Abstract
The biologics have revolutionized the treatment of rheumatoid arthritis (RA). However, there are still patients that are difficult to control and a cure is still not achievable. Tofacitinib, a Janus kinase (JAK) inhibitor is an orally available, new-in-class, disease-modifying anti-rheumatic drug with similar efficacy to biologics. JAK is activated by multiple cytokines involved in the pathology of RA, and affects non-immune and immune cells, mainly the lymphocytes. Besides its anti-rheumatic effect, the recent focus has been on adverse events. As with other biologics, serious infections have been observed especially with patients with lymphopenia, consistent with the mechanism of action. The major difference in adverse events from other disease-modifying anti-rheumatic drugs is the prominent increase in the occurrence of herpes zoster; it is increased worldwide, especially in Asia. There are other concerns such as malignancies and hyperlipidemia that may cause cardiovascular events that deserve further attention. The first JAK inhibitor for RA is demonstrating great benefit along with some risk, providing insights into the post-biologic era.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tofacitinib has the ability to regulate rheumatoid arthritis activity similar to biologics. |
Tofacitinib is associated with a prominently increased herpes zoster incidence with unknown cause. |
Tofacitinib is the leading drug in the post-biologic era, although its safety profile deserves close attention. |
1 Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by persistent synovitis of unknown etiology. Uncontrolled synovitis can cause cartilage and bone destruction, resulting in joint deformity. Not only the deformity of the joint but also joint swelling and pain lead to considerable limitations in daily living, which can also cause a socioeconomic burden. Great advances have been achieved in the treatment of RA with biologics targeting specific proinflammatory cytokines (e.g., tumor necrosis factor [TNF], interleukin-6) or immune cells (e.g., T cells, B cells). Irrespective of the biological target, approximately 30 % of the patients treated with biologics, in most cases with concomitant methotrexate (MTX), achieve remission with minimal limitations in their daily living. However, the rate of remission decreases after the patient does not respond to at least one TNF inhibitor and further failure with TNF inhibitors results in decreased treatment response [1]. However, only limited information is available and well-designed controlled studies are lacking for patients who do not respond to non-TNF inhibitors [2]. Accordingly, there is a large number of patients with uncontrolled synovitis even with these active treatments. Therefore, continuous development is conducted for biologics with a new mechanism of action such as a bispecific antibody that is able to target two cytokines instead of one. However, biologics require parenteral administration with a financial burden.
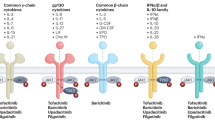
The most recent advance in the treatment of RA is the Janus kinase (JAK) inhibitors. JAK is a tyrosine kinase activated immediately after the cytokine binds to its original receptor and is known to play important roles in cytokine receptor binding-triggered signal transduction. Following the activation of JAK, transcription factor signal transducer and activator of transcription (STAT) is activated and translocates to the cell nuclei and regulates the transcription of the target genes in conjunction with other transcription factors. The JAK family consists of four members: JAK1, JAK2, JAK3, and Tyk2. Over 40 different cytokines and growth factors have been shown to activate specific combinations of JAKs and STATs, some are involved in the pathogenesis of RA while the same cytokine can be involved in the immune system of the host (e.g., interferon [IFN]-α, IFN-γ, interleukin [IL]-6). Among the JAKs, JAK3 is specifically expressed on hematopoietic cells and is crucial for the development, differentiation, and survival of lymphocytes, mainly T cells and natural killer (NK) cells. Deletion of JAK3 in mice and loss-of-function mutations in humans result in severe combined immunodeficiency [3–5]. Thus, a specific JAK3 inhibitor was considered an ideal immunosuppressant with minimal side effects depending on the limited expression of JAK3 (Table 1).
Tofacitinib is the first JAK inhibitor (Jakinib) applied to RA and is also the first to demonstrate similar efficacy to a TNF inhibitor as an orally available drug and has been approved in 40 countries for the treatment of patients with moderate-to-severe RA who did not respond to one or more disease-modifying antirheumatic drugs (DMARDs). It was first developed as a JAK3 inhibitor, and subsequently became apparent as a JAK1/JAK3 inhibitor. Depending on the mechanism of action, tofacitinib is now classified into a new category of DMARDs: targeted synthetic DMARDs [6]. The latest treatment guidelines for the treatment of RA including tofacitinib were developed by the American College of Rheumatology (ACR) and the European Union League Against Rheumatism [7, 8]. In the ACR guideline, tofacitinib is placed as a second-line DMARD following treatment with conventional synthetic DMARDs in established RA with moderate or high disease activity, a coequal status with TNF and non-TNF inhibitors. Regarding the European Union League Against Rheumatism guideline, tofacitinib may be considered after biological treatment has failed. Recent clinical trials with other JAK inhibitors, such as the JAK1/JAK2 inhibitor baricitinib, the JAK1/JAK3 inhibitor peficitinib, the JAK1 inhibitor filgotinib and ABT-494, and the JAK3 inhibitor decernotinib are demonstrating similar efficacy. In particular, the JAK1/JAK2 inhibitor baricitinib has demonstrated better efficacy compared with both MTX and adalimumab, the representative TNF inhibitor; the third drug to outperform the efficacy of MTX subsequent to tocilizumab (anti-IL-6 receptor antibody) and tofacitinib and the first-ever drug to outperform the efficacy of a TNF inhibitor for RA [9].
The specific nature of RA almost always leads to a complexity in treatment. Treating the ‘bad’ immune system is always associated with the suppression of the ‘good’ immune system. For instance, blocking the IFN pathway is efficacious for RA treatment while it can harm the anti-viral system in the host. On that note, in 2013, the European Medicines Agency did not approve tofacitinib based on safety concerns of an increased risk of serious infections, cancers, gastrointestinal perforation, and liver dysfunction. Furthermore, clinical studies were considered to be not sufficient to show a consistent reduction in disease activity and structural damage, particularly at the US Food and Drug Administration-approved dosage of 5 mg twice daily [10]. In addition, the approval by the US Food and Drug Administration includes warnings on serious infections and malignancies [11]. However, those warnings are somewhat similar to those described in the labeling for biologics. The clinical use of tofacitinib is expanding in USA; however, it seems to be limited in other countries, presumably owing to the new mechanism of action with the warnings described above and limited experience. This review focuses on both the benefit and risk of tofacitinib to take an overall view of JAK inhibitors in the treatment of RA.
2 Mechanism of Action of Tofacitinib
Depending on the selectivity of tofacitinib on JAK1 and JAK3 along with the well-known immunodeficient phenotype of JAK3 deficiency in both humans and mice has implicated that the major effect could be on lymphocytes. In human, mutations in the JAK3 gene result in the T-B+NK- phenotype and stem cell transplantation restores normal T-cell function [3, 12]. However, expression of JAK3 is observed in other immune cells such as monocytes and dendritic cells and JAK1 is broadly expressed in the entire body, suggesting that tofacitinib suppresses the immune system in a variety of ways [13, 14].
2.1 Effect on Lymphocytes
The prominent immunosuppressive effect of tofacitinib was initially proved by an animal model with graft vs. host disease [15]. Analysis of T cells in patients treated with tofacitinib revealed that the proliferative response of circulating CD4+ T cells to antigenic stimulation was significantly reduced in correlation with clinical parameters of RA activity [16]. RA synoviocytes have also been investigated and revealed that RA synovial fibroblasts and CD14+ monocytes were not directly affected, whereas IL-17 and IFN-γ production by CD4+ T cells were markedly decreased. In addition, supernatant from tofacitinib-treated CD4+ T cells significantly suppressed the production of IL-6 by RA synovial fibroblasts and IL-8 by CD14+ monocytes, suggesting an indirect action on myeloid cells [17]. Importantly, tofacitinib also reduced receptor activator of nuclear factor kappa ligand (RANKL) production, a crucial factor for osteoclastogenesis, in human CD4+ T cells [18].
In contrast to CD4+ T cells, the effect of tofacitinib on NK cells and CD8+ T cells is largely unknown. Investigation with non-human primates resulted in the reduction of peripheral blood NK cells and CD8+ T cells in a dose-dependent manner with tofacitinib. A retrospective survey of RA patients treated with tofacitinib revealed that a low number of CD8+ T cells before the initiation of tofacitinib was a risk factor for infection (mainly upper respiratory tract infection) [16].
B cells are also affected by JAK inhibition. JAK3 dysfunction in humans did not affect the number of B cells but attenuated its function [19, 20]. In an animal model, tofacitinib suppressed antibody responses to Pseudomonas exotoxin A, with reduced numbers of CD127+ pro-B cells and germinal center B cells [21]. Accordingly, treatment of healthy human B cells with tofacitinib resulted in suppressed B-cell activation, differentiation, and class switching while regulatory function was preserved [22].
2.2 Effect on Myeloid Cells
All JAKs are expressed and up-regulated by the stimulation of myeloid cells. An increased number of invasive dendritic cells (DCs) has been observed in the synovial tissues and an increase in the number of DCs expressing high levels of TLR4 ligands in synovial fluid has been reported [23, 24]. In vitro evaluation of human monocyte-derived DCs (MoDCs) treated with tofacitinib revealed reduced clustering and expression of CD80 and CD86 that was dependent on a type-I IFN-mediated mechanism [25]. Production of TNFα, IL-1β, and IL-6 from MoDC was induced by a lipopolysaccharide and was significantly suppressed by tofacitinib, and co-culture of tofacitinib-treated MoDCs with naïve CD4+ T cells resulted in reduced T-cell proliferation and IFN-γ production. In another study, tofacitinib suppressed the activation and expression of STAT1 and downstream inflammatory target genes in TNF-stimulated and RA synovial macrophages. Accordingly, an arthritis model dependent on macrophages, but not on lymphocytes was strongly suppressed with tofacitinib along with decreased joint destruction. These findings suggest that suppression of cytokine/chemokine production and innate immunity contribute to the therapeutic efficacy of tofacitinib [26].
Another important myeloid cell involved in the pathogenesis of RA is osteoclasts. Osteoclastogenesis is enhanced owing to the overproduction of proinflammatory cytokines and overexpression of RANKL constructing the bone destruction pathology. However, tofacitinib did not affect human osteoclast differentiation or function, but decreased human T-lymphocyte RANKL production, suggesting that tofacitinib suppresses osteoclastogenesis in an indirect manner [18]. Most recent investigations with TNF-α and/or IL-6-induced osteoclast-like cells demonstrated that the addition of tofacitinib suppressed osteoclast-like cell differentiation and bone resorption activity [27]. However, the differentiation and function of osteoclast-like cells induced with TNF-α alone have been shown to increase in the presence of tofacitinib [26].
2.3 Effect on Non-Immune Cells
Even though immune cells are the major contributor in the pathogenesis of RA, non-immune cells such as synoviocytes, particularly fibroblast-like synoviocytes have emerged for the relationship with the inflammatory pathway. Synovial biopsies were performed in patients treated with MTX before and after receiving tofacitinib 10 mg twice daily (BID; bis in die) or placebo for 4 weeks. Although there were no overall changes in the presence of immune cells (T cells, B cells, macrophages), expression of metalloproteinase and the IFN-regulated gene (e.g., CXCL10) were suppressed, and clinical improvement correlated with reductions of STAT1 and STAT3 activation, the transcription factors activated downstream of JAKs. These results suggest that IFN- and IL-6-mediated signaling are preferentially suppressed by tofacitinib in the synovium [28] (Table 1). In RA-fibroblast-like synoviocytes, IL-6 trans-signaling induced JAK2, STAT3 activation, and acute-phase serum amyloid A expression, which was abrogated with tofacitinib, also indicating the IL-6-mediated pathway as a major target for tofacitinib [29].
3 Anti-rheumatic Effect of Tofacitinib
MTX is currently the most effective and widely used first-line anti-rheumatic drug. Even though biologics are highly effective and have revolutionized the treatment of RA, most of them except for tocilizumab, an anti-IL-6 receptor antibody, have limited clinical efficacy compared with MTX as for monotherapy. Tofacitinib is the first JAK inhibitor approved for the treatment of RA and is widely recognized as a new class in DMARDs with a nomenclature of targeted synthetic DMARDs [6]. The efficacy and safety of tofacitinib have been demonstrated in phase II and III randomized controlled trials and long-term extension studies in patients with moderate-to-severe RA with insufficient response to MTX, DMARDs, and/or biologics, namely TNF-inhibitors [30–39]. Clinical efficacy was observed from the early phases of the treatment, starting from 1 to 2 weeks with sustained efficacy up to 48 months as follows; ACR20 response 70–80 %, ACR70 response 26–40 %, and disease activity score (DAS)28- erythrocyte sedimentation rate (DAS28(ESR)) <2.6 18–29 % [38]. It is noteworthy that tofacitinib demonstrated similar efficacy to adalimumab, a representative TNF inhibitor, in two randomized clinical trials with or without concomitant MTX. As already mentioned, the effectiveness of TNF inhibitors as monotherapy is limited. Therefore, the results of a clinical trial in patients receiving concomitant MTX demonstrating that tofacitinib has a similar DAS28(ESR) remission rate to adalimumab (5 mg BID; 6.2 %, 10 mg BID; 12.5 %, adalimumab; 6.7 %) with numerically higher ACR20 response rate with both tofacitinib 5 mg and 10 mg BID (5 mg BID; 51.5 %, 10 mg BID; 52.6 %, adalimumab; 47.2 %) was a new observation as an oral DMARD [36]. Another memorable effect with tofacitinib is that it was also effective in RA patients with insufficient response to biologics, mostly TNF inhibitors. Both monotherapy and concomitant DMARD therapy were effective in these patients [33, 40]. When active RA patients with an inadequate response to TNF inhibitors and concomitant MTX were treated with tofacitinib and MTX, the ACR20 response rates at 3 months were 5 mg BID; 55 %, 10 mg BID; 64 %, and placebo; 32 %, DAS28(ESR) remission (<2.6) rate were 5 mg BID; 6.7 %, 10 mg BID; 8.8 %, and placebo; 1.7 %. Boolean-based defined remission rates were 5 mg BID; 6.1 %, 10 mg BID; 4.5 %, and placebo; 0 % [33]. Clinical trials with tofacitinib monotherapy also revealed better efficacy compared with MTX monotherapy, a second DMARD exceeded efficacy compared with MTX [39–43]. When MTX-naïve active RA patients were treated with MTX or tofacitinib 5 or 10 mg BID, ACR 20 response rates at month 6 were MTX; 50.5 %, 5 mg; 71.3 %, 10 mg; 76.1 %, ACR70 response rates were MTX; 12.0 %, 5 mg; 25.5 %, 10 mg; 37.7 %, and DAS28(ESR) remission rates were MTX; 7.6 %, 5 mg; 14.6 %, 10 mg; 21.8 %, demonstrating significant efficacy of tofacitinib compared with MTX [42]. Efficacy on structural damage has been performed in three studies. The first study was the ORAL Scan study where patients with inadequate response to MTX were randomized to tofacitinib 5 mg BID, 10 mg BID, or placebo. At month 3, non-responder placebo-treated patients were randomized to tofacitinib 5 or 10 mg BID and the remaining placebo-treated patients advanced to randomization at month 6. Mean changes in total modified Sharp/van der Heijde score at months 6 and 12 were placebo; 0.47, 0.92, 5 mg BID; 0.12, 0.29, 10 mg BID; 0.06, 0.05, and statistical difference compared with placebo was observed only with the 10-mg BID treatment arm [37]. ORAL Start randomly assigned MTX-naïve active RA patients to receive tofacitinib 5 mg, 10 mg BID, or MTX. Mean changes of modified Sharp/van der Heijde score from baseline at month 6 were 5 mg; 0.2, 10 mg; <0.1, MTX; 0.8, at month 12 were 5 mg; 0.4, 10 mg; 0.2, MTX; 1.2, at month 24 were 5 mg; 0.6, 10 mg; 0.3, MTX; 2.1 [42]. The third study involved MTX-naïve active RA patients randomized to tofacitinib 10 mg BID + MTX, tofacitinib 10 mg BID monotherapy, or MTX monotherapy and the effect on structure was analyzed with magnetic resonance imaging. Bone marrow edema significantly improved and less deterioration of erosive damage was seen at months 6 and 12 [44]. These studies have demonstrated that the effect on structural damage is mainly observed with tofacitinib 10 mg BID that is mostly not approved and the effect of 5 mg BID was only observed in MTX-naïve patients. These treatment effects were sustained up to 7 years with acceptable safety [45]. These results provide us with some new treatment strategies; an additional treatment choice after not responding to MTX treatment, monotherapy in patients who cannot tolerate MTX, after an insufficient response to a single or multiple biologic. In reality, monotherapy seems to be conducted in a reasonably large proportion of cases [46]. However, tofacitinib has not yet been approved by the European Medicines Agency owing to the committee’s view that the benefits of treatment did not outweigh significant and unresolved concerns about safety [10].
4 Safety of Tofacitinib
The clinical efficacy of tofacitinib is generally accepted although the safety is still an open question. The most common treatment-emergent adverse events (AEs) were infections (nasopharyngitis, upper respiratory tract infection, bronchitis, urinary tract infection, and herpes zoster [HZ]) and gastrointestinal disorders (nausea, diarrhea) [45]. Owing to the mechanism of action, serious infection events (overall 2.93/100 patient-years [PYs]) and malignancies (excluding non-melanoma skin cancer [NMSC]) (overall 0.85/100 PYs) were numerically higher than placebo that were the most common causes of death during the clinical trials [47, 48]. The most common malignancy was lung cancer followed by breast cancer, lymphoma, and gastric cancer. However, gastric cancer was only observed in Japan, which has the highest gastric cancer rate worldwide [49]. NMSC that is reported to associate with RA, was higher in patients previously treated with TNF inhibitor compared to TNF inhibitor naive (1.01 vs. 0.47/100 PYs), patients aged ≥65 compared to <65 years (1.67 vs. 0.38/100 PYs), and patients of White ethnicity had the highest incidence rate compared to Asian, Black, or other ethnicity (0.86 vs. 0.03, 0.00, 0.14/100 PYs) [50, 51]. The incidence rate of malignancies was stable over time with increasing tofacitinib exposure and was within the expected range of patients with moderate-to-severe RA patients [45]. Serious infection events were the most common severe AEs and the most commonly observed were pneumonia, HZ, and urinary tract infection [48]. Opportunistic infections were observed in 32 patients; oesophageal candidiasis (n = 9), cytomegalovirus (n = 6), disseminated/multi-dermal HZ (n = 6), pneumocystis pneumonia (n = 4), Cryptococcus (n = 3), non-tuberculosis (Tb) mycobacterium (n = 2), toxoplasmosis (n = 1), and BK virus encephalitis (n = 1). Tb was reported in 26 patients (0.205/100 PYs, pulmonary [n = 15], extra-pulmonary/disseminated [n = 11]) varying by regional background incidence of Tb. In particular, HZ was increased (4.22/100 PYs) generally across the tofacitinib clinical trial. Serious HZ was reported in 35 patients, and multi-dermatomal HZ (n = 6), ophthalmic HZ (n = 6), and HZ oticus (n = 1). Although safety is generally acceptable, DMARDs as with other effective therapies for autoimmune diseases have some class or molecule-related effects.
4.1 Serious Infection
Serious infections have been reported causing death during the RA clinical trial. The incidence rate of serious infections across the tofacitinib clinical trial was 3.09/100 PYs (2.73–3.49). Although differences in patient backgrounds can affect the outcome, the incident rate was comparable to pre-existing biologics (adalimumab; 4.9–5.1/100 PYs [52], rituximab 3.9–4.3/100 PYs [53], tocilizumab 3.8–5.1/100 PYs [54], etanercept 3.8/100 PYs [55], abatacept 2.0–3.1/100 PYs [56], golimumab 5.09/100 PYs [57]) and stable over time. The risk factors were age, corticosteroid dose, diabetes mellitus, and tofacitinib dose [58]. There are limited data available directly comparing the rates of serious infections with tofacitinib relative to a biologic agent; adalimumab was employed as an active control agent in only two randomized controlled trials. A recent systematic literature search was conducted to investigate the risk of serious infections with tofacitinib compared with biologics. Estimated incidence rates (95 % confidence intervals [CIs]) for each biologics were abatacept 3.04/100 PYs (2.49, 3.72), rituximab 3.72/100 PYs (2.99, 4.62), tocilizumab 5.45/100 PYs (4.26, 6.96), and TNF inhibitors 4.90/100 PYs (4.41, 5.44), whereas with tofacitinib 5 mg BID was 3.02/100 PYs (2.25, 4.05) and 10 mg BID was 3.00/100 PYs (2.24, 4.02) in phase III trials and 2.50/100 PYs (2.05, 3.04) and 3.19/100 PYs (2.74, 3.72) in long-term extension studies. At least in these interventional studies, in which patients with risk factors for infections are excluded depending on the inclusion criteria, the risk of serious infections with tofacitinib is comparable to published rates for biologics in patients with moderate-to-severe active RA [59].
4.2 Herpes Zoster
Primary infection of varicella zoster virus (VZV) usually results in varicella (chickenpox) and thereafter becomes latent in neurons of ganglia. The natural decline of cell-mediated immunity with age causes VZV reactivation producing HZ. HZ occurs as a result of the reactivation of latent VZV and the incident is well known to increase with age depending on the decline of VZV-specific cell-mediated immunity [60]. The age-adjusted incidence of HZ is increasing with unknown reasons in most reported countries [61, 62]. HZ can occasionally cause postherpetic neuralgia and also substantial mortality among older and immunodeficient patients. While tofacitinib provided good evidence on anti-rheumatic activity and some possibility in new treatment strategies as a new class in DMARDs, it has also provided the other side of a double-edged sword. There was a clear increase of HZ in the tofacitinib-treated patients with differences in ethnicity [63]. This can cause further difficulties for a rheumatologist to decide which patients to treat with doubtful skin lesions and further handling of post-herpetic neuralgia. Therefore, it is time to review the risk factor and what is known in relation to HZ in RA patients.
A higher incidence of HZ in RA patients compared with the general population has been reported previously [64–67]. Various reports on cohorts and databases have been conducted. Table 2 summarizes the incidence rate and extracted risk factors from each report for HZ in RA patients treated with DMARDs, specifically enumerating the previously reported risk factors; age and use of corticosteroids and MTX. The first report that indicated the increase of HZ in RA patients was a small series of patients from a single center in USA [64]. Thereafter, reports from USA, UK, Australia, and Japan has been published with different incidence rates from 6.8 to 19.7/1000 PYs (by multiplying the original 1.97/100 PYs by ten [68]). Epidemiology studies of HZ in the general population has been conducted in several countries including USA, Canada, Europe, Asia, and Australia. The overall median HZ incidence rate was 4–4.5/1000 PYs. The incidence rate in the similar age group depicted in Table 2 were 2–4/1000 PYs in the 40 s, 4–5/1000 PYs in the 50 s, and 5–10/1000 PYs in the 60 s, indicating some increase in RA patients treated with DMARDs [60]. An increased risk of HZ in RA patients has been found to be associated with the use of corticosteroids and non-biologic DMARDs while exposure to MTX can differ in ethnicity [65, 69, 70]. The risk of HZ with TNF inhibitors has been demonstrated with conflicting results. Some studies have shown the difference in risk between monoclonal antibodies and a receptor-Fc fusion protein, while one report showed no clear difference among the biologics and also without any difference among non-biologic DMARDs, and another report demonstrated a clear increased risk with all TNF inhibitors without differences [71–73]. The most recent retrospective investigation reported the high risk of HZ in older RA patients treated with biologics [68]. The proportion of RA patients vaccinated for HZ prior to biologic agent initiation ranged from 0.4 % in 2007 to 4.1 % in 2011 [68]. The crude incidence rate of HZ was 1.97 per 100 PYs overall. Comparison of each agents resulted in no difference in the crude incidence rate nor the adjusted hazard ratio, suggesting that the risk of HZ was similar across biologic agents, including those with different modes of actions, namely TNF inhibitors and non-TNF inhibitors [68, 73, 74]. Of note, corticosteroid use had a significant association with HZ and patients who had the vaccination had a lower rate. Another recent RA cohort study was performed with patients participating in the Australian Rheumatology Association Database. Crude incidence in the entire RA cohort was 15.9/1000 PYs (95 % CI 13.5–18.8). An increased risk of HZ was found for all TNF inhibitors combined (hazard ratio 1.71; 95 % CI 1.00–2.92) compared with those who have not been exposed [75].
4.3 Adverse Events as a Protein Kinase Inhibitor
A systematic review has been performed and summarized on AEs in patients with RA treated with protein kinase inhibitors (PKis) to identify family and molecule-related AEs. The comparator included the placebo arm in clinical trials and studies on healthy individuals, RA patients, and patients with diseases other than RA with a similar comorbidity burden. Compounds targeting JAK, SYK, p38, and cKit families were selected. Tofacitinib had a significantly increased risk for hypercholesterolemia and other PKis were related to other AEs with a numerically higher risk. Serious infections and malignancies were not significantly more frequent in PKi-treated patients than in comparator groups [76]. It is intriguing to note that JAK inhibitors are the only PKi currently approved or on clinical trial among the PKis investigated in this report. In consideration of other JAK inhibitors currently on clinical trials and in the preclinical phase; the anti-rheumatic effect could be a class effect rather than a molecule-related effect.
The rate of infection and all-cause mortality across tofacitinib clinical trials have been reported. Tofacitinib was administered as monotherapy or in combination with MTX or other non-biologic DMARDs. Lymphopenia of <500/µL was rare but was associated with an increased risk of treated and/or serious infection. Overall, all-cause mortality rates were 0.30/100 PYs (0.20–0.44). The overall risk of infection and mortality in RA patients treated with tofacitinib appear to be similar to those observed in RA patients treated with biologic agents [58].
5 Importance of VZV-Specific Cell-Mediated Immunity
The incidence of HZ is increasing in the general population and is also known for its association with various medical conditions such as diabetes mellitus, human immunodeficiency virus infection, stroke, brain injury, cancer, use of statins, and autoimmune diseases. Patients with systemic lupus erythematosus and granulomatosis with polyangiitis (Wegener’s) have a 3- to 20-fold increased risk of HZ compared with the general population [77, 78]. Analysis of humoral immunity with measurement of serum IgG and IgM antibodies and cellular immunity with IFN-γ production and cell proliferation in response to VZV revealed that cellular immunity was specifically decreased compared with healthy controls in systemic lupus erythematosus patients but not in granulomatosis with polyangiitis GPA patients [79]. This indicates that a specific suppression of the cellular immunity could be associated with the incidence of HZ.
The importance of cell-mediated immunity was also observed with other medical conditions. For instance, patients with agammaglobulinemia cannot produce VZV-specific antibodies but are protected from reactivation due to cell-mediated immunity. Patients treated with immunosuppressants leading to suppression of cell-mediated immunity or patients with cell-mediated immunodeficiency disorders are known with increased and/or severe HZ and VZV, and VZV vaccine is protective for stem cell recipients depending on the induction of cell-mediated immunity but not anti-VZV antibodies. A prospective study on the relationship between VZV-specific cell-mediated immunity and the severity of HZ has been performed in Japan. A total of 12,522 people aged over 50 years were enrolled and cell-mediated immunity assessed by a skin test resulted in a significant inverse relationship to the severity of skin lesions, and acute and subacute pain. Conversely, weak response to the VZV skin test was associated with a high risk of post-herpetic neuralgia. The VZV-specific antibody titer was not associated with the severity of skin lesions and HZ-associated pain [80]. Therefore, measureable VZV antibodies are not protectable and establishing the T-cell-mediated specific immunity is important for protection from VZV reactivation. In fact, a prominent increase in the incidence rate of HZ has been observed in tofacitinib clinical trials, irrespective to concomitant DMARDs (Table 3).
6 Herpes Zoster in Patients Treated with Tofacitinib
HZ cases were collected from the tofacitinib clinical trials and incidence rates and potential risk factors for HZ were evaluated (Table 3) [45, 81–84]. The striking result was the prominent increase in incidence rate (2.12–8.6/100 PYs) compared with the previously reported incidence rate in the general population (4–4.5/1000 PYs) and in RA patients treated with DMARDs (6.8–19.7/1000 PYs, Table 2) [60]. Among 4789 patients, 239 were identified as having tofacitinib-associated HZ. The crude incidence rate was 4.4/100 PYs (95 % CI 3.8–4.9), with a substantial increase in Asia (7.7/100 PYs, 95 % CI 6.4–9.3) particularly in Japan and Korea (9.2/100 PYs, 95 % CI 7.5–11.4). Older age was associated with HZ (odds ratio 1.9, 95 % CI 1.5–2.6), but previously reported risk factors such as corticosteroids, MTX, DMARDs, disease duration, disease severity, diabetes mellitus, and smoking habit were not extracted. Importantly, the absolute lymphocyte count was also not a risk factor [63]. Among the clinical trials of tofacitinib, ORAL Start was a trial that initiated MTX or tofacitinib in MTX-naïve patients, a group of patients younger in age and presumably with less risk for HZ. HZ developed in 31 of 770 patients who received tofacitinib (4.0 %) and in 2 of 186 patients who received MTX (1.1 %). Even though the incidence rate is not available and it is premature to conclude, a somewhat similar incident proportion of HZ with other trials was observed. This suggests that tofacitinib could increase the risk of HZ in young RA patients even with monotherapy [42].
The clear increase in Asia, especially in Japan and Korea, led to the interest in ethnic differences of HZ. The incidence rate in the general population in Korea seems to be comparable to other Western countries [60]. Likewise the mean incidence rate in the general population in Japan is reported as 4.15/1000 PYs based on a large-scale survey conducted in Miyazaki Prefecture [85]. The incidence rate stratified by age was also comparable to what has been reported from other Western countries [60]. To clarify the risk and incidence in Japanese RA patients, a large observational cohort study has been performed based on patient self-reports. Among the 7986 patients (30,140 PYs), 366 cases of HZ were confirmed. The incidence rate was 9.1/1000 PYs overall, 7.8 in men, and 10.3 in women. The risk factors were age, high disease activity, corticosteroids, and MTX [69]. Hence, the incidence rate of HZ in Japanese RA patients was also comparable to that of other Western countries (Table 2). However, the analysis of Japanese patients participating in the tofacitinib clinical trial revealed that the incidence rate was near to twice as high as that from the global study (Table 3) [39, 41]. Study A3921041 was an open-label, long-term extension study of patients treated with monotherapy or with background MTX. Patients received tofacitinib 5 mg BID or tofacitinib 10 mg BID. A total of 486 patients were treated (1439.9 PYs of exposure) and HZ was diagnosed in 94 patients (19.3 %). The incidence rate for HZ was 7.4/100 PYs, which was apparently higher compared with the global population [39]. Currently, the reason for this difference in unclear. The pharmacokinetics of tofacitinib does not vary with ethnicity. It could involve genetic, cultural, or environmental differences or differences in immunization, viral strain, and importantly, the sensitivity and specificity of diagnosis, which seems to be a common problem in studies with HZ. The suppression of cell-mediated immunity by tofacitinib could be a reason for the increase in the overall patient population but does not explain the ethnic difference. Tofacitinib exhibits anti-rheumatic activity by suppressing the production of IL-6 and IL-6 signaling that could contribute to HZ occurrence. However, the rate of HZ with tocilizumab is 2.3/100 PYs, which is comparable to the usual RA population [86]. Therefore, a complex mechanism is involved in the occurrence of HZ and some unknown factors are involved.
7 Recurrence of Herpes Zoster
Usually, the therapeutic process of HZ is straightforward and the reactivation of the VZV-specific immunity would prevent recurrence of HZ. However, decisions on doubtful skin lesions and management of postherpetic neuralgia can be difficult. Another important issue is whether the occurrence of HZ suggests any excessive immunosuppression or further AEs during treatment with tofacitinib. In that regard, the recurrence of HZ could suggest overtreatment. There are only a few reports on the recurrence rate of HZ (Table 2). Recurrence of HZ has also been observed in the tofacitinib clinical trial. The most recent report investigated the recurrence rates among adults. Medical records of Olmsted County, Minnesota were reviewed. Of the 1669 persons with a medically documented episode of HZ, 95 had 105 recurrences (eight persons with more than one recurrence). The recurrence rate was 6.2 % and the time between HZ episodes in the same person varied from 96 days to 10 years. Importantly, recurrences were significantly more likely in immunocompromised individuals, women, and in those aged over 50 years [87]. In a recent report at a convention, HZ cases were identified from the tofacitinib clinical trial and evaluated if HZ could predict subsequent AEs. As a result, the incidence rate was stable over 90 months and HZ was no more likely to associated with the development of a subsequent AE or malignancy [81].
8 Herpes Zoster and Cancer
A particular issue of RA treatment could be whether HZ predicts further AEs in conjunction with immunosuppression. On that note, both HZ and cancer are associated with immunosuppression [88]. In fact, HZ occurs more often in patients with a cancer diagnosis. In a retrospective cohort study with a primary care database, the hazard ratio for cancer diagnosis after HZ was 2.42, highest in younger patients. Prior immunosuppression was not associated with risk, and the diagnosis of HZ did not affect survival [88]. However, there are studies that conclude that HZ is not a good indicator for underlying cancer [89]. The definition of immunosuppression can be also difficult in HZ cases. Psychological stress and/or long-term stress can result in premature aging of the immune system. Major depression is also reported with lower VZV-specific cellular immunity and recent HZ cases reported a significantly higher number of stressful life events within 6 months prior to a rash onset. These conditions are not rare in RA patients and in addition to these factors, RA itself is known to be associated with an increased cancer incidence. Therefore, current evidence suggests some risk of cancer after HZ but is inconclusive. The incidence rate of malignancies (excluding NMSC) in a tofacitinib clinical trial conducted in Japan was 1.29/100 PYs. Although it is numerically higher compared with the global study, the number of patients is still small with a large width of a 95 % CI (Table 4) [86, 90–96]. Long-term follow-up is necessary because there could be some lag time for the development of cancer.
9 Reactivation of the VZV-Specific Immunity
VZV-specific immunity can be boosted by subclinical reactivation of the virus or environmental exposure. Some investigations identified the relationship between contact with varicella and the risk of HZ. In one study, the association of the two diseases was not observed at a weekly level but an increase in varicella in children aged under 5 years significantly decreased HZ incidence in individuals aged 15–44 years [97]. In another study, people living with a child or increased contact with multiple children was associated with a reduced risk of HZ [98]. Occupational exposure to ill children (pediatrician, general practitioner) was also a protective factor. A study with pediatricians resulted in enhanced specific cellular immunity to VZV compared with the general population, which may be because of re-exposure to VZV from children with chickenpox. The incidence of HZ in their 50 s and 60 s was one-half to one-eighth of the general population. In fact, more than half of them lived without their children or grandchildren, suggesting the booster effect by re-exposure to VZV through their occupational environment [99]. Based on these reports, it is of interest whether environmental or occupational factors would affect HZ incidence in patients treated with tofacitinib.
Because a live attenuated vaccine (ZOSTAVAX®) is indicated for the prevention of HZ in individuals aged 50 years or older, immunization prior to tofacitinib treatment would be something to consider, although safety could be an issue. Live vaccine is contraindicated in individuals treated with biologics owing to the safety concern that the HZ vaccine may be associated with a short-term HZ risk. Clinical guidelines recommend using a live HZ vaccine to prevent shingles in RA patients, but there is only limited information. In reality, immunizing patients with autoimmune disease has been reported. A study with patients aged 50 years and older with RA, psoriasis, psoriatic arthritis, ankylosing spondylitis, and/or inflammatory bowel diseases collected data from administrative claims. Approximately 6 % of vaccinated patients were treated with TNF inhibitors at the time of vaccination and the incidence rates of HZ were similar regardless of immunization. Therefore, the short-term risk of HZ did not increase in vaccinated patients, even under immunosuppressive therapies such as biologics at the time of vaccination [100]. However, in this study, the use of concomitant corticosteroids or non-biologic DMARDs was mostly lower than 10 %. Another study analyzed the effect of tofacitinib on a humoral or cell-mediated live vaccine. RA patients aged ≥50 years with active RA despite MTX therapy were immunized with ZOSTAVAX® and randomized to either tofacitinib 5 mg BID (n = 55) or placebo (n = 57) with concomitant MTX. Humoral immunity was assessed by VZV-specific IgG and cell-mediated immunity was assessed by VZV-specific T-cell response. Both the VZV-specific IgG titer and the T-cell response increased in tofacitinib-treated patients and was comparable to patients with placebo. Of particular note, one patient that lacked immunity to VZV developed cutaneous dissemination with vaccine-strain VZV (Oka strain virus) 2 days following tofacitinib initiation (16 days post-vaccination) consistent with vaccine-induced disease. Therefore, a normal response to immunization can be expected in RA patients treated with tofacitinib and MTX; however, people who lack pre-existing VZV immunity should be avoided [101]. Recently, a new form of VZV vaccine has been developed. A recombinant subunit vaccine containing VZV glycoprotein E and the adjuvant system AS01B (HZ/su) has demonstrated a clinically acceptable safety profile and elicited a robust immune response for all age groups and was more immunogenic than a live attenuated VZV vaccine [102]. This vaccine may be an alternative especially for immunocompromised individuals because they can avoid the risk of vaccine-induced disease. Likewise HZ/su also demonstrated immunogenicity and was well tolerated in immunocompromised individuals who underwent hematopoietic stem-cell transplantation [103]. Therefore, ZOSTAVAX® may be used prior to tofacitinib except for those without VZV immunity, and the new VZV vaccine may provide better solutions in the future to avoid complications associated with HZ.
10 Other Viral Infection and Immunization
Owing to the increased rate of HZ, the status of other herpes group viruses is also of interest. Cytomegalovirus (CMV) reactivation occurs asymptomatically in most cases frequently with age, associated with increased circulating CMV-specific B cells, antibody titers, and CMV-specific CD8 T cells with a mature and differentiated phenotype. Along with these changes, alterations in other virus-specific T-cell immunity such as VZV, Epstein-Barr virus (EBV), and HSV have been shown. CMV seropositivity has been shown to have a negative effect on the number of EBV-specific CD8 T cells, while an increase in the HSV-1 antibody titer was observed with age. In regard to VZV, antibody titers positively associate with age presumably owing to reactivation and CMV infection further amplifies the positive association between age and reactivation [104]. These reports indicate that the herpes group virus reactivation can affect the immune status against one another with some phenomenon related to immunosenescence in older patients. Considering the age of patients we treat with DMARDs, immunosenescence could be an important factor. However, it is also known that RA patients have an impaired immune response to EBV [105] and reactivation of latent viruses with the involvement of immunosuppressive agents is more common. In addition, MTX treatment is reported to increase the risk of EBV reactivation and the development of lymphoproliferative disorders [106]. However, previous studies revealed that EBV load was not affected in RA patients treated with TNF inhibitors or juvenile idiopathic arthritis patients treated with tocilizumab, an anti-IL-6 receptor antibody [107, 108]. The most recent report performed a prospective study on EBV, CMV, and VZV loads in 20 RA patients initiating tocilizumab. Positivity in viral load (VL) were: EBV eight patients, CMV one patient, and none for VZV. Although half of the patients received concomitant MTX, treatment did not affect VL but rather turned CMV negative and EBV negative in six patients [109]. Therefore, inhibition of an inflammatory cytokine does not seem to directly affect the anti-herpes virus immunity. However, the JAK inhibitor tofacitinib exerts anti-rheumatic activity predominantly inhibiting the lymphocytes, which would make one imagine that tofacitinib would strongly affect the anti-herpes immunity. In another study with plaque psoriasis patients treated with tofacitinib followed with CMV and EBV VL, modest dose-dependent percentage increases from baseline in B-cell counts and percentage reductions from baseline in NK cell counts were observed. The proportion of patients with detectable CMV and EBV VL increased but there was no relationship with lymphocyte sub-populations or tofacitinib treatment [109]. Even though this was a study with patients without autoimmunity and who were not treated with MTX, it is of interest that there was a certain amount of patients that VL turned positive during this 12-week period.
The effect of tofacitinib on pneumococcal and influenza vaccine immunogenicity has been evaluated. RA patients treated with tofacitinib 10 mg BID or placebo with or without MTX were immunized. Similar proportions of patients developed influenza responses (tofacitinib; 56.9 %, placebo; 62.2 %), although fewer patients developed protective influenza titers in the tofacitinib-treated group, particularly with concomitant MTX compared with placebo (76.5 and 91.8 %). In the same study, a different treatment sequence was performed with a 2-week withdrawal of tofacitinib prior to immunization. Similar proportions of patients responded to both PPSV-23 and influenza, demonstrating that temporal discontinuation has a limited effect upon vaccine response. Therefore, immunization with PPSV-23 can be preferred prior to initiation of tofacitinib whereas influenza vaccine can be performed under medication [110].
11 Dyslipidemia
Systemic inflammation such as RA is well known to alter the lipid metabolism. Patients with active RA have reduced low-density lipoprotein (LDL)-cholesterol, altered composition of high-density lipoprotein (HDL)-cholesterol, with a reduced level of total cholesterol [111]. Soluble mediators such as inflammatory cytokines and chemokines cause the redistribution of fatty acids from the peripheral blood to tissues, establishing an atherogenic pattern of dyslipidemia. In consequence, RA patients are associated with significantly increased cardiovascular (CV) morbidity and mortality compared with the general population [111]. Reduction of inflammation with DMARDs is followed by significantly elevated cholesterol levels and an improvement in endothelial function and CV mortality. The IL-6 antagonist, tocilizumab, is known for its greater magnitude in increasing HDL, LDL-cholesterol, and total cholesterol levels [112]. However, the morbidity and mortality of CV events in RA patients treated with tocilizumab is similar to that of other biologics [113]. As mentioned previously, the anti-rheumatic of tofacitinib partly depends on the inhibition of IL-6 production and signaling. A dose-dependent increase in HDL, LDL-cholesterol, and total cholesterol was seen with the treatment of tofacitinib, a similar profile to tocilizumab [112]. Hence, the mechanism of dyslipidemia could be explained by the inhibition of IL-6. However, there is a difference in the mechanism of action (e.g., suppression of CD80/CD86 expression, TNF/chemokine production) and AEs (higher incidence of HZ) [17, 25, 28, 63]. Whether tofacitinib would increase the risk of CV events will require long-term observation, but there has not been any increase to date [114]. Interestingly, the assessment of cholesterol and lipoprotein kinetics in active RA patients and healthy individuals receiving tofacitinib was shown to alter the lipoprotein kinetics, improve HDL function, and increase LDL particle size [112]. Tofacitinib also converts fat-storing cells into metabolically active cells. [115]. Hence, tofacitinib-related dyslipidemia could be cardioprotective but deserves further attention.
12 Conclusion
Even with advances in the understanding of cytokine biology in connection with the pathology of RA, there is still a continuing burden of RA requiring new forms of drugs and treatment strategies. Inhibition of a single cytokine with biologics has proven that a proportion of patients can achieve a favorable outcome while a large proportion cannot. Hence, inhibiting more than one cytokine is an alternative treatment to be considered. Tofacitinib is the first targeted synthetic DMARD approved worldwide and is providing a new opportunity for patients with RA to achieve a similar outcome with biologics with an oral drug. At the same time, the new mechanism of anti-rheumatic action is providing thought-provoking AEs. We are now looking into a new era with a new class in DMARDs that requires knowledge not only on cytokine biology but also signaling that hopefully will bring patients closer to a cure.
References
Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nature Rev Rheumatol. 2015;11(5):276–89.
Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016. doi:10.1007/s10067-016-3227-8.
Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature. 1995;377(6544):65–8.
Nosaka T, van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, et al. Defective lymphoid development in mice lacking Jak3. Science. 1995;270(5237):800–2.
Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3(6):771–82.
Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewe R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis. 2014;73(1):3–5.
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Yamaoka K. Janus kinase inhibitors for rheumatoid arthritis. Curr Opin Chem Biol. 2016;16(32):29–33.
European Medicines Agency. Refusal of the marketing authorisation for Xeljanz (tofacitinib). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002542/WC500142485.pdf. Accessed 10 Apr 2016.
U.S. Food and Drug Administration. FDA news release: FDA approves Xeljanz for rheumatoid arthritis. Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm327152.htm. Accessed 10 Apr 2016.
Roberts JL, Lengi A, Brown SM, Chen M, Zhou YJ, O’Shea JJ, et al. Janus kinase 3 (JAK3) deficiency: clinical, immunologic, and molecular analyses of 10 patients and outcomes of stem cell transplantation. Blood. 2004;103(6):2009–18.
Musso T, Johnston JA, Linnekin D, Varesio L, Rowe TK, O’Shea JJ, et al. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J Exp Med. 1995;181(4):1425–31.
Yamaoka K, Min B, Zhou YJ, Paul WE, O’Shea JJ. Jak3 negatively regulates dendritic-cell cytokine production and survival. Blood. 2005;106(9):3227–33.
Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302(5646):875–8.
Sonomoto K, Yamaoka K, Kubo S, Hirata S, Fukuyo S, Maeshima K, et al. Effects of tofacitinib on lymphocytes in rheumatoid arthritis: relation to efficacy and infectious adverse events. Rheumatology. 2014;53(5):914–8.
Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-gamma and interleukin-17 production by human CD4+ T cells. Arthritis Rheumatol. 2012;64(6):1790–8.
LaBranche TP, Jesson MI, Radi ZA, Storer CE, Guzova JA, Bonar SL, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheumatol. 2012;64(11):3531–42.
Oakes SA, Candotti F, Johnston JA, Chen YQ, Ryan JJ, Taylor N, et al. Signaling via IL-2 and IL-4 in JAK3-deficient severe combined immunodeficiency lymphocytes: JAK3-dependent and independent pathways. Immunity. 1996;5(6):605–15.
Taylor N, Candotti F, Smith S, Oakes SA, Jahn T, Isakov J, et al. Interleukin-4 signaling in B lymphocytes from patients with X-linked severe combined immunodeficiency. J Biol Chem. 1997;272(11):7314–9.
Onda M, Ghoreschi K, Steward-Tharp S, Thomas C, O’Shea JJ, Pastan IH, et al. Tofacitinib suppresses antibody responses to protein therapeutics in murine hosts. J Immunol. 2014;193(1):48–55.
Wang SP, Iwata S, Nakayamada S, Sakata K, Yamaoka K, Tanaka Y. Tofacitinib, a JAK inhibitor, inhibits human B cell activation in vitro. Ann Rheum Dis. 2014;73(12):2213–5.
Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheumatol. 2005;52(8):2313–22.
Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171(11):5736–42.
Kubo S, Yamaoka K, Kondo M, Yamagata K, Zhao J, Iwata S, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73(12):2192–8.
Yarilina A, Xu K, Chan C, Ivashkiv LB. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheumatol. 2012;64(12):3856–66.
Yokota K, Sato K, Miyazaki T, Kitaura H, Kayama H, Miyoshi F, et al. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014;66(1):121–9.
Boyle DL, Soma K, Hodge J, Kavanaugh A, Mandel D, Mease P, et al. The JAK inhibitor tofacitinib suppresses synovial JAK1-STAT signalling in rheumatoid arthritis. Ann Rheum Dis. 2015;74(6):1311–6.
Migita K, Koga T, Komori A, Torigoshi T, Maeda Y, Izumi Y, et al. Influence of Janus kinase inhibition on interleukin 6-mediated induction of acute-phase serum amyloid A in rheumatoid synovium. J Rheumatol. 2011;38(11):2309–17.
Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheumatol. 2009;60(7):1895–905.
Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheumatol. 2012;64(3):617–29.
Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheumatol. 2012;64(4):970–81.
Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–60.
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159(4):253–61.
Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH. Tofacitinib Study I. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res. 2011;63(8):1150–8.
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–19.
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheumatol. 2013;65(3):559–70.
Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41(5):837–52.
Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18(1):34.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507.
Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25(4):514–21.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86.
Strand V, Kremer J, Wallenstein G, Kanik KS, Connell C, Gruben D, et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther. 2015;17:307.
Conaghan PG, Ostergaard M, Bowes MA, Wu C, Fuerst T, van der Heijde D, et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann Rheum Dis. 2016. doi:10.1136/annrheumdis-2015-208267.
Wollenhaupt J, Silverfield J, Lee E, Terry K, Kwok K, Lazariciu I, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and clinical and radiographic efficacy in open-label, long-term extension studies over 7 years. Arthritis Rheumatol. 2015. http://acrabstracts.org/abstract/tofacitinib-an-oral-janus-kinase-inhibitor-in-the-treatment-of-rheumatoid-arthritis-safety-and-clinical-and-radiographic-efficacy-in-open-label-long-term-extension-studies-over-7-years/. Accessed 11 Apr 2016.
Chastek B, Harnett J, Curtis J, Gerber R, Gruben D, Song R, et al. Real world evaluation of patients with rheumatoid arthritis initiating tofacitinib vs. adalimumab and etanercept. Arthritis Rheumatol. 2015. http://acrabstracts.org/abstract/real-world-evaluation-of-patients-with-rheumatoid-arthritis-initiating-tofacitinib-vs-adalimumab-and-etanercept/. Accessed 12 Apr 2016.
Curtis JR, Lee EB, Kaplan IV, Kwok K, Geier J, Benda B, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis. 2016;75(5):831–41.
Cohen S, Tanaka Y, Mariette X, Curtis J, Kwok K, Lee E, et al. Integrated safety analysis of tofacitinib in RA clinical trials with a cumulative exposure of 12,664 patient-years. Ann Rheum Dis. 2014;73(Suppl. 2):119–20.
Yamamoto S. Stomach cancer incidence in the world. Jpn J Clin Oncol. 2001;31(9):471.
Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32(11):2130–5.
Curtis J, Lee EB, Martin G, Mariette X, Terry K, Chen Y, et al. Analysis of non-melanoma skin cancer across the tofacitinib rheumatoid arthritis clinical programme. Ann Rheum Dis. 2015;74(Suppl. 2):257.
Schiff MH, Burmester GR, Kent JD, Pangan AL, Kupper H, Fitzpatrick SB, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(7):889–94.
van Vollenhoven RF, Emery P, Bingham CO 3rd, Keystone EC, Fleischmann RM, Furst DE, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72(9):1496–502.
Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13(5):R141.
Gottlieb AB, Gordon K, Giannini EH, Mease P, Li J, Chon Y, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol. 2011;10(3):289–300.
Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, et al. Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: open-label extension of the ATTEST Study. Ann Rheum Dis. 2011;70(11):2003–7.
Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Mack M, et al. Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann Rheum Dis. 2015;74(3):538–46.
Cohen S, Radominski SC, Gomez-Reino JJ, Wang L, Krishnaswami S, Wood SP, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(11):2924–37.
Strand V, Ahadieh S, French J, Geier J, Krishnaswami S, Menon S, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther. 2015;17(1):362.
Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–30.
Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic Proc. 2007;82(11):1341–9.
Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older, in the U.S., 2008. Am J Prev Med. 2011;40(2):e1–6.
Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(10):2675–84.
Antonelli MA, Moreland LW, Brick JE. Herpes zoster in patients with rheumatoid arthritis treated with weekly, low-dose methotrexate. Am J Med. 1991;90(3):295–8.
Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheumatol. 2007;57(8):1431–8.
Veetil BM, Myasoedova E, Matteson EL, Gabriel SE, Green AB, Crowson CS. Incidence and time trends of herpes zoster in rheumatoid arthritis: a population-based cohort study. Arthritis Care Res. 2013;65(6):854–61.
Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology. 2006;45(11):1370–5.
Yun H, Xie F, Delzell E, Chen L, Levitan EB, Lewis JD, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapy. Arthritis Care Res. 2015;67(5):731–6.
Nakajima A, Urano W, Inoue E, Taniguchi A, Momohara S, Yamanaka H. Incidence of herpes zoster in Japanese patients with rheumatoid arthritis from 2005 to 2010. Mod Rheumatol. 2015;4:1–4.
Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301(7):737–44.
Galloway JB, Mercer LK, Moseley A, Dixon WG, Ustianowski AP, Helbert M, et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2013;72(2):229–34.
Che H, Lukas C, Morel J, Combe B. Risk of herpes/herpes zoster during anti-tumor necrosis factor therapy in patients with rheumatoid arthritis: systematic review and meta-analysis. Jt Bone Spine. 2014;81(3):215–21.
Pappas DA, Hooper MM, Kremer JM, Reed G, Shan Y, Wenkert D, et al. Herpes zoster reactivation in patients with rheumatoid arthritis: analysis of disease characteristics and disease-modifying antirheumatic drugs. Arthritis Care Res. 2015;67(12):1671–8.
McDonald JR, Zeringue AL, Caplan L, Ranganathan P, Xian H, Burroughs TE, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48(10):1364–71.
Segan J, Staples MP, March L, Lassere M, Chakravarty EF, Buchbinder R. Risk factors for herpes zoster in rheumatoid arthritis patients: the role of tumour necrosis factor-alpha inhibitors. Intern Med J. 2015;45(3):310–8.
Salgado E, Maneiro JR, Carmona L, Gomez-Reino JJ. Safety profile of protein kinase inhibitors in rheumatoid arthritis: systematic review and meta-analysis. Ann Rheum Dis. 2014;73(5):871–82.
Bond D, Mooney J. A literature review regarding the management of varicella-zoster virus. Musculoskelet Care. 2010;8(2):118–22.
Chen HH, Chen YM, Chen TJ, Lan JL, Lin CH, Chen DY. Risk of herpes zoster in patients with systemic lupus erythematosus: a three-year follow-up study using a nationwide population-based cohort. Clinics (Sao Paulo). 2011;66(7):1177–82.
Rondaan C, de Haan A, Horst G, Hempel JC, van Leer C, Bos NA, et al. Altered cellular and humoral immunity to varicella-zoster virus in patients with autoimmune diseases. Arthritis Rheumatol. 2014;66(11):3122–8.
Asada H, Nagayama K, Okazaki A, Mori Y, Okuno Y, Takao Y, et al. An inverse correlation of VZV skin-test reaction, but not antibody, with severity of herpes zoster skin symptoms and zoster-associated pain. J Dermatol Sci. 2013;69(3):243–9.
Winthrop K, Tanaka Y, Yamaoka K, Curtis J, Nduaka C, Fan H, et al. Herpes Zoster during the Tofacitinib Clinical Development Program for RA: characterization of herpes zoster incidence and evaluation of whether herpes zoster predicts subsequent serious infections or malignancy. Arthritis Rheumatol. 2015. http://acrabstracts.org/abstract/herpes-zoster-during-the-tofacitinib-clinical-development-program-for-ra-characterization-of-herpes-zoster-incidence-and-evaluation-of-whether-herpes-zoster-predicts-subsequent-serious-infections-or/. Accessed 12 Apr 2016.
Fleischmann R, Yazici Y, Wollenhaupt J, Wang L, Maniccia A, Kwok K, et al. Clinical outcomes for rheumatoid arthritis patients receiving tofacitinib monotherapy in the open-label long-term extension over 6 Years. Arthritis Rheumatol. 2015. http://acrabstracts.org/abstract/clinical-outcomes-for-rheumatoid-arthritis-patients-receiving-tofacitinib-monotherapy-in-the-open-label-long-term-extension-over-6-years/. Accessed 12 Apr 2016.
Winthrop K, Curtis J, Lindsey S, Valdez H, Fan H, Wang L, et al. Herpes zoster and tofacitinib: the risk of concomitant nonbiologic therapy. Arthritis Rheumatol. 2015. http://acrabstracts.org/abstract/herpes-zoster-and-tofacitinib-the-risk-of-concomitant-nonbiologic-therapy/. Accessed 12 Apr 2016.
Charles-Schoeman C, Burmester G, Nash P, Zerbini CA, Soma K, Kwok K, et al. Efficacy and safety of tofacitinib following inadequate response to conventional synthetic or biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2015; doi:10.1136/annrheumdis-2014-207178.
Toyama N, Shiraki K, Society of the Miyazaki Prefecture D. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J Med Virol. 2009;81(12):2053–8.
Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20(3):222–32.
Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clinic Proc. 2011;86(2):88–93.
Cotton SJ, Belcher J, Rose P, Jagadeesan SK, Neal RD. The risk of a subsequent cancer diagnosis after herpes zoster infection: primary care database study. Br J Cancer. 2013;108(3):721–6.
Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Risk of cancer after herpes zoster: a population-based study. N Engl J Med. 1982;307(7):393–7.
Keystone EC, Genovese MC, Hall S, Miranda PC, Bae SC, Palmer W, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: results through 2 years of the GO-FORWARD study extension. J Rheumatol. 2013;40(7):1097–103.
Smolen JS, Kay J, Landewe RB, Matteson EL, Gaylis N, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann Rheum Dis. 2012;71(10):1671–9.
Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, de Longueville M, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis. 2015;74(1):96–103.
Simon TA, Smitten AL, Franklin J, Askling J, Lacaille D, Wolfe F, et al. Malignancies in the rheumatoid arthritis abatacept clinical development programme: an epidemiological assessment. Ann Rheum Dis. 2009;68(12):1819–26.
Weinblatt ME, Moreland LW, Westhovens R, Cohen RB, Kelly SM, Khan N, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol. 2013;40(6):787–97.
Takeuchi T, Miyasaka N, Kawai S, Sugiyama N, Yuasa H, Yamashita N, et al. Pharmacokinetics, efficacy and safety profiles of etanercept monotherapy in Japanese patients with rheumatoid arthritis: review of seven clinical trials. Mod Rheumatol. 2015;25(2):173–86.
Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheumatol. 2007;56(9):2886–95.
Garnett GP, Grenfell BT. The epidemiology of varicella-zoster virus infections: the influence of varicella on the prevalence of herpes zoster. Epidemiol Infect. 1992;108(3):513–28.
Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet. 2002;360(9334):678–82.
Terada K, Hiraga Y, Kawano S, Kataoka N. Incidence of herpes zoster in pediatricians and history of reexposure to varicella-zoster virus in patients with herpes zoster. Kansenshogaku zasshi. 1995;69(8):908–12.
Zhang J, Delzell E, Xie F, Baddley JW, Spettell C, McMahan RM, et al. The use, safety, and effectiveness of herpes zoster vaccination in individuals with inflammatory and autoimmune diseases: a longitudinal observational study. Arthritis Res Ther. 2011;13(5):R174.
Winthrop K, Wouters A, Choy E, Soma K, Hodge J, Nduaka C, et al. Assessment of immunogenicity of live zoster vaccination (Zostavax®) in rheumatoid arthritis patients on background methotrexate before and after tnitiating tofacitinib or placebo. Arthritis Rheumatol. 2015. http://acrabstracts.org/abstract/assessment-of-immunogenicity-of-live-zoster-vaccination-zostavax-in-rheumatoid-arthritis-patients-on-background-methotrexate-before-and-after-initiating-tofacitinib-or-placebo/. Accessed 12 Apr 2016.
Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96.
Stadtmauer EA, Sullivan KM, Marty FM, Dadwal SS, Papanicolaou GA, Shea TC, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood. 2014;124(19):2921–9.
Ogunjimi B, Hens N, Pebody R, Jansens H, Seale H, Quinlivan M, et al. Cytomegalovirus seropositivity is associated with herpes zoster. Hum Vaccin Immunother. 2015;11(6):1394–9.
Toussirot E, Roudier J. Epstein-Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol. 2008;22(5):883–96.
Yamakawa N, Fujimoto M, Kawabata D, Terao C, Nishikori M, Nakashima R, et al. A clinical, pathological, and genetic characterization of methotrexate-associated lymphoproliferative disorders. J Rheumatol. 2014;41(2):293–9.
Balandraud N, Guis S, Meynard JB, Auger I, Roudier J, Roudier C. Long-term treatment with methotrexate or tumor necrosis factor alpha inhibitors does not increase Epstein-Barr virus load in patients with rheumatoid arthritis. Arthritis Rheumatol. 2007;57(5):762–7.
Kawada J, Iwata N, Kitagawa Y, Kimura H, Ito Y. Prospective monitoring of Epstein-Barr virus and other herpesviruses in patients with juvenile idiopathic arthritis treated with methotrexate and tocilizumab. Mod Rheumatol. 2012;22(4):565–70.
Mourgues C, Henquell C, Tatar Z, Pereira B, Nourisson C, Tournadre A, et al. Monitoring of Epstein-Barr virus (EBV)/cytomegalovirus (CMV)/varicella-zoster virus (VZV) load in patients receiving tocilizumab for rheumatoid arthritis. Joint Bone Spine. 2015; doi:10.1016/j.jbspin.2015.07.009.
Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75(4):687–95.
Navarro-Millan I, Yang S, DuVall SL, Chen L, Baddley J, Cannon GW, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum Dis. 2016;75(2):341–7.
Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67(3):616–25.
Yamamoto K, Goto H, Hirao K, Nakajima A, Origasa H, Tanaka K, et al. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol. 2015;42(8):1368–75.
Souto A, Salgado E, Maneiro JR, Mera A, Carmona L, Gomez-Reino JJ. Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 2015;67(1):117–27.
Moisan A, Lee YK, Zhang JD, Hudak CS, Meyer CA, Prummer M, et al. White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nat Cell Biol. 2015;17(1):57–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Kunihiro Yamaoka has received consultant fees from Pfizer, Chugai Pharma, Mitsubishi-Tanabe Pharma, and Abbvie; has received honoraria from Pfizer, Chugai Pharma, Mitsubishi-Tanabe Pharma, Bristol-Myers Squibb, Takeda Industrial Pharma, GlaxoSmithkline, Nippon Shinyaku, Eli lilly, Janssen Pharma, Eisai Pharma, Astellas Pharma, and Actelion Pharmaceuticals; and has received research support from Chugai Pharma and Mitsubishi-Tanabe Pharma.
Rights and permissions
About this article
Cite this article
Yamaoka, K. Benefit and Risk of Tofacitinib in the Treatment of Rheumatoid Arthritis: A Focus on Herpes Zoster. Drug Saf 39, 823–840 (2016). https://doi.org/10.1007/s40264-016-0430-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0430-0