9. https://doi.org/10.1080/21678421.2019.1587634.
Zucchi E, Ticozzi N, Mandrioli J. Psychiatric symptoms in amyotrophic lateral sclerosis: beyond a motor neuron disorder. Front Neurosci. 2019;13:175. https://doi.org/10.3389/fnins.2019.00175.
Le Bastard Q, Al-Ghalith GA, Grégoire M, Chapelet G, Javaudin F, Dailly E, et al. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment Pharmacol Ther. 2018;47:332–45. https://doi.org/10.1111/apt.14451.
Tuteja S, Ferguson JF. Gut microbiome and response to cardiovascular drugs. Circ Genomic Precis Med. 2019;12: e002314. https://doi.org/10.1161/CIRCGEN.119.002314.
Acknowledgements
The authors are supported by institutional research funds (Ricerca Fondamentale Orientata) from the University of Bologna. The authors thank Ippazio Cosimo Antonazzo from the University of Milano-Bicocca for his support in data analysis planning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was received for this research.
Conflicts of interest/competing interests
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data that support the findings of this study were downloaded from the FAERS quarterly data, available to the public at the following link: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Code availability
The R script developed for the analyses is available from the corresponding author upon reasonable request.
Author contributions
EB, FN, FD, JM, and EP conceived the project and reviewed the manuscript. AG and FM wrote the original draft of the manuscript. AG and FM performed the investigation, carried out the analyses, and interpreted data. FM performed the investigation, carried out the analyses, interpreted data, and administered the project. EB, LV, FN, JM, and EP contributed to methodological issues, supervised the project, and reviewed the manuscript. FD and EP administered the project. All the authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaimari, A., Fusaroli, M., Raschi, E. et al. Amyotrophic Lateral Sclerosis as an Adverse Drug Reaction: A Disproportionality Analysis of the Food and Drug Administration Adverse Event Reporting System. Drug Saf 45, 663–673 (2022). https://doi.org/10.1007/s40264-022-01184-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01184-1