Abstract
Introduction
Active surveillance pharmacovigilance is a systematic approach to medicine safety assessment and health systems strengthening, but has not been widely implemented in low- and middle-income countries. This study aimed to assess the cost effectiveness of a national active surveillance pharmacovigilance system for highly active antiretroviral therapy (HAART) compared with the existing spontaneous reporting system in Namibia.
Methods
A cost–utility analysis from a governmental perspective compared active surveillance pharmacovigilance to spontaneous reporting. Data from a sentinel site active surveillance program in Namibia from August 2012 to April 2013 was projected to all HIV-infected adults initiating HAART in Namibia. Costs (pharmacovigilance program, HAART, adverse event [AE] treatment), quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs, dollars/QALY) were evaluated. Analysis was completed for (i) cohort analysis: a single cohort beginning HAART in 1 year in Namibia followed over their remaining lifetime, and (ii) population analysis: patients continued to enter and leave care and treatment over 10 years.
Results
For the cohort analysis, totals were US$21,267,902 (2015 US dollars) and 116,224 QALYs for care and treatment under active surveillance pharmacovigilance versus US$15,257,381 and 116,122 QALYs for care and treatment under spontaneous reporting pharmacovigilance, resulting in an ICER of US$58,867/QALY for active surveillance compared with spontaneous reporting pharmacovigilance. The population analysis ICER was US$4989/QALY. Results were sensitive to quality of life associated with AEs.
Conclusion
Active surveillance pharmacovigilance was projected to be highly cost effective to improve treatment for HIV in Namibia. Active surveillance pharmacovigilance may be valuable to improve lives of HIV patients and more efficiently allocate health resources in Namibia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The estimated program start-up costs for nationwide active surveillance pharmacovigilance for highly active antiretroviral therapy (HAART) in Namibia was estimated to be US$97,200, while the recurring annual program costs were estimated to be US$263,400 compared with US$49,800 for spontaneous reporting pharmacovigilance. |
A single cohort of patients monitored through active surveillance pharmacovigilance compared with the existing spontaneous reporting pharmacovigilance system in Namibia was projected to cost an additional US$6,010,521 in total cost of care and accrue an additional 102 quality-adjusted life-years (QALYs) over their remaining lifetime. |
The entire affected HIV population in active surveillance pharmacovigilance compared with the existing spontaneous reporting pharmacovigilance system in 10 years was projected to cost an additional US$1,654,543 in total cost of care and accrue an additional 331 QALYs, and hence to be a highly cost-effective system to improve HIV treatment in Namibia. |
1 Introduction
Pharmacovigilance is the science and activities relating to the detection, evaluation, understanding, and prevention of adverse events (AEs) associated with medicine use [1]. Pharmacovigilance provides information in assessing the inevitable tradeoff between benefits and potential harm from medicine use. This is accomplished through the collection and analysis of information on AEs and communication to those that have the knowledge to interpret the information and act in order to minimize harm to patients [1]. Additional potential benefits of a pharmacovigilance system include improved understanding of the burden of and risk factors for iatrogenic illnesses, a greater understanding of population health, and improved healthcare provision and outcomes. Although some of these population-level benefits are difficult to quantify, it may be feasible to quantify benefits to individual health. For example, if an AE is detected early, a healthcare provider can alter treatment to minimize negative effects on a given patient’s quality of life, improve medication adherence, and/or slow disease progression [2].
Public health systems, including those in low- and middle-income countries (LMICs), traditionally use spontaneous reporting pharmacovigilance systems to identify and report AEs [3, 4]. In a spontaneous reporting system, healthcare providers, pharmaceutical companies and/or patients passively report a suspected AE to a public health or governmental organization via various mechanisms, including phone, internet, or postal systems. Spontaneous reporting systems, relatively inexpensive to run, are useful for signal generation, but lack a well defined population denominator and, therefore, lack the ability to calculate incidence. Spontaneous reporting systems also suffer from significant underreporting of AEs [5–7].
Active surveillance is a type of pharmacovigilance whereby active measures are taken to detect the presence or absence of adverse events on an ongoing basis within a defined group of people. It involves the ongoing systematic collection, analysis, and interpretation of data [3, 8–10]. In LMICs, healthcare systems without active surveillance tend not to have the capacity for high-quality AE detection [11]. Among other limitations, not enough trained providers are available to detect many AEs. An active surveillance system raises the index of suspicion of AEs and hence raises the potential rate of detection and the treatment of AEs. In addition to enhancing individual level safety, active surveillance methods allow for calculation of population-based rates of AEs [12]. Active surveillance pharmacovigilance can contribute local data by providing estimates of the incidence of AEs and risk factors for medicine-associated AEs, and may allow for more effective and efficient use of resources to reduce the burden of disease [10, 13]. Through active surveillance, potential medicine-associated safety problems and their risk factors can be identified. Active surveillance is particularly well suited for HIV medications, especially when patients are monitored at antiretroviral therapy (ART) clinics and assessed by healthcare providers on a routine basis.
Despite potential population health improvements and society’s willingness to pay for medicine regulation and to avoid AEs [14–18], active surveillance systems have yet to be implemented on a large scale in LMICs. Active surveillance systems can be complex and costly to implement. While a framework to assess cost effectiveness of pharmacovigilance has been proposed [19], data have yet to be presented on the health benefits and medical costs of an active pharmacovigilance program at a national level. National-level costs and benefits of active pharmacovigilance can be difficult to estimate since the benefits of a national program cut across multiple medicines, indications, and disease areas.
In the Republic of Namibia, an upper-middle-income country, the Systems for Improved Access to Pharmaceuticals and Services (SIAPS) program supports the implementation of initiatives to improve HIV and AIDS treatment outcomes through strategies that promote pharmacovigilance at the health facility and community levels [20]. The Therapeutics Information and Pharmacovigilance Centre (TIPC) is the designated national pharmacovigilance program responsible for the promotion of the rational and safer use of medicines in Namibia. TIPC is part of the Ministry of Health and Social Services (MoHSS), which also administers a network of ART clinics throughout the country.
In this study, we evaluated the projected health outcomes and costs of pharmacovigilance in Namibia using first-line highly active antiretroviral therapy (HAART) as a case study. We assessed the cost effectiveness of a national active surveillance pharmacovigilance system for HAART, as compared with the existing spontaneous AE reporting pharmacovigilance system.
2 Methods
From August 2012 to April 2013, the MoHSS, through the TIPC, initiated a prospective sentinel site active surveillance activity for first-line HAART at two MoHSS ART outpatient clinic sites located at the Windhoek Central Hospital and the Katutura Intermediate Hospital in Windhoek, Namibia, with technical support from the SIAPS program [21]. The study population for this analysis was based on patients included in the sentinel site active surveillance activity; that is, HIV-infected patients naïve to HAART who were newly placed on a first-line HAART regimen in two publicly financed ART clinics in Namibia. Briefly, patients were 41 % male and had an average age of 37 years (Table 1). As previously described, the mean CD4 count was 216 and 51 % had WHO stage 1 disease. The most common HAART regimen was tenofovir/lamivudine/nevirapine [21].
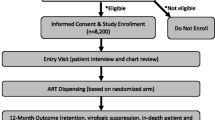
The costs of a pharmacovigilance system, including care and treatment delivered, were derived from the governmental perspective of MoHSS. This perspective includes costs to the government, including costs to the ART clinics for implementation and management of a pharmacovigilance system and care and treatment associated with managing patients with HIV (Fig. 1). We compared active surveillance with the existing spontaneous reporting system based on two methods: (i) a cohort analysis and (ii) a population analysis. First, we used a cohort analysis, in which a single cohort of all patients eligible to begin HAART in 1 year in Namibia entered care within the pharmacovigilance system and were followed over a lifetime horizon. Because health systems affect not just a single cohort, but the entire catchment population, we also used a second, population-based analysis. In this analysis, patients continued to enter (via beginning HAART) and leave (via mortality or loss-to-follow-up) care in the presence of the pharmacovigilance system each year as they became eligible for HAART over a 10-year time horizon. In both analyses, patients could be lost to follow up in any year of the model, and hence no longer included in the population. Both costs and health outcomes were discounted at 3 % per annum, as recommended [22].
2.1 Outcomes
The effectiveness of each pharmacovigilance system is dependent on the probability of detecting an AE in that system. In the active surveillance system, this data was acquired from the sentinel active surveillance activity, which has been described elsewhere [23]. In brief, a specially designed active surveillance data collection form was developed and placed into patient treatment charts, called the ART Patient Care Booklet. Adults naïve to ART were enrolled. Physicians recorded ART and health information during each follow-up visit, including presence or absence of AEs. In the spontaneous reporting system, healthcare providers are expected to report suspected AEs via fax or mail to the TIPC through the national spontaneous AE reporting system. To avoid the influence of active surveillance on spontaneous AE reports, data for the spontaneous reporting system was acquired from the year prior to the sentinel active surveillance activity.
The health outcomes of each pharmacovigilance system were evaluated in quality-adjusted life-years (QALYs), which combine morbidity and mortality. Underlying mortality was defined from life tables for treated HIV-infected patients in Namibia [24]. Patients with a detected or undetected AE had a higher probability of death (Table 2). Morbidity values for patients with and without AEs were defined from existing literature (Table 2) [18, 25–28]. The reduction in QALYs due to a severe AE when the regimen was switched was calculated using quality-of-life weights, AE durations, and AE probabilities of lipoatrophy, anemia, renal failure, neuropathy, lactic acidosis, myocardial infarction, and hepatotoxicity [29]. We assumed the QALY reduction when the regimen was not switched to be double that when the regimen was switched. We assumed the QALY reduction for a mild/moderate AE was half that of a severe AE. Values from sub-Saharan Africa were used when available.
Costs included two categories: program costs and medical care costs (Fig. 1). Program costs of an active surveillance system implementation and annually were acquired from the sentinel active surveillance activity and combined with population data to model countrywide estimates [30, 31]. For active surveillance, start-up costs were annuitized over the first 7 years of the program. Program costs of a spontaneous reporting system were acquired from TIPC (Table 2). The spontaneous reporting system did not have any start-up costs, as it is an existing program. This is a true marginal analysis of a health system in Namibia; therefore we aim to compare active surveillance to the current ‘standard of care’, in this case, the steady-state spontaneous reporting system that currently exists in Namibia, which does not have start-up costs. Medical-care costs, which included HAART and treatment of AEs, for both pharmacovigilance systems were calculated on a per-patient basis based on costs of HAART and inpatient and outpatient care for AEs acquired from TIPC (Table 2). All costs are shown in 2015 US dollars (US$).
2.2 Model
Cost effectiveness was defined in terms of cost compared with the quantity of the reductions in mortality and morbidity as a result of increased detection and treatment of AEs [32]. In an active surveillance program in an LMIC, we assume that more AEs will be detected, leading to an increase in number of patients switched to other medicines, which improve health but also increase costs for these patients. For active surveillance, we assumed that all detected cases were reported, as observed in the sentinel site activity. For spontaneous reporting, we used a hazard ratio comparing detected cases for spontaneous reporting with active surveillance. Because this data was not available for an HIV-infected population, we used a hazard ratio assumption based on methicillin-resistant Staphylococcus aureus (MRSA) control (Table 2). A percentage of these switches may be to more expensive second-line medicines, while the majority is to alternative first-line medicines. Depending on the pharmacovigilance system and healthcare provider, detection of an AE may or may not have an influence on treatment. In this model, we assumed that AE detection is by the healthcare provider and therefore its detection can influence treatment.
We adapted a previously described decision-analytic framework to a cost–utility model of costs and outcomes of pharmacovigilance at the national level (Fig. 2) [19]. We made several simplifying assumptions in the model framework and inputs. We assumed that all AEs can be categorized as either severe or mild/moderate, probability of detection of an AE is independent of severity of the AE, and among mild/moderate AEs no death or hospitalizations occur due to the event. We also assumed that if the AE is not detected there would be no healthcare utilization related to the event. We assumed that AEs occur at the beginning of the first year of treatment and, if treated, resolve within 1 year. We assumed that inpatient and outpatient visits do not affect quality of life (i.e., quality of life is only affected by AE severity and switching HAART regimens). Finally, we assumed that the probability of outpatient visits is independent of switching HAART regimens or severity of the AE, probability of death is independent of inpatient or outpatient visits, and probability of death without switching HAART regimen is double the probability of death with HAART regimen switch. Assumptions were evaluated in sensitivity analyses.
Decision analytic framework used to model costs and outcomes comparing active surveillance pharmacovigilance and spontaneous reporting pharmacovigilance at the national level in Namibia. Under each branch is listed the probability (specified by p=). Additionally, any branch with a corresponding decrease in quality of life (specified by u=) or cost (specified by c=) is defined. Those probabilities that differ by pharmacovigilance system are specified by AS for active surveillance and SR for spontaneous reporting. Each branch has an underlying utility value for quality of life, as well as a cost of first- or second-line medication, as defined in Table 2, which is not listed in the figure. AE adverse event, HAART highly active antiretroviral therapy, PV pharmacovigilance
2.3 Analytical Methods
A probability was calculated for the average patient to be in each of the final health states (shown as the white triangles in Fig. 2). Each health state was assigned a value for QALYs and cost. The probability of being in each final state was applied to the annual incident HAART cohort of 8910 patients [33] in the 213 existing public ART clinics (includes main ART sites, Integrated Management of Adulthood Illnesses sites, and outreach sites) [34] in Namibia to calculate population total costs and total QALYs associated with pharmacovigilance. The HIV incidence rate in Namibia has remained essentially constant since 2007, therefore we assumed a constant annual cohort size in the model [33].
We applied the per-patient results for medical costs and health outcomes to each of the two methods described above: (i) the lifetime cohort analysis, and (ii) the 10-year population analysis. We then calculated an incremental cost-effectiveness ratio (ICER) in which the numerator is the incremental program and medical costs for an active surveillance pharmacovigilance system compared with the existing spontaneous reporting pharmacovigilance system, and the denominator is the incremental QALYs gained due to active surveillance pharmacovigilance compared with spontaneous reporting pharmacovigilance. An intervention is considered cost effective if the ICER is less than three times the gross domestic product (GDP) per capita, and highly cost effective if the ICER is under the GDP per capita [35]. The current GDP per capita in Namibia is US$9736 purchasing power parity [30].
To assess uncertainty in the model, we conducted a one-way sensitivity analysis. For this analysis, we evaluated the ICER results when each input value was varied from a low to high range, and summarized the input variables that had the greatest effect on ICERs in a tornado diagram. We also conducted a probabilistic sensitivity analysis.
Data analysis was done using Stata version 10 and Microsoft Excel version 14. The study was approved by the Institutional Review Board at the University of Washington as well as the Ministry of Health and Social Services of Namibia as part of the duties of TIPC.
3 Results
3.1 Program Costs
The estimated national-level program start-up cost for a nationwide active surveillance pharmacovigilance program for HAART was determined to be US$97,200. There were no start-up costs for the existing spontaneous reporting pharmacovigilance program (see Sect. 2 for explanation). The estimated annual recurring program cost for active surveillance pharmacovigilance was US$263,400 versus US$49,800 for spontaneous reporting pharmacovigilance (Table 3).
3.2 Per-Patient Costs and Health Outcomes
In the year the patient entered the program, per-patient total medical costs (including HAART and treatment of AEs) incurred in an active surveillance pharmacovigilance system were US$130 versus US$123 within a spontaneous reporting pharmacovigilance system (Table 3). In an active surveillance system, more adverse events are assumed to be detected; therefore, more patients are switched to alternative drugs, which should provide better health outcomes. The mean expected QALYs per patient in the first year of active surveillance pharmacovigilance was 0.90 versus 0.89 in spontaneous reporting pharmacovigilance (Table 3).
3.3 Cohort Analysis
For the cohort analysis, the total costs included program costs for pharmacovigilance as well as HAART costs and costs for treatment of AEs for 8910 patients [34] who began HAART and therefore entered the pharmacovigilance system at the same time. Data for these patients were modeled for their remaining lifetime, over which time total costs for the cohort including pharmacovigilance program, HAART, and associated medical costs were US$21,267,902 for active surveillance pharmacovigilance versus US$15,257,381 for spontaneous reporting pharmacovigilance (Table 3). Over their remaining lifetime, this cohort accumulated 116,224 QALYs within an active surveillance pharmacovigilance system versus 116,122 QALYs within a spontaneous reporting pharmacovigilance system. This resulted in an ICER of US$58,867 per QALY for active surveillance pharmacovigilance compared with spontaneous reporting pharmacovigilance.
3.4 Population Analysis
For the population analysis, total costs included program costs for pharmacovigilance as well as HAART costs and costs for treatment of AEs for 89,100 patients (8910 entering the pharmacovigilance system each year for 10 years). These patients’ costs and outcomes were modeled over 10 years, over which time total costs including program, HAART, and associated medical costs were US$41,705,190 for care and treatment under an active surveillance pharmacovigilance system versus US$40,050,647 under a spontaneous reporting pharmacovigilance system (Table 3). Over 10 years, these patients accumulated 328,580 QALYs within an active surveillance system versus 328,249 QALYs within a spontaneous reporting system. This resulted in an ICER of US$4989 per QALY for active surveillance pharmacovigilance compared with spontaneous reporting pharmacovigilance. This is considered highly cost effective.
3.5 Sensitivity Analysis
The ICER for the cohort analysis was most sensitive to the number of patients in the cohort, the hazard ratio for detecting an AE in spontaneous reporting compared with active surveillance, the probability of detecting an AE in active surveillance, and the QALYs lost due to a severe AE (Fig. 3). The ICER for the population analysis was most sensitive to the probability that a regimen switch was to second line, the probability of hospitalizations, the number of new HAART patients annually, the hazard ratio for detecting an AE in spontaneous reporting compared with active surveillance, and the probability of detecting an AE in active surveillance (Fig. 3). Results were robust to probabilistic sensitivity analysis. Active surveillance pharmacovigilance was projected to be more likely to be cost effective than spontaneous reporting pharmacovigilance at a willingness-to-pay threshold of US$6500 per QALY in the population analysis (Fig. 4). Based on the current GDP per capita in Namibia [30], active surveillance had a 16 % probability of being cost effective in the cohort analysis, and a 65 % probability of being highly cost effective in the population analysis (Fig. 4). An exploratory Value of Information (VoI) analysis found that the expected value of perfect information would be US$938,444 using a willingness-to-pay threshold to three times GDP in Namibia.
Tornado diagram for sensitivity of the ICER comparing active surveillance with spontaneous reporting for a cohort of patients in a pharmacovigilance system in Namibia (top) and for the entire population over 10 years (bottom). All costs are shown in 2015 US dollars. AE adverse event, HAART highly active antiretroviral therapy, ICER incremental cost-effectiveness ratio, PV pharmacovigilance, QALYs quality-adjusted life-years
Cost-effectiveness acceptability curves for active surveillance pharmacovigilance versus spontaneous reporting pharmacovigilance at varying levels of willingness to pay for one QALY based on probabilistic sensitivity analysis for a single cohort over the remaining patient lifetime (top) and for the entire population over 10 years (bottom). All costs are shown in 2015 US dollars. GDP gross domestic product per capita (purchasing power parity), PV pharmacovigilance, QALYs quality-adjusted life-years
4 Discussion
The ICER for a single cohort of 8910 patients over their lifetime for active surveillance pharmacovigilance compared with the existing spontaneous reporting pharmacovigilance system was US$58,867 per QALY, which would not be considered cost effective based on the current GDP of US$9736 per capita in Namibia. However, for the entire population of 89,100 patients beginning HAART in Namibia over 10 years, the ICER was only US$4989 per QALY, which would be considered highly cost effective. Although the cost of active surveillance pharmacovigilance system was projected to be higher than that of spontaneous reporting, active surveillance would save more lives and improve patient quality of life over time for the population, resulting in a cost-effective program.
The primary purpose of pharmacovigilance is generally considered to be generation of additional knowledge on the effectiveness and safety of medicines in order to eventually improve the therapeutic and preventive approaches towards single diseases. Active surveillance pharmacovigilance may improve treatment guidelines and policies and ultimately health by generating information that leads to the use of medicines with a relatively lower incidence or severity of AEs [36]. Though this benefit is difficult to incorporate into a traditional economic evaluation such as the current model, it would likely be important for improving health. A future analysis might analyze the VoI for having higher quality data to influence treatment guidelines. The VoI represents the monetary value of decreasing uncertainty around treatment decisions. The VoI is dependent on the probability that the treatment decision based on existing information will be wrong (probability of error), the monetary consequences of a wrong decision, the useful lifetime (time horizon) for the new guidelines, and the size of the affected population. For example, though a more thorough model for VoI would need to be completed to fully address this issue, by implementing a VoI analysis into our population-based model, we found that the expected value of perfect information would be US$938,444 using a willingness-to-pay threshold to three times GDP in Namibia.
The population analysis results presented here represent a program analysis over a 10-year time horizon. We chose this approach in order to provide useful population-level information about potential program implementation in Namibia. Under the assumptions discussed above, we conclude that an active surveillance pharmacovigilance system would be projected to be highly cost effective in Namibia. This is one of the first studies of the cost effectiveness of an active surveillance pharmacovigilance system. The analytic methods used here may also be applied to other health systems or other settings to assess potential cost effectiveness of these health system improvements.
This study had several limitations. One limitation of our approach is the assumption that active surveillance leads to a higher rate of detection and treatment of AEs in LMIC. If this assumption is incorrect, the validity of the model is decreased. We utilized a hazard ratio for this assumption that was based on MRSA. The difference in these two disease states is a limitation of this assumption. A second limitation of our method is a lack of inclusion of morbidity and mortality outcomes that occur after 10 years in the population analysis. An additional limitation of this model is the lack of inclusion of other medicines, indications, and disease areas. The existing spontaneous reporting system in Namibia includes all medicines, though we have modeled the pharmacovigilance system only for HAART. In contrast, a national active surveillance program might only have the capacity to evaluate HAART.
The cost-effectiveness results presented here were sensitive to the input values used for quality-of-life reductions associated with an AE. Unfortunately, a paucity of high-quality data on quality of life exists in this area. This is an important limitation of our results. If incorrect disutility values were used, it could change the estimated cost effectiveness of active surveillance. Although we performed a wide range of sensitivity analyses around these values, it will be important for future work to address this area. Additionally, the data inputs for detection of AEs were limited by the relatively small sample size of the active surveillance sentinel site activity.
Additional benefits of active surveillance pharmacovigilance could lead to identification of incidence rates of and risk factors for adverse events, which improves knowledge of medicine safety. Additionally, data from active surveillance pharmacovigilance could be used to guide high-quality studies of risks associated with HAART and other treatments in Namibia. Finally, an active surveillance program is a health system—the implementation of which could strengthen the existing network of health systems. Currently with the UNAIDS 90-90-90 Strategy, there is a push towards ‘test and treat’ for many HIV/AIDS programs in Africa as a pathway towards epidemic control [37]. As ‘test and treat’ policies are implemented, a larger population will be treated with HAART and therefore also eligible for pharmacovigilance programs. Based on the results presented here, the larger the population affected by active surveillance, the more cost effective it is projected to be. Additionally, with a larger population on treatment, high-quality data on AEs and risk factors will be more necessary than ever to maximize population health. Based on our results, we recommend implementing or scaling up active surveillance programs wherever feasible as HAART is scaled up.
At a population level, active surveillance pharmacovigilance was projected to be a highly cost-effective system to improve safety of HIV treatment in Namibia compared with the existing spontaneous reporting system. The health outcomes and costs of an active surveillance system in Namibia could be used to assess feasibility of active surveillance in other settings, as well as provide information on the potential cost effectiveness of improving health systems. Knowledge of costs and benefits of a health program prior to implementation is of particular importance when resources are limited. Using the results presented here, along with the motivation and government support to improve health systems in Namibia, it may be feasible to improve medicine safety for HIV patients and more efficiently allocate resources for pharmacovigilance in Namibia.
5 Conclusions
Though not projected to be cost effective for a single cohort of patients, at a population level, active surveillance for first-line HAART in Namibia was projected to be highly cost effective compared with the existing spontaneous reporting system. Future work should addresses identified weaknesses in the methods presented here, including quantifying the probability of detecting AEs using different pharmacovigilance systems, and measuring quality of life associated with AEs in Namibia. Future studies in other settings could utilize the methods presented here to assess cost effectiveness of pharmacovigilance, or compare cost effectiveness of other health system-strengthening interventions.
References
World Health Organization WHO. The Importance of Pharmacovigilance—Safety Monitoring of medicinal products. 2002 [Internet]. Essential Medicines and Health Products Information Portal A World Health Organization resource; 2002;3–44. Available from: http://apps.who.int/medicinedocs/en/d/Js4893e/.
Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care [Internet]. 2013;25(4):400–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22908886.
Bakare N, Edwards IR, Stergachis A, Pal S, Holmes CB, Lindquist M, et al. Global pharmacovigilance for antiretroviral drugs: overcoming contrasting priorities. PLoS Med [Internet]. 2011 [cited 2013 Oct 28];8(7):e1001054. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18070223.
Patel H, Parthasarathi G, Ramesh M. Pharmacovigilance of anti-neoplastic agents in a developing country—a report through a spontaneous reporting & continuous monitoring system. Value Health [Internet]. 2015 [cited 2016 Jan 5];18(7):A429–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26532417.
Ruud KW, Srinivas SC, Toverud E-L. Addressing gaps in pharmacovigilance practices in the antiretroviral therapy program in the Eastern Cape Province, South Africa. Res Social Adm Pharm [Internet]. 2010;6(4):345–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21111391.
Centres P. A Practical Handbook on the Pharmacovigilance of Antiretroviral medicines. 2009 [cited 2013 Oct 28];150. Available from: http://books.google.com/books?hl=en&lr=&id=GlTtJsXfb9QC&oi=fnd&pg=PR1&dq=hiv+pharmacovigilance&ots=PqWSKdKWT_&sig=1LEZbWu8qyePV045FOyLCW69LwE#v=onepage&q&f=true.
Holtz L, Cecilio L, Minowa E, Julian G. Pharmacovigilance in Oncology: Knowledge and Perception on Adverse Events Reporting in Brazil. Value Health [Internet]. 2015 [cited 2016 Jan 5];18(7):A815. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26534347.
Management Sciences for Health. Strengthening Pharmaceutical Systems [Internet]. 2007 [cited 2014 Mar 11]. Available from: https://depts.washington.edu/deptgh/globalmed/projects/strengthening-pharmaceutical-systems-sps/.
World Health Organisation. World Health Organization. Pharmacovigilance for antiretrovirals in resource-poor countries. Geneva: World Health Organization; 2007.
Nsubuga P, White ME, Thacker SB, Anderson MA, Blount SB, Broome CV, et al. Public health surveillance: a tool for targeting and monitoring interventions. Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al., editors. Dis control priorities dev ctries [Internet]. 2nd ed. Washington (DC): World Bank; 2006 [cited 2014 Jan 21];2:997–1018. Available from: http://www.ncbi.nlm.nih.gov/books/NBK11770/.
Kouadio KI, Clement P, Bolongei J, Tamba A, Gasasira AN, Warsame A, et al. Epidemiological and surveillance response to Ebola virus disease outbreak in Lofa county, Liberia (March–September, 2014); lessons learned. PLoS Curr [Internet]. 2015 Jan [cited 2016 Mar 14];7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4447624&tool=pmcentrez&rendertype=abstract.
Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes: Complementing spontaneous reporting systems. Drug Saf. 2013;36(2):75–81.
Miller V, Nwokike J, Stergachis A. Pharmacovigilance and global HIV/AIDS. Curr Opin HIV AIDS [Internet]. 2012;7(4):299–304. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22627711.
Eichler H-G, Bloechl-Daum B, Brasseur D, Breckenridge A, Leufkens H, Raine J, et al. The risks of risk aversion in drug regulation. Nat Rev Drug Discov [Internet]. 2013;12(12):907–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24232377.
Bouvy JC, Ebbers HC, Schellekens H, Koopmanschap MA. The cost-effectiveness of periodic safety update reports for biologicals in Europe. Clin Pharmacol Ther [Internet]. 2013;93(5):433–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23549148.
Bouvy JC, Koopmanschap MA, Shah RR, Schellekens H. The cost-effectiveness of drug regulation: the example of thorough QT/QTc studies. Clin Pharmacol Ther [Internet]. 2012;91(2):281–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22205197.
Bouvy JC, Koopmanschap MA, Schellekens H. Value for money of drug regulation. Expert Rev Pharmacoecon Outcomes Res [Internet]. 2012;12(3):247–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22812545.
Bouvy J, Weemers J, Schellekens H, Koopmanschap M. Willingness to pay for adverse drug event regulatory actions. Pharmacoeconomics [Internet]. 2011;29(11):963–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21910513.
Babigumira JB, Stergachis A, Choi HL, Dodoo A, Nwokike J, Garrison LP. A framework for assessing the economic value of pharmacovigilance in low- and middle-income countries. Drug Saf [Internet]. 2014;37(3):127–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24550105.
Namibia | SIAPS Program [Internet]. [cited 2015 Nov 30]. Available from: http://siapsprogram.org/wherewework/namibia/.
Mann M, Mengistu A, Gaeseb J, Sagwa E, Mazibuko G, Baeten JM, Babigumira JB, Garrison LP, Stergachis A. Sentinel site active surveillance of safety of first-line antiretroviral medicines in Namibia. Pharmacoepidemiol Drug Saf. 2016. doi:10.1002/pds.4022.
Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine [Internet]. J Ment Health Policy Econ. New York: Oxford University Press; 1996. p. 425. Available from: http://books.google.com/books?id=HWttErwnBHsC&pgis=1.
Mengistu A, Gaeseb J, Stergachis A, Mann M, Sagwa E, Mazibuko G. Technical Report of the Active Surveillance of Safety of First line Antiretroviral Medicines in Windhoek Central & Katutura Intermediate Hospital. [Internet]. Submitted to the US Agency for International Development by the Systems for Improved Access to Pharmaceuticals; 2014. Available from: http://siapsprogram.org/publication/sentinel-site-active-surveillance-of-the-safety-of-first-line-antiretroviral-medicines-in-windhoek-central-hospital-and-katutura-intermediate-hospital/.
IHME Viz Hub. Mortality Viz [Internet]. [cited 2016 Apr 11]. Available from: http://vizhub.healthdata.org/mortality/.
Louwagie GM, Bachmann MO, Meyer K, Booysen F le R, Fairall LR, Heunis C. Highly active antiretroviral treatment and health related quality of life in South African adults with human immunodeficiency virus infection: a cross-sectional analytical study. BMC Public Health [Internet]. 2007;7:244–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17854510.
Pitt J, Myer L, Wood R. Quality of life and the impact of drug toxicities in a South African community-based antiretroviral programme. J Int AIDS Soc [Internet]. 2009;12:5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19393051.
Robberstad B, Olsen JA. The health related quality of life of people living with HIV/AIDS in sub-Saharan Africa— a literature review and focus group study. Cost Eff Resour Alloc [Internet]. 2010;8:5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20398367.
Wouters E, Heunis C, van Rensburg D, Meulemans H. Physical and emotional health outcomes after 12 months of public-sector antiretroviral treatment in the Free State Province of South Africa: a longitudinal study using structural equation modelling. BMC Public Health [Internet]. 2009;9:103–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19368714.
Bendavid E, Grant P, Talbot A, Owens DK, Zolopa A. Cost-effectiveness of antiretroviral regimens in the World Health Organization’s treatment guidelines: a South African analysis. AIDS [Internet]. 2011;25(July 2010):211–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21124202.
World Bank. Namibia | Data [Internet]. [cited 2015 May 16]. Available from: http://data.worldbank.org/country/namibia.
UNAIDS. Namibia [Internet]. [cited 2013 Oct 28]. Available from: http://www.unaids.org/en/regionscountries/countries/namibia/.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–Explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Guidelines Task Force. Value Heal. 2013;16:231–50.
Republic of Namibia Ministry of Health and Social Services. The Namibia AIDS Response Progress Report 2015 [Internet]. [cited 2016 Apr 8]. Available from: http://www.unaids.org/sites/default/files/country/documents/NAM_narrative_report_2015.pdf.
Republic of Namibia Ministry of Health and Social Services. GLOBAL AIDS RESPONSE PROGRESS REPORTING 2013 Monitoring the 2011 Political Declaration on HIV/AIDS [Internet]. Directorate of Special Programmes Division Expanded National HIV/AIDS Coordination Subdivision: Response Monitoring and Evaluation. [cited 2015 Dec 8]. Available from: http://www.unaids.org/sites/default/files/country/documents/NAM_narrative_report_2014.pdf.
WHO. WHO CHOICE: cost effectiveness thresholds [Internet]. [cited 2015 May 28]. Available from: http://www.who.int/choice/costs/CER_thresholds/en/.
Services M of H and S. Namibia Standard Treatment Guidelines. First Edition, 2011 [Internet]. 2011. Available from: http://apps.who.int/medicinedocs/documents/s19260en/s19260en.pdf.
UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014;40. http://www.unaids.org/en/resources/documents/2014/90-90-90.
Masenyetse LJ, Manda SO, Mwambi HG. An assessment of adverse drug reactions among HIV positive patients receiving antiretroviral treatment in South Africa. AIDS Res Ther [Internet]. 2015;12:6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25745501.
Rosen S, Long L, Fox M, Sanne I. Cost and cost-effectiveness of switching from stavudine to tenofovir in first-line antiretroviral regimens in South Africa. J Acquir Immune Defic Syndr [Internet]. 2008;48(3):334–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18545151.
Anwikar SR, Bandekar MS, Smrati B, Pazare AP, Tatke PA, Kshirsagar NA. HAART induced adverse drug reactions: A retrospective analysis at a tertiary referral health care center in India. Int J Risk Saf Med [Internet]. 2011;23(3):163–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22020396.
Marshall C, Richards M, McBryde E. Do active surveillance and contact precautions reduce MRSA acquisition? A prospective interrupted time series. PLoS One [Internet]. Public Library of Science; 2013 [cited 2016 Apr 11];8(3):e58112. doi:10.1371/journal.pone.0058112.
Republic of Namibia Ministry of Health and Social Services. Namibia Standard Treatment Guidelines [Internet]. 2011. Available from: http://apps.who.int/medicinedocs/documents/s19260en/s19260en.pdf.
Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review. Trop Med Int Health [Internet]. 2010 [cited 2016 Apr 1];15 Suppl 1:1–15. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2948795&tool=pmcentrez&rendertype=abstract.
Disease-specific Toolkits. Pharmacovigilance Toolkit. [cited 2014 Feb 23];(January 2012):1–117. Available from: http://www.pvtoolkit.org.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This report was made possible by the generous support of the American people through the US Agency for International Development (USAID), under the terms of cooperative Agreement Number AID-OAA-A-11-00021. The contents are the responsibility of Management Sciences for Health and do not necessarily reflect the views of USAID or the United States Government.
Conflict of interest
Marita Mann, Assegid Mengistu, Johannes Gaeseb, Evans Sagwa, Greatjoy Mazibuko, Joseph B. Babigumira, Louis P. Garrison, Jr., and Andy Stergachis have no conflicts of interest that are directly relevant to the content of this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Mann, M., Mengistu, A., Gaeseb, J. et al. Active Surveillance versus Spontaneous Reporting for First-Line Antiretroviral Medicines in Namibia: A Cost–Utility Analysis. Drug Saf 39, 859–872 (2016). https://doi.org/10.1007/s40264-016-0432-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0432-y