Abstract
Growing evidence indicates that many drugs have the ability to cause a potentially lethal cardiac arrhythmia, torsades de pointes (TdP). This necessitates the development of a compilation of drugs that have this potential toxicity. Such a list is helpful in identifying the etiology of TdP in patients taking multiple drugs and assists decision making by those caring for patients at high risk of TdP. The Arizona Center for Education and Research on Therapeutics (AZCERT) has developed a process to standardize the identification of drugs and place them in risk categories for their clinical ability to cause TdP and QT prolongation. AZCERT’s Adverse Drug Event Causality Analysis (ADECA) utilizes 16 types of data drawn from four sources to compile an open-source knowledge base, QTdrugs, which is maintained on the CredibleMeds.org website. Because the evidence for most drugs is incomplete, the ADECA process is used to place drugs into one of three categories that represent different levels of certainty: known TdP risk, possible TdP risk, and conditional TdP risk. Each category has strict evidentiary requirements for clinical evidence of TdP and/or QT prolongation. These are described in this paper. Because evidence can evolve over time, the ADECA process includes the continuous gathering and analysis of newly emerging evidence to revise the lists. The QTdrugs lists have proven to be a valued, readily available, commercial influence-free resource for healthcare providers, patients, researchers, and authors of consensus guidelines for the safe use of medicines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Arizona Center for Education and Research on Therapeutics (AZCERT) has developed a process to identify and standardize the placement of marketed drugs according to their clinical ability to cause torsades de pointes (TdP) and QT prolongation. |
The QTdrugs knowledge base is a compilation of drugs placed into risk categories that represent different levels of certainty: known TdP risk, possible TdP risk and conditional TdP risk. |
QTdrugs lists are open source and made available online at the CredibleMeds.org website, in smart phone or tablet applications, and to programmers through an Application Program Interface. |
The QTdrugs knowledge base is a resource for healthcare providers, patients, and researchers that has been valuable in clinical practice, clinical and epidemiologic research, and in developing clinical decision support systems that improve the safe prescribing of QT-prolonging medications. |
1 Introduction
In 1999, the Agency for Healthcare Research and Quality launched a network of Centers for Education and Research on Therapeutics (CERTs) [1] as part of a national strategy to address the growing problem of preventable adverse reactions occurring with prescription drugs [2,3,4,5,6], a problem that, at the time, was estimated to cause over 100,000 deaths each year in US hospitals [6]. The number of these centers eventually grew to 14, with each focusing on a different aspect of drug (and device) safety. One of the first created was the Arizona Center for Education and Research on Therapeutics (AZCERT), a university-based program that became a non-profit 501(c)(3) organization in 2012. For almost two decades, AZCERT has performed research, analyzed evidence, and conducted educational programs to reduce the negative health impact of preventable adverse drug reactions (ADRs) and drug–drug interactions (DDIs). In 2015, AZCERT was awarded a contract by the US Food and Drug Administration’s Safe Use Initiative to develop clinical decision support systems (CDSSs) designed to improve the safe use of antibiotics, especially those that prolong the QT interval on the electrocardiogram (ECG) and thereby increase the risk of ‘torsades de pointes’ (TdP) arrhythmias and sudden cardiac death [7, 8].
Prior to the early 1990s, TdP was considered to be an uncommon event that was only associated with a few cardiac drugs, such as quinidine, that prolonged the QT interval [9]. Unexpectedly, in the mid-1990s, QT prolongation and TdP was found to occur with a growing number of non-cardiac drugs (e.g., terfenadine, astemizole, and others) that had been prescribed for many years with presumed safety [10,11,12]. Because TdP is relatively uncommon and difficult to diagnose without an ECG, there was concern that other non-cardiac QT-prolonging drugs might also have this potential [13, 14].
As AZCERT began developing education and research programs to detect and prevent drug-induced TdP, it became necessary to develop a rigorous and reliable process to analyze a broad array of available evidence in order to determine which drugs induced TdP and under what clinical conditions.
QT prolongation by drugs has been used as a biomarker to improve detection of risk and to facilitate TdP prevention [15]. However, because many patients taking these drugs develop measurable QT prolongation and only a relatively small number of those patients develop TdP, QT prolongation is a highly sensitive but non-specific predictor of risk [15]. Furthermore, evaluation of causality for specific drugs is often difficult because many patients who may have developed TdP do not have an ECG at the time of the event. Even when ECGs are available, the diagnosis of drug-induced TdP is often missed [15, 16].
In recent years, the FDA has required drug developers to perform laboratory (hERG testing) and human studies (thorough QT studies in normal subjects) to identify drugs that can cause QT prolongation and therefore identify those that might cause TdP [17]. These tests have found that many of the important drugs on the market today and many in development can prolong QT intervals. Some QT-prolonging medications are effective for the treatment of serious illnesses such as cancer or life-threatening infections and must remain available because they cannot be replaced by safer medicines. For these drugs to remain available for patients, it is essential to know which drugs can cause TdP and how that risk can be minimized [16].
2 The Adverse Drug Event Causality Analysis (ADECA) Process to Evaluate Evidence and Assign Torsades de Pointes (TdP) Risk Categories
Because of the complexity of drug toxicology, the human biologic variability that exists, and the ever-changing clinical practice environment, analysis of diverse and evolving evidence is an essential part of drug safety assessment. Traditional drug safety resources have sought to summarize the toxicity of specific drugs. Few have focused on a specific form of toxicity and summarized the drugs likely to cause that toxicity. Because of growing evidence that many classes of drugs have the ability to cause TdP, the need arose for a compilation of those drugs that have the potential to cause this adverse event. Among its applications, such a list could be helpful in the differential diagnosis of the cause of TdP in patients taking multiple drugs and could assist decision making regarding therapy for patients at high risk for TdP.
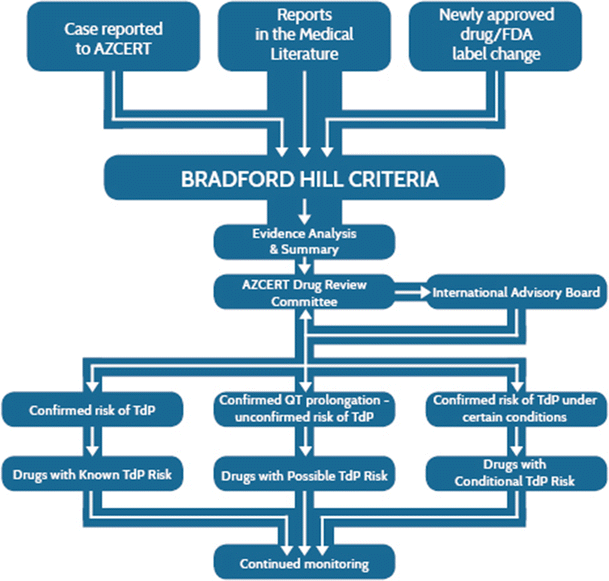
With funding from the US Agency for Healthcare Research and Quality, AZCERT has developed a process to accelerate and standardize the identification of drugs for their ability to cause QT prolongation and/or TdP. AZCERT’s Adverse Drug Event Causality Analysis (ADECA, shown schematically in Fig. 1) has become a model for evaluation of evidence to stratify drugs according to their risk for these adverse events (AEs) [18, 19]. The process utilizes the time-honored Bradford Hill criteria to differentiate causality from association through systematic analysis of laboratory and clinical evidence [20,21,22]. Because the evidence of AE risk may be incomplete, the ADECA process places drugs into one of three categories that represent different levels of certainty: known TdP risk, possible TdP risk and conditional TdP risk. Also, because evidence is very likely to evolve over time, the process includes the continual gathering and analysis of any newly emerging evidence. When justified by new evidence, assignments of drugs to these categories are changed or removed.
2.1 Selection of Drugs for ADECA Review
The review process for a new drug begins when AZCERT scientists become aware of any evidence suggesting that a drug might have the potential to prolong the QT interval and/or cause TdP. Drugs identified from the following sources undergo full ADECA review:
-
1.
A new drug enters the US or European Union (EU) market or a previously marketed drug’s label is revised to include mention that it prolongs the QT interval or that cases of TdP have been reported.
-
2.
A case report or inquiry about a drug is received by email at info@azcert.org or to the http://www.CredibleMeds.org website.
-
3.
A drug is identified during a standard monthly literature search (PubMed search terms: ‘QT’ or torsad*) in which a scientific article reports that a drug causes blockade of hERG ion channels or cardiac potassium current (I Kr), QT prolongation, TdP, hypokalemia, or hypomagnesemia.
2.2 Evaluation of Evidence for QT Prolongation or TdP Causality
To evaluate the evidence for a drug’s risk of causing QT prolongation and/or TdP, the following elements in the Bradford Hill causality analysis are utilized to identify a causal relationship and distinguish it from a simple association between a drug’s use and an AE:
-
Strength is assessed by the numerical associations of a drug with reports of QT prolongation or TdP in the published medical literature and/or the spontaneous AE reports of QT prolongation or TdP to the FDA Adverse Event Reporting System (FAERS) and/or the World Health Organization (Vigibase).
-
Coherence refers to similar signal strength with reports in FAERS or other published reports of related events (QT prolongation, cardiac arrest, ventricular tachycardia, syncope etc.) [15, 16].
-
Plausibility is assessed by whether a drug has a relevant pharmacologic action that is known to cause TdP (e.g., hERG channel, I Kr blockade or increased late sodium current, I NaL) [15].
-
Biological gradient, i.e., the dose or exposure–response relationship.
-
Experimental evidence consists of in vitro data, clinical trials, and challenge/de-challenge/re-challenge experience.
-
Temporality considers the interval between drug exposure and subsequent development of QT increase and/or TdP.
-
Consistency comes from consistent trends in FAERS or Vigibase as well as across reports of QT prolongation or TdP in the scientific literature (PubMed searches).
-
Specificity considers identifiable factors known to predispose to TdP, e.g., hypokalemia, hypomagnesemia, extreme bradycardia, or genetic predisposition (congenital long QT syndrome) [15].
-
Analogy includes comparison of the evidence to that for other drugs that have chemical, pharmacologic, or toxic similarities to the drug of interest.
These criteria are applied in the evaluation of each piece of evidence found from the sources described in Sect. 2.3. In some cases, AZCERT has performed laboratory or clinical experiments to fill specific gaps in the available evidence, often through collaboration [23,24,25,26,27,28,29].
2.3 Sources of Evidence for ADECA
2.3.1 The Medical Literature
AZCERT investigators monitor biomedical research publications of relevance by reviewing monthly reports generated by the National Library of Medicine’s National Center for Biotechnology Information (NCBI). Each month, AZCERT scientists review a list of approximately 80–150 newly published articles that are captured with the search term: ‘QT’ OR Torsad*. The abstracts of these publications are scanned to identify reports that are relevant to the drugs currently being monitored by AZCERT or to identify new drugs that will undergo full evaluation.
AZCERT monitors these monthly reports from NCBI for evidence of drug-induced hERG block, I Kr block, QT prolongation, induction of hypokalemia, hypomagnesemia, or TdP for any of the drugs that are being followed or under evaluation at that time. When these drugs undergo full evaluation, the following search terms are used for specific PubMed searches: drug_name AND (‘QT’ or Torsad*), drug_name AND hERG and drug_name AND ‘potassium channel’
2.3.2 Official Drug Labels Approved by Regulatory Agencies
Initially, only drugs marketed in the US were the focus of the ADECA process. However, in recent years, drugs have been evaluated that were marketed first in the EU, Japan, Canada, or Australia and many have been added to the lists of drugs with QT and TdP risk. For drugs marketed in the US, official FDA labels are downloaded from the Drugs@FDA website (https://www.accessdata.fda.gov/scripts/cder/drugsatfda/) and searched for mention of either QT prolongation or TdP associated with use of the drug. Positive evidence of QT prolongation or risk of TdP requires that the label include a clear statement that QT prolongation or TdP has been observed in patients during clinical use. A hypothetical statement that QT prolongation or TdP “may occur” or “has been seen with similar drugs” is not considered to be positive evidence in labeling. Mention of either QT prolongation or TdP after intentional or accidental overdose is considered supportive evidence for conditional risk of TdP (see risk categories in Sect. 2.4).
To obtain information and labels for new drugs marketed in countries outside the US, a search is conducted using country-specific search engines, as well as the labels (when available) from international regulatory agencies (European Medicines Agency [EMA], Japan’s Pharmaceuticals and Medical Devices Agency [PMDA], Australia’s Therapeutic Goods Administration [TGA], Swissmedic, Health Canada, and the UK Medicines and Healthcare Products Regulatory Agency [MHRA], etc.)
2.3.3 The FDA Adverse Event Reporting System (FAERS) Database
The FDA maintains a publicly available database of AEs associated with use of drugs and biologics that have been reported to the FAERS. The database is one of the FDA’s primary post-marketing safety surveillance tools and is perhaps better known to the public as the MedWatch reporting system. The FAERS data are made available to the public and updates are released every 3 months. In the ADECA process, FAERS data are analyzed by AZCERT’s scientific reviewers using Empirica Signal® software (Oracle Health Sciences, Redwood City, CA, USA), a data mining tool initially developed by Oracle Health Systems and the FDA for use by FDA safety scientists [30, 31].
2.3.4 The World Health Organization (WHO) Adverse Events Database
The WHO’s Vigibase database of adverse events is also available publicly and is searched and analyzed using Empirica Signal®. Vigibase serves as a secondary or confirmatory source for the FAERS data and is especially useful for evaluation of drugs developed and marketed outside the US.
While FAERS and Vigibase data are extremely useful and have been the primary tools for the FDA and other regulatory agencies to detect many serious ADRs, both have limitations that have been documented and described [30]. For example, because the reports are spontaneous and voluntary, it is not possible to estimate the number of people exposed to the drug, thus making comparison of drug signals in these databases virtually impossible. Duplication, under-reporting of AE, and incomplete reports further complicate analysis of the data. Nevertheless, statistical approaches that adjust for these limitations have been developed and enable reliable identification of pharmacovigilance signals [30].
To mine these databases for signals associated with specific drugs or drug combinations, AZCERT scientists use two approaches. The first method is based on a multi-item gamma Poisson shrinker (MGPS) approach that is utilized to find unexpectedly frequent associations between a given AE and a drug. It uses the empirical Bayesian geometric mean (EBGM) to estimate the relative reporting ratio [32] with a 90% credible interval (CrI). The lower and upper bounds of the Crl are designated as EB05 and EB95, respectively. In this approach, a signal in FAERS or Vigibase is considered relevant if the EBGM and the entire 90% CrI are ≥2.0 (i.e., EB05 ≥2.0). The analyses focus on the drug–AE combinations in which the drug of interest was designated as the ‘suspect product’ by the reporter to FAERS or WHO.
A second approach is used to evaluate potential DDIs as contributors to QT prolongation or TdP. Emperica Signal® calculates an Interaction Signal Score (INTSS) that is essentially a way of measuring the strength of a higher-order association beyond what would be expected from any of the component pairs of items of different types. INTSS is computed as follows:
where
-
EB05 is the conservative estimate score for the three-dimensional combination of AE and two drugs associated with the AE (i.e., QT or TdP).
-
EB95MAX is the highest EB95 score found for the component pairs of items of different types.
An INTSS >1.00 indicates a stronger association than that for the component pairs of items.
Figure 2 demonstrates the type of data display generated by the Empirica analysis. The drug in this case is methadone, a drug whose risk of TdP was identified because of cases reported to AZCERT [33], and was added to the list of drugs with known risk of TdP in 2002.
Comparative signals for five adverse events of interest associated with methadone. Bar lengths represent observed EBGM magnitudes; error bars represent 90% CrI; color shades represent magnitude categories for EB05. Data shown are as of 2016 Quarter 2 and the graph was generated by Oracle’s Empirica Signal® program. An EB05 ≥2.0 for an adverse event is considered a relevant signal. CrI credible interval, EBGM Empirical Bayesian Geometric Mean, EB 05 lower 5% CrI for the EBGM, EB 05 -EB 95 90% CrI for the EBGM, N number of reports to FAERS in which methadone was designated as the suspected drug
2.4 Risk Categories for Drugs: The QTdrugs Lists
Sixteen different types of evidence from the sources described in Sect. 2.3 are analyzed and, when specific risk criteria are met, the drugs are placed into one of the following three categories as shown in Fig. 3:
Three categories of risk and the type of evidence that is required for drugs that prolong the QT interval or cause TdP. FAERS/WHO FDA Adverse Event Data System or World Health Organization’s Vigibase of adverse event reports, Med. Lit. published medical literature, TdP torsades de pointes, TQT study Thorough QT/QTc study
Known risk of TdP (KR) Substantial evidence supports the conclusion that these drugs can prolong the QT interval and are associated with TdP when used as directed in labeling.
Possible risk of TdP (PR) Substantial evidence supports the conclusion that these drugs can prolong the QT but there is insufficient evidence at this time that the drugs, when used as directed in labeling, are associated with TdP.
Conditional risk of TdP (CR) Substantial evidence supports the conclusion that these drugs are associated with TdP BUT only under conditions or circumstances of their use (e.g., excessive dose, or when given to patients with conditions such as hypokalemia, hypomagnesemia, or when taken with interacting drugs) OR because the drug has shown the ability to create one or more conditions that facilitates induction of TdP (e.g., by inhibiting metabolism of QT-prolonging drugs or by causing an electrolyte disturbance that induces TdP).
The ADECA process has rigid requirements for the types of evidence that must be positive in order for a drug to be placed in any one of the three categories. These requirements are indicated by the checked boxes in Fig. 3 and are described in greater detail in Sect. 2.5.
Lists of the drugs that have been placed in these three categories are posted on the CredibleMeds website and have become generally known as the QTdrugs Lists. The lists can be accessed using the URLs http://www.QTdrugs.org, http://www.torsades.org, or http://www.CredibleMeds.org.
In addition to the lists of drugs placed in these three categories, a fourth list is maintained and includes all drugs that should be avoided (if at all possible) by patients with congenital long QT syndrome (CLQTS). This list is created by combining the three lists of QT-prolonging drugs (KR, PR, and CR) and adding any drugs that possess another pharmacologic action that could facilitate TdP in these high-risk patients. These additional drugs include central nervous system stimulants and adrenergic stimulants that are known to increase TdP risk in CLQTS patients, even though they may not prolong the QT interval per se. Commonly known as the Drugs to Avoid list, it is often recommended as a resource for patients with CLQTS and their families (http://www.sads.org/living-with-sads/drugs-to-avoid#.WDTO1LIrLb0).
2.5 Evidence Requirements for Risk Categories
Table 1 lists the sixteen types of evidence used by AZCERT and how, if positive, the evidence can fulfill requirements or can support the placement of a drug into one of the three primary risk categories as shown in Fig. 4. Each risk category has certain types of evidence that are required (R) to be positive but certain other types of positive evidence are considered only supportive (S). In addition, some types are considered to be equivalent and any one of these can be the ‘required alternative’ (RA) evidence. For example, in order for a drug to be placed in the KR category there must be positive evidence of TdP (with the caveat that the drug was used as directed in labeling). The evidence could be from either (1) cases reported in the published medical literature or (2) a positive signal for TdP in FAERS or Vigibase. Drugs in the TdP KR category and TdP PR category require positive evidence of QT prolongation from either (1) the published medical literature, (2) a thorough QT study, (3) the drug label, or (4) a positive signal for QT prolongation in FAERS or Vigibase. For the TdP CR category, there are also alternatives for the types of TdP evidence but the preponderance of the evidence must include the presence of confounding or interacting factors such as concomitant use with drugs that are metabolic inhibitors of QT-prolonging drugs, excessive dose, overdose, hypokalemia, hypomagnesemia, bradycardia, or a diagnosis of CLQTS.
The types of evidence that are either ‘Required’ or ‘Supportive’ for a drug to be placed in one of the three risk categories for QT prolongation and/or TdP. EB 05 lower 5% credible interval for the empirical bayesian geometric mean, FAERS/WHO FDA Adverse Event Data System or World Health Organization’s Vigibase of adverse event reports, I NaL late sodium current, KR known risk, Med. Lit. published medical literature, TdP torsades de pointes, TQT study Thorough QT/QTc study
2.6 Continuous ADECA Review
A systematic analysis of the available data and evidence is conducted continuously by AZCERT’s clinical scientists and trained staff to maintain the accuracy of the lists of drugs. Evidence summaries for drugs are reviewed by the AZCERT scientific review team at regularly scheduled meetings and when there is unanimous agreement that there is adequate evidence to support adding a drug to a category (or moving a drug from one category to another), the changes are recommended to the QTdrugs Advisory Board. If any of the 39 members of the Advisory Board request additional data or recommend reconsideration, the decision is reevaluated and can be presented to the entire Board for discussion and reconsideration. If the recommended changes are approved by the Advisory Board, the lists on the website are updated and an email notification is sent to the 86,000 registered users. In recent years, the lists have been revised approximately every 30–45 days. Figure 5 is a graph that demonstrates the growing number of drugs that have been added to the lists since their inception.
3 Free, Public Access to the QTdrugs Lists and Knowledge Base
The QTdrugs lists are available open source at http://www.QTdrugs.org, http://www.torsades.org, or http://www.crediblemeds.org and can be searched by brand or generic name, sorted according to drug class or therapeutic use, downloaded, and printed. Because the lists are updated frequently and use of outdated lists could potentially result in harm to patients, AZCERT requires users who wish to view or download the entire lists to register so that they can be notified by email whenever the lists have been changed. The lists and other content on the website are copyrighted to protect them from reproduction in ways that might endanger patients. Permission to reproduce the lists can be obtained with the stipulation to include specific statements regarding limitations to their use and a recommendation that users check the CredibleMeds website for the latest versions of the lists. Importantly, the CredibleMeds website includes a request for user feedback and advice for how the QTdrugs lists and the website can better support their needs and interests. Emails and questions sent to info@azcert.org are answered by members of the Scientific Review Committee.
The QTdrugs lists are also freely available as smart phone or iPAD applications and through an Application Program Interface that can support CDSSs based in electronic medical records.
4 Clinical and Research Applications and Limitations
Since its creation in 1999, the website has experienced steady growth in utilization by patients/public (35%), healthcare providers (59%), and research scientists (6%). Over 702,000 unique visitors accessed the site in 2016 and 56% were from one of 200 countries outside the US. Over 86,000 registered users download the QTdrugs lists regularly and the number of registrants has increased by ~750/week for the last 3 years. Each month, 10,000 visitors return and another 20,000 new visitors access the QTdrugs lists on the website.
While the majority of registered visitors use the lists of drugs for clinical purposes, the lists have also been found useful in clinical research and have been cited in numerous publications that examine the causes and epidemiology of TdP [34,35,36]. Consensus panels and multiple authorities have recommended the QTdrugs lists as important resources for patients with CLQTS and for assessing the cause of drug-induced LQTS [15, 18, 37]. Investigators at the Mayo Clinic and Indiana University have used the QTdrugs lists to develop and validate a ‘QT score’ that can quantify a patient’s risk of death [38] or excessive QT prolongation [39, 40]. These validated scores are based on the patient’s risk factors, such as biological sex, hypokalemia, hypomagnesemia, baseline QT interval, sepsis, other QT-prolonging medicines, bradycardia, cardiac diagnoses, and age. Using QT scores as part of a CDSS, Tisdale et al. [39] and Sorita et al. [40] significantly reduced the prescribing of several QT-prolonging drugs to patients who had a high risk for TdP.
A limitation of the site and the drug lists is the absence of a list of safe alternatives for those drugs that cause QT prolongation or TdP. However, this is not practical because TdP is a rare event and quantifying that risk would require targeted research for that specific purpose; research that is only rarely available. To address the need for alternatives to be considered, the website’s drug search function can return the following message: “Drug Not Classified: This drug has been reviewed by CredibleMeds but the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories. This is not an indication that this drug is free of a risk of QT prolongation or torsades de pointes since it may not have been adequately tested for these risks in patients”.
A second limitation of the lists is the placement of drugs into broad categories (KR, PR, etc.) and the lack of quantification of relative risk within each category. However, such quantification is not possible since it would require prospective, comparative studies which are not available.
5 Conclusions
Unlike most traditional drug safety resources which are compilations of all adverse events that have been associated with a given drug, AZCERT takes a different approach and focuses on a single adverse event, TdP, and places drugs in risk categories that represent different levels of certainty for causing that adverse event. The ADECA process for analysis of evidence and the open-source display of the resulting knowledge base could have broad application in programs to prevent other serious, potentially preventable adverse events. Scientific rigor, the absence of a commercial influence, and the readily available, open-source nature of the knowledge base are positive attributes for a source of biomedical information.
References
Woosley RL. Centers for education and research in therapeutics. Clin Pharmacol Ther. 1994;55(3):249–55.
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group [see comments]. JAMA. 1995;274(1):29–34.
Leape LL, Bates DW, Cullen DJ, Cooper J, Demonaco HJ, Gallivan T, et al. Systems analysis of adverse drug events. ADE Prevention Study Group [see comments]. JAMA. 1995;274(1):35–43.
Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group [see comments]. JAMA. 1997;277(4):307–11.
Bates DW. Frequency, consequences and prevention of adverse drug events. J Qual Clin Pract. 1999;19(1):13–7.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–90.
Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351(11):1089–96.
Woosley RL, Roden DM. Pharmacologic causes of arrhythmogenic actions of antiarrhythmic drugs. Am J Cardiol. 1987;59(Supp):19E–25E.
Honig PK, Woosley RL, Zamani K, Conner DP, Cantilena LRJ. Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant administration of erythromycin. Clin Pharmacol Ther. 1992;52(3):231–8.
Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269(12):1532–6.
Barbey JT, Anderson M, Ciprandi G, Frew AJ, Morad M, Priori SG, et al. Cardiovascular safety of second-generation antihistamines. Am J Rhinol. 1999;13(3):235–43.
Tran HT. Torsades de pointes induced by nonantiarrhythmic drugs [published erratum appears in Conn Med 1994 Aug; 58(8):494]. Conn Med. 1994;58(5):291–5.
Drici MD, Knollmann BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998;280(20):1774–6.
Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047–60.
Woosley RL. Discovering adverse reactions: why does it take so long? Clin Pharmacol Ther. 2004;76(4):287–9.
Shah RR. Drug-induced QT interval prolongation: regulatory perspectives and drug development. Ann Med. 2004;36(Suppl 1):47–52.
Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34(40):3109–16.
Danielsson B, Collin J, Jonasdottir BG, Borg N, Salmi P, Fastbom J. Antidepressants and antipsychotics classified with torsades de pointes arrhythmia risk and mortality in older adults—a Swedish nationwide study. Br J Clin Pharmacol. 2016;81(4):773–83.
Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300.
Perrio M, Voss S, Shakir SAW. Application of the Bradford-Hill criteria to assess the causality of cisapride-induced arrhythmia: a model for assessing causal association in pharmacovigilance. Drug Saf Int J Med Toxicol Drug Exp. 2007;30(4):333–46.
Shakir SAW, Layton D. Causal association in pharmacovigilance and pharmacoepidemiology: thoughts on the application of the Austin Bradford-Hill criteria. Drug Saf Int J Med Toxicol Drug Exp. 2002;25(6):467–71.
Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67(4):413–8.
Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285(10):1322–6.
Shuba YM, Degtiar VE, Osipenko VN, Naidenov VG, Woosley RL. Testosterone-mediated modulation of HERG blockade by proarrhythmic agents(1). Biochem Pharmacol. 2001;62(1):41–9.
Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther. 2002;303(2):688–94.
Curtis LH, Østbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al-Khatib SM, et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003;114(2):135–41.
Kornick CA, Kilborn MJ, Santiago-Palma J, Keefe DL, Katchman AN, Schulman G, et al. Electrocardiographic changes and life-threatening ventricular arrhythmias with intravenous methadone. Pain. 2003;105(3):499–506.
Katchman AN, Koerner J, Tosaka T, Woosley RL, Ebert SN. Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther. 2006;316(3):1098–106.
Szarfman A, Tonning JM, Doraiswamy PM. Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy. 2004;24(9):1099–104.
Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25(6):381–92.
Almenoff JS, DuMouchel W, Kindman LA, Yang X, Fram D. Disproportionality analysis using empirical Bayes data mining: a tool for the evaluation of drug interactions in the post-marketing setting. Pharmacoepidemiol Drug Saf. 2003;12(6):517–21.
Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14(11):747–53.
Straus SM, Bleumink GS, Dieleman JP, van der LJ, ’t Jong GW, Kingma JH, et al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004;164(12):1293–7.
van der Sijs H, Kowlesar R, Klootwijk AP, Nelwan SP, Vulto AG, van GT. Clinically relevant QTc prolongation due to overridden drug-drug interaction alerts: a retrospective cohort study. Br J Clin Pharmacol. 2009;67(3):347–54.
Poluzzi E, Raschi E, Koci A, Moretti U, Spina E, Behr ER, et al. Antipsychotics and torsadogenic risk: signals emerging from the US FDA adverse event reporting system database. Drug Saf. 2013;36(6):467–79.
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace. 2013;15(10):1389–406.
Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88(4):315–25.
Tisdale JE, Jaynes HA, Kingery JR, Overholser BR, Mourad NA, Trujillo TN, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014;7(3):381–90.
Sorita A, Bos JM, Morlan BW, Tarrell RF, Ackerman MJ, Caraballo PJ. Impact of clinical decision support preventing the use of QT-prolonging medications for patients at risk for torsade de pointes. J Am Med Inform Assoc. 2015;22(e1):e21–7. doi:10.1136/amiajnl-2014-002896 (Epub 2014 Oct 16).
Acknowledgements
The authors gratefully acknowledge the contributions of Kristin Black and Marius Petriu to the CredibleMeds.org website and the QTdrugs knowledge base.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work reported here has been supported by the US Food and Drug Administration’s Safe Use Initiative, HHSF223201400189C, and a Grant from the Bert W. Martin Foundation.
Conflict of interest
The authors, Raymond L. Woosley, Klaus Romero, Craig W. Heise, Tyler Gallo, Jared Tate, Raymond David Woosley, and Sophie Ward have no conflicts of interest that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
Woosley, R.L., Romero, K., Heise, C.W. et al. Adverse Drug Event Causality Analysis (ADECA): A Process for Evaluating Evidence and Assigning Drugs to Risk Categories for Sudden Death. Drug Saf 40, 465–474 (2017). https://doi.org/10.1007/s40264-017-0519-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-017-0519-0