Abstract
Introduction
Assessing the significance of pharmacist interventions (PIs) is essential to demonstrate the added value of pharmacists. Methods and tools for assessing the potential significance of PIs are diverse and their properties are questionable.
Objectives
We aimed to systematically review the tools available to assess the potential significance of PIs.
Methods
We conducted a systematic search for English- or French-language publications from 1986 to 2013 in PubMed, PsycINFO, PASCAL, and CINAHL. Studies were screened by two independent reviewers based on inclusion/exclusion criteria and were abstracted for content, structure of tools, and validation process.
Results
Of 873 citations screened, 82 distinct tools were identified from 133 studies. While clinical aspects were often defined quite clearly, terminology regarding humanistic, economic, and process-related aspects of PIs was omitted, incomplete, or ambiguous in most tools. The probabilities of consequences of PIs/drug-related problems were evaluated in 20/82 tools. Few tools simultaneously measured economic, clinical, humanistic, and process-related variables. Structure of the tools varied from an implicit, mono-dimensional tool to an explicit, multi-dimensional algorithm. Validation processes were diverse in terms of quantification and number of raters, rating method, and psychometric parameters. Of 133 identified studies, there was limited evidence of validity (8/133, 6.0 %), inter-rater reliability (49/133, 36.8 %), and intra-rater reliability (2/133, 1.5 %).
Conclusions
The majority of tools focused primarily on assessing clinical aspects and failed to detect comprehensive impacts. The heterogeneity of tools and assessment processes hindered our ability to synthesize the results of evaluations. Limited results for their validity and reliability cast doubt on the credibility of this methodology for justification of the value of PIs. Recommendations for development of tools with optimal theoretical, pragmatic, and psychometric properties are proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The role of pharmacists should be to determine the value of pharmacist interventions (PIs) and to target those with the most value. |
The majority of tools for assessing the potential significance of PIs focused primarily on assessing clinical aspects and failed to detect other impacts. |
We propose optimal pragmatic, psychometric, and theoretical properties for the development of new tools assessing the potential significance of PIs. |
1 Introduction
Adverse drug events (ADEs) are a major problem relating to patient safety. They are associated with increased morbidity and mortality, prolonged hospitalizations, and higher costs of care [1, 2]. Nearly half of all ADEs are considered preventable [1]. Therefore, the detection, resolution, and prevention of actual or potential drug-related problems (DRPs) through pharmacist interventions (PIs) are considered key to reducing ADEs [1]. In this article, a DRP is commonly defined as “an event or circumstance involving drug treatment that actually or potentially interferes with the patient experiencing an optimum outcome of medical care” [2], and PIs are defined as “discrete activities by pharmacists related to patient care” [3].
Assessing the significance of a PI is now recognized as essential for demonstrating the added value of pharmacists to the healthcare system and justification for obtaining additional resources in clinical pharmacy practice. This assessment is also used as an indicator of a pharmacist’s performance and continuing quality improvement, research, and education [1].
Through studies in the literature, it is possible to classify approaches to assessing the significance of an individual PI into three main types: Approach 1—the evaluation of actual consequences of DRPs (e.g., actual severity of harm); Approach 2—the evaluation of actual consequences after performing a PI and follow-up with the patient (e.g., actual clinical outcomes); or Approach 3—the estimation of potential significance of a PI (Fig. 1). The term ‘actual’ is understood to mean the entity that has appeared in the patient, while the term ‘potential’ refers to a situation in which it is possible that the entity could appear in the patient [4].
According to ‘Approach 1’, the earlier the pharmacist intervenes to prevent harm to the patient, the more significant a PI is likely to be. In fact, harm as a result of DRPs in the patient is rare. For example, Vessal [5] found that about 90 % of the prescription errors resulted in no harm in patients because a great majority of errors were corrected early by pharmacists. Two limitations of this approach are that it offers little guidance to improve the quality of a PI in the future or to reflect the quality of the whole system of patient care rather than only the contribution of a PI [6].
According to ‘Approach 2’, the assessment of actual consequences, commonly clinical outcomes, in the patient after a PI and follow-up of the patient is the only valid indicator of the quality of a PI. It is helpful in the daily decision making of physicians and pharmacists [1]. However, assessment of an actual clinical outcome in patients is associated with some primary difficulties: criteria/technology of follow-up, timeframe, and determination of causal relationships between PIs and health outcomes [7–10].
According to ‘Approach 3’, the potential significance of PIs may be assessed via two sub-types: Approach 3A—prediction of the potential consequences of DRPs in the absence of a PI; and Approach 3B—prediction of the potential consequences of an implemented PI [11, 12]. The assessment of the potential significance of a PI is associated with metrological problems such as subjectivity, validity, and reliability of predictions. However, this method is frequently used as a means of commenting on the significance and quality of a PI because of its practicability when data are lacking for evaluation of actual consequences and its usefulness in guidance for improving the quality of a PI (e.g., hierarchy of potential significance of a PI and targeting the potentially most significant PIs). Therefore, for this review, we only synthesized tools for assessing the potential significance of PIs—Approach 3.
Methods and tools to assess the significance of PIs are diverse, and their pragmatic, psychometric, and theoretical properties are questionable. The only literature review of tools for rating PIs was reported in 1999 by Overhage and Lukes [12], who noted that only ten of 51 identified articles included an explicit description of the rating tool used. Thus, the authors developed a two-dimensional tool that could characterize a hospital pharmacist’s recommendations based on the severity of the DRP and the value of that intervention. A broad variation of this validated tool has been adopted for characterizing clinical activities in different settings. However, to our knowledge, no other up-to-date literature review has been conducted. Furthermore, since then, with increases in economic constraints, aging, burden of chronic disease, and patient lack of compliance, the quality assessment of PIs is shifting from solely clinical to include economic and humanistic impacts (e.g., patient quality of life, compliance, and satisfaction) [13]. Therefore, the purpose of this systematic review is to summarize the tools available for assessment of the potential significance of a PI and to propose the pragmatic, psychometric, and theoretical properties of ideal tools.
2 Methods
2.1 Research Strategy
We performed a systematic search of the databases MEDLINE (PubMed) (1986–February 2013), PASCAL (1997–February 2013), PsycINFO (1999–February 2013), and CINAHL with full-text (1993–February 2013) to collect studies using tools to assess the potential significance of an individual PI.
We combined two groups of keywords for the following search: drug-related problems AND pharmacist interventions (‘drug related problems’ OR ‘drug therapy problems’ OR ‘medication therapy problems’ OR ‘medication inappropriateness’ OR ‘pharmaceutical care issues’ OR ‘medicine related problems’ OR ‘medication related problems’ OR ‘medication errors’) AND (‘pharmaceutical care’ OR ‘pharmaceutical services’ OR ‘medication order review’ OR ‘medication review’ OR ‘pharmacotherapy interventions’ OR ‘pharmacy interventions’ OR ‘drug utilization review’ OR ‘pharmacist recommendations’ OR ‘pharmacist interventions’).
2.2 Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) original articles published in English or French; (2) abstract available; (3) published in peer-reviewed journals; (4) involved pharmacists alone or in cooperation with other healthcare professionals; and (5) included an explicit description of a method for rating the impacts of a PI, called ‘a tool’ in this review.
The exclusion criteria for articles included the following: (1) literature reviews; (2) studies related to one specific type of DRPs/PIs (e.g., administration errors, drug information service); (3) tools only assessing the actual consequences of DRPs [e.g., ADEs/adverse drug reactions (ADRs)]; (4) tools only assessing the actual consequences of a PI; (5) studies assessing economic impact only; and (6) non-accessible articles. In addition, reference lists of articles that met our inclusion criteria, of systematic reviews, and of review articles were assessed and, if relevant, were retrieved; 11 additional articles were also retrieved from a thesis by Quélennec [14], which performed a literature review of tools for evaluation of potential clinical impacts of medication errors (MEs) intercepted through medication conciliation. Finally, a hand-search was conducted to identify articles that had not been captured in the electronic database search.
2.3 Screening and Data Extraction
In February 2013, one author (THV) screened all titles, abstracts, and then full-text articles for the first time. Another author (CC) independently screened with the same strategies. Additional articles retrieved by the second reviewer were added to the final results. The second reviewer also verified the extraction of relevant data from articles included by the first reviewer. We resolved any disagreement through discussion until consensus was reached.
2.3.1 Content of Tools
To identify the indicators used in existing tools, theoretical models that are able to be applied to assess PIs were reviewed. The conceptual models “structure-process-outcome model” by Donabedian [15] suggested that the quality of healthcare interventions be assessed through three types of indicators related to “structural features” (appropriate resources and system design); “process of care” (the method by which healthcare is provided); and “outcome” (the consequence of the healthcare provided). The model provided by Kozma et al. [16] placed outcomes into three categories—Economic, Clinical, and Humanistic Outcomes (ECHO model)—depicting the value of pharmaceutical services. Figure 2 demonstrates the combination of the above two models.
According to a risk model [17], risks are analyzed by combining the severity of consequences and probability in the context of an existing situation. Risk matrices are used predominantly in safety risk management of MEs, for example, the National Patient Safety Risk Matrix in the UK [17], the Safety Assessment Code Matrix in the USA [18], and the Standard for Risk Management in Australia [19]. An original safety–risk matrix assesses a broad range of risks, including clinical, financial risks, risks related to reputation, business processes, and system, etc. The matrix of clinical risk was simplified to develop some tools assessing the potential significance of a PI [20–24].
According to a basic pharmacoeconomic model [25], the value of a PI considers both inputs and outputs of a PI compared with the absence of a PI (Fig. 3). Inputs can be thought of as resources required to implement the PI. Outputs can be thought of as consequences of a PI, in the form of clinical, humanistic, or process-related consequences. The difference between the cost of the original therapy and the new therapy gives the cost savings (or the increase in the cost of therapy). Cost avoidance refers to the prevention of additional health resources that are required to treat ADEs if a pharmacist does not intervene, such as hospitalization or a medical visit. The cost of implementation of a PI refers to the expenses of providing the PI such as pharmacist’s time, phone calls, etc. In some studies [26, 27], the economic value of a PI is estimated through cost savings plus cost avoidance less cost of implementation of a PI.
Regarding the content of tools, after combination of the above four models that are able to be applied to assess IPs, we determined and classified indicators used in existing tools into five main types of indicators: those related to economic, clinical, and humanistic outcomes, and process and probability of the impact.
2.3.2 Structure of Tools
We classify the structure of tools as mono-dimensional or multi-dimensional. One dimension was defined as an independent rating to answer one question related to impacts of a PI. Each dimension was also classified as nominal (two or more categories, but there is no intrinsic ordering to the categories, for example, rating PIs into two categories: technical or clinical problems [28]) or ordinal (there is a clear ordering of the dimension, for example, ordering clinical impacts of PIs into three categories such as minor, moderate, or major significance [29]). Each aspect of impact of a PI (e.g., clinical, economic aspect) was evaluated independently in one dimension or combined within ‘significance’ dimension with other aspects. For example, clinical impact was evaluated independently into six category dimensions (adverse significance, no significance, somewhat significant, very significant, extremely significant), and drug cost saving of a PI was evaluated independently in three category dimensions (drug cost reduction, drug cost increase, no change), respectively, in the tool by Briceland et al. [30]. Conversely, drug cost savings was integrated with clinical impact into a four-category dimension (low, mild, moderate, high significance) in the tool by Williams et al. [31].
2.3.3 Psychometric Parameters of Tools
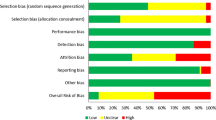
Regarding the psychometric parameters of tools, validity aims to check whether the tool is measuring what it is supposed to measure; inter-rater reliability measures whether the same results are produced when the same test is applied to the same scenarios by different raters; intra-rater reliability measures whether the same results are produced when the same test is applied to the same scenarios by the same rater on two different occasions [32]. We assessed risk of bias in studies that reported validity and/or reliability results according to the Cochrane Handbook for Systematic Reviews of Interventions [33]. We addressed the following main components: selection bias, performance bias, detection bias, and other biases. We classified each study as having a low, high, or unclear/unknown risk of bias [see the Electronic Supplementary Material (ESM) 1].
2.3.4 Assessment of Quality of Tools
We assessed the quality of each tool used in included studies using the criteria outlined in the ESM 2. One point is awarded when a criterion is clearly satisfied. The sum of scores represents the quality of a tool for assessing the significance of PIs in an included study.
We designed two forms to extract data. The articles were evaluated and summarized by (1) authors, published year, country; (2) structure of tools; (3) approach of assessment; (4) content of tools; (5) notes (see ESM 3); and by (6) setting, number of sample, sampling; (7) qualification and number of raters; (8) rating methods; (9) definitions of consensus; (10) validation; (11) inter-rater reliability; (12) intra-rater reliability, (13) risk of bias, and (14) score of quality of a tool (see ESM 4). For eligible studies, at least two review authors (THV and CC) independently extracted the data using these forms. We resolved discrepancies through discussion until consensus was reached. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details. We conducted this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [34].
3 Results
3.1 Studies Identified
A total of 873 articles were retrieved from PubMed (646), PASCAL (96), PsycINFO (33), and CINAHL with full-text (98). Of these, 833 articles were removed because of repetition or irrelevance, and 93 articles were added from reference lists, the review by Quélennec [14], an independent search by the second reviewer, and other sources. Finally, 133 articles [3, 12, 20–24, 28–30, 35–157] were selected for inclusion in the reviewed dataset (see ESM 3, 4). Some studies used a tool or multiple tools that were described in previous studies; therefore, the study comprises only 82 distinct tools in 133 selected articles. Figure 4 presents the systematic review flowchart.
Tools were created by research teams in the USA (43 studies), the UK (19 studies), Canada (16 studies), Australia (15 studies), France (seven studies), the Netherlands (five studies), Sweden (four studies), Norway (four studies), Spain (four studies), Germany (three studies), Switzerland (three studies), Belgium (two studies), Denmark (one study), Iran (one study), Israel (one study), Taiwan (one study), Ethiopia (one study), India (one study), Malaysia (one study), and UK and Saudi Arabia (one study).
3.2 Content of Tools
3.2.1 Main Approaches for Assessment of Significance of Pharmacist Interventions (PIs)
Of 82 distinct tools identified, 30 tools assessed the potential consequences of DRPs (Approach 3A in Fig. 1), while 46 tools assessed the potential significance of a PI (Approach 3B). Six tools applied multiple approaches [3, 12, 56, 86, 100, 120]. For example, the tool by Overhage and Lukes [12] assessed both the potential consequences of DRPs (approach 3A) and the potential significance of a PI (approach 3B).
3.2.2 Indicators Used in the Content of Existing Tools
The tools could cover one aspect or a range of aspects of impacts simultaneously. Indicators (not exhaustive) used in existing tools for assessment of potential significance of PIs are summarized in the following (see also ESM 5).
3.2.2.1 Clinical Impact
All tools reported clinical aspects as indispensable when rating the significance of a PI. Ranking the clinical significance of PI was realized by assessing effects of DRPs/PIs on safety (e.g., adverse health consequence [48], toxicity [44, 55], morbidity [21, 29, 86, 106, 113]); effectiveness (e.g., response to medication [87], disease control [53]); and necessity [134] of drug therapy; or characteristics of effects (e.g., short-term/long-term [106], permanent/temporary [23, 105, 113]), etc.
3.2.2.2 Humanistic Impact
Humanistic outcomes, also called patient-reported outcomes, are the consequences of the disease and/or its treatment as expressed by the patient. Humanistic outcomes are now more commonly used in clinical practice [158]. In this review, distinct tools clearly stated some indicators of humanistic outcomes: patient’s knowledge, compliance, patient’s satisfaction, inability to work, and quality of life. Humanistic aspects were often evaluated in combination with clinical aspects as a ‘significance’ dimension and classified as ‘low significance’ [31, 59, 71, 77, 79, 82, 108, 130], while some distinct tools evaluated certain indicators of humanistic impact of a PI independently [55, 87, 111, 120, 150].
3.2.2.3 Economic Impact
Different studies on the economic impact of a PI employ different terminologies, leading to some confusion in the perspective and components of costs, making the comparison of studies difficult. Cost savings and/or cost avoidance were rated independently in some tools [20, 30, 38, 41, 43, 48, 50, 53, 55, 56, 61, 65, 68, 73, 87, 93, 111, 123, 134]. In some studies, independent rating of the economic impact of a PI was used as the first step to determine the monetary value of a PI program [38, 41, 43, 48, 50, 68, 73, 80, 94, 123]. Cost avoidance was estimated through the types of healthcare resources avoided (e.g., readmission [102, 105, 113] or a scheduled visit to the physician [31, 48]), while cost savings were evaluated through costs related to drug therapy [20, 38, 43, 61, 66, 109, 111], drug therapy monitoring [38, 61], treatment cost [29], patient cost [43], or reimbursement [66].
3.2.2.4 Process-Related Impact
Like humanistic impacts, the process-related impacts of a PI were often ignored or reporting was incomplete or ambiguous or only mentioned arbitrarily in some tools. They may be grouped into resolving technical problems [28, 57, 82, 91], informational intervention [31, 38, 53, 57, 71, 75, 82, 94], physician’s satisfaction [120], facilitation of continuity of care [55], teamwork support [82], adherence to evidence-based therapy [104, 135], and others [93].
3.2.2.5 Structure-Related Impact
No structure-related indicators (e.g., a comprehensive inventory, record-keeping amenities such as a computer database, a designated area of the pharmacy, trained pharmacists/technicians [159]) were found in the reviewed tools.
3.2.2.6 Probability
The determination of probability of a consequence for each DRP/PI was used in 20 of 82 distinct tools [3, 20–24, 48, 56, 70, 86, 89, 109, 112, 113, 115, 116, 121, 132, 135, 138]. The definitions of each level of probability were based on concrete terms with or without a range of numeric probabilities or a Likert score. The number of levels ranged from 2 to 11. Evaluation of the probability of a consequence of a DRP was useful to evaluate the confidence of judgment [70, 116, 135]; classify the risk of an adverse heath consequence by combining the severity and the probability of occurrence [20–24]; and/or clarify the estimation of cost avoidance of a PI by combining the type of healthcare resources required to treat an adverse health consequence and its probability [48, 56].
3.3 Structure of Tools
The tools were multi-dimensional (one dimension with 2–20 categories, 39/82) or mono-dimensional (2–9 dimensions, 43/82), ordinal or nominal (see ESM 3). The majority were presented as classification systems with associated definitions, but other tools were based on visual analog scales [69, 132] or ordinal Likert scales [127].
3.4 Validation Process
The validation process was heterogeneous in terms of qualification and number of raters, rating methods, determination of psychometric parameters, etc. (see ESM 4).
3.4.1 Raters and Rating Methods
The profile of raters differed—internal or external, blinded or not, junior or senior, generalists or specialists—and they had various qualifications (e.g., pharmacist, physician, nurse, or pharmacologist). Rating methods varied—some studies were simply based on a single professional’s view (individual-based rating), while others used an inter-disciplinary group (group-based rating) with up to 30 raters and up to five different specialties.
There were a few instances in which a clear definition was presented outlining precisely what constituted consensus. For example, asking a panel of experts to independently judge an event and then combining their opinions using various mathematical approaches (e.g., mode [38, 39, 41, 56, 81, 100, 101, 119], median [24, 100, 130], mean [39, 41, 53, 56, 60, 69, 81, 83, 89, 100, 122, 136], sum [59]). Alternatively, a conservative approach was used taking the lower category of significance [138, 139] or an hierarchical approach in which a more senior expert was consulted when there was a disagreement among the clinical panel [37, 48, 49, 54, 55, 65, 68, 77, 91, 99, 103, 108, 113, 116, 124, 125, 128, 144, 151–153, 156]. In most studies, the consensus may have been arbitrarily determined; in other words, it was defined simply as a consensus-based approach (reached through discussion) [3, 22, 37, 38, 43, 44, 46, 48, 49, 54, 62, 68, 72, 77, 80, 82, 84, 91, 92, 97, 103, 104, 107, 108, 110, 113, 115–118, 120, 121, 123, 124, 132, 134, 135, 150, 152, 153, 160, 161].
3.4.2 Psychometric Parameters of Tools
Validity was only reported in eight studies (8/133, 6 %) [23, 45, 61, 69, 83, 106, 127, 131]. These explored face validity [127] or criteria-based validity (the results of coding by raters were compared with known outcomes in the literature [69, 83] or evidence in patients’ medical records [61] or with those of other skilled people or the consensus of an expert panel [23, 45, 106, 131]. Dean and Barber [69] and Taxis et al. [83] found a clear relationship between potential harm as assessed using their tools and actual harm. Eadon [45] found no significant difference between a pharmacist’s scores and those of three physicians (Mann–Whitney U test, U = 933.5, z = 0.034). Elliott and Woodward [23] found 93–100 % agreement between two pharmacists and one geriatrician, while Knez at al. [131] found 46 % agreement between a panel of three pharmacists and a physician. In three studies [61, 106, 127], descriptive information was given but no statistical information presented.
Measures of inter- and intra-rater reliability were established in 49 studies (36.8 %) (see ESM 4). High inter-rater reliability was found in 24 studies: Lesar et al. [63], Rupp [48], Overhage and Lakes [12], Caleo et al. [56], Lewinski et al. [24], Gleason et al. [128], Kwan et al. [110], Wong et al. [118], Chua et al. [146], Midlov et al. [115], Pippins et al. [116], Granas et al. [120], Lee et al. [132] with κ ≥ 0.7; Chedru et al. [59] with sigma x, y ≥ 0.7; Goarin at al. [129] with t test p < 0.05; Hawkey et al. [20] with Spearman’s rank correlation p < 0.05; Bayliff and Einarson [41], Strong and Tsang [50], and Virani and Crown [87] with coefficient of agreement ≥0.7; Khalili et al. [151], Hick et al. [81], and Bobb et al. [88] with agreement ≥80 %; Gisev et al. [127] with W ≥ 0.3; and Coffey et al. [119] with AC1 = 0.69, p < 0.01. Intra-rater reliability was only reported in two studies (1.5 %), with poor agreement in the study by Cousins et al. [61] and good agreement in a study by Dean and Barber [69].
While many studies showed that reliability was not affected by the profession of the rater [45, 69, 102, 124, 129], others found that physicians rated DRPs/PIs with lower severity/value than did pharmacists [12, 23, 38, 98]; or conversely, pharmacists tended to score PIs as being less clinically significant than physicians [53, 79]. A study by Lee and McPherson [100] found that ratings were more consistent between pharmacists than between physicians and pharmacists. However, even within the same profession, reliability was difficult to obtain. Fernández-Llamazares et al. [149] demonstrated that senior pharmacists rated more consistently than junior pharmacists.
3.5 Assessment of Quality of Tools
The scores of quality of tools for assessing significance of PIs in 133 included studies were presented in Table 1.
4 Discussion
4.1 Limitations of this Review
It was difficult to identify all tools in the literature. We retrieved only four available databases. Tools were sometimes mentioned but not described in detail [162]. Tools only assessing the actual consequences of DRPs (Approach 1 in Fig. 1) or the actual consequences of a PI (Approach 2 in Fig. 1) were not used for this review because these cover different concepts. We used the outcome terminology proposed by Holdford and Smith [13]. However, the identification of classifications of indicators mentioned in existing tools was complicated because of the different terminologies used by authors and institutions. For example, determining whether a tool evaluated humanistic impacts of a PI was difficult for the following reasons (1) not all indicators of humanistic outcomes are theoretically well defined; (2) in some tools, the terminology of humanistic indicators is confusing; and (3) the complex relationships between humanistic, clinical, and economic outcomes. An assessment of the significance of PIs is key to justifying value of pharmacy services. However, methods differ between studies, which hinders their review and synthesis. Our review is a first attempt to (1) distinguish different approaches used to assess the significance of PIs, (2) evaluate the quality of tools based on theoretical models, and (3) discuss the strengths and weaknesses of existing tools and validation process. We suggest recommendations for an optimal method of evaluation of the significance of PIs.
4.2 Content of tools
The principal indicators of the impact of a PI concern the process; the clinical, humanistic, and economic outcomes; and probability. These indicators are inconsistently mentioned in tools. Some tools cover many indicators, but a comprehensive tool is not available. One reason for this may be that few tools were constructed based on theoretical models, a systematic literature review, and input from healthcare professionals.
Pharmacy practitioners and pharmacy managers need to demonstrate that for each PI the benefits outweigh the costs for a given patient, healthcare system, and society. According to the economic model, the cost of implementing a PI, cost savings, and cost avoidance should be evaluated. Tools should be constructed so as to capture the potential significance of a PI with an estimation of its economic impact (e.g., using the Williams et al. [31] tool, the potential significance had a fairly good correlation with the economic value) and is the first step to conducting a more sophisticated economic evaluation [38, 48, 73, 77, 93, 123].
Most tools focus on patient outcomes. However, PIs are also useful for the health practitioner. Tools therefore should reflect the possible impacts on both. In order to assign a probability for a potential consequence, it is ideal to know how often it has been described in the literature as well as how often it occurs at the local healthcare facility. However, in most cases, the determination of this probability was difficult to estimate. This is primarily because such probabilities are rarely available in the literature and can vary based on patient risk, co-morbidities, or other factors [138]. Generally, in order to improve the consistency of judgment of probability between raters, studies only select and code the most likely harm prevented [19, 23, 48, 56] and request the opinions of staff most familiar with these events. A multi-dimensional matrix of risk that considers many aspects of impacts and the probability of each aspect, such as the matrix developed by National Patient Safety Agency [17] could be used as a framework to construct a new tool for assessing PIs.
Assessing the potential significance of a PI is primarily based on the potential severity of consequences of DRPs that might have occurred if a pharmacist had not intervened. It makes sense to use the same definitions, terminology, and grading systems for both the potential significance of a PI and the actual severity of consequence of MEs, ADEs, or ADRs [19, 93, 163]. Indeed, the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) Index [164] has been used to design new tools to assess PIs [84, 86, 88, 128]. Furthermore, most tools use a variety of similar terminologies without precise definitions, which risks inconsistent rating.
4.3 Structure of Tools
One can argue that a tool for evaluating impacts of a PI should be as simple as possible. However, a simple tool can hardly detect all possible impacts of PIs and would not provide enough information for practice and research. Therefore, a well-structured tool should provide the main dimensions and the main levels. A stepwise instruction should be developed to guide the use of tools in practice, so results of different studies can be compared.
An ordinal tool is preferred to prioritize the most significant PIs. Half of the tools were mono-dimensional and often concentrated on clinical impacts of PIs, failing to detect other impacts. Multi-dimensional tools and the independent evaluation of different impacts of a PI improve the sensitivity and flexibility of evaluation methods. For example, the tool by Lindblad et al. [111] separates the evaluation of economic impacts (cost savings) and clinical impacts, thereby facilitating the estimation of cost savings by the whole PI program. The number-based levels facilitate interpretation of results.
Although many studies used multi-dimensional tools, the results of each dimension were interpreted separately. Only Lindblad et al. [111] used the method of simultaneous interpretation of mean impacts of many dimensions for all PI. For all interventions, this study found a mean of 1.4 clinical, 0.8 humanistic, and 0.1 economic outcomes. This method of interpretation of results gives the added value of the whole PI program rather than the individual PI. There is no method for determining these multi-dimensional impacts of each PI.
Many authors adapted existing tools in the literature to their study. In the ESM 3 and 4, we grouped studies into sub-groups that used the same or a slightly modified tool. The most commonly adapted tools used in other studies include the following: Folli et al. in 1987 [36] (eight studies), Hatoum et al. in 1988 [38] (26 studies), Lesar et al. in 1990 [42] (four studies), Western Australian Clinical Pharmacists Group in 1991 [44] (three studies), Rupp in 1992 [48] (three studies), Chedru and Juste in 1997 [59] (five studies), Alderman in 1997 [29] (three studies), Overhage and Lukes in 1999 [12] (11 studies), Dean and Barber in 1999 [69] (six studies), Hawksworth et al. in 1999 [70] (three studies), NCC MERP Index in 2001 [164] (five studies), Society of Hospital Pharmacists of Australia guideline in 2005 [19] (four studies), Cornish et al. in 2005 [22] (five studies), and Blix et al. in 2006 [97] (three studies). The advantages of using existing structured measures include that they have already been validated and their reliability confirmed, and using measures that have been applied by others allows comparison between studies. However, limitations include difficulties in finding a suitable tool for local use, and that reproducibility of the reliability of a specific tool is not always obvious. For example, the tool by Overhage and Lukes [12] showed high inter-rater reliability in their study, but when adapted by Bosma et al. [98], Lee and McPherson [100], Fernández-Llamazares et al. [149], and Somers et al. [157] exhibited low inter-rater reliability.
4.4 Validation Process
The criteria-based validity of any method measuring the potential significance of a PI is difficult to assess because there is no generally accepted standard with which to compare [12]. The comparison of the scores given to MEs with known outcomes has limitations because errors resulting in more severe outcomes may be more likely to be reported in the literature [69]. Nonetheless, the comparison of the individual scores with the consensus results of a group of experts has other limitations. The existence of a consensus does not mean that the ‘correct’ answer has been found [165]. The consensus method is just a means of identifying current medical opinion and areas of disagreement. It recommends that the results should, when possible, be matched to other data in the literature [102], to the actual outcomes in the patient after follow-up [61], to observable events [165], or to other systems of reporting such as MEs and ADEs [89].
Measuring the inter- and intra-rater reliability of methods for assessment of impacts of PIs is a scientific and practical requirement. Indeed, this information not only provides useful data about the reliability of a subjective assessment but can also be used for teaching, peer review, and audit purposes [65, 149]. However, this measure has not been established for all tools. It is not possible to directly compare the reliability of tools as they used different methods to assess reliability.
Like the actual severity ratings of ADEs [166–168] or MEs [169, 170], literature shows many inter-rater and intra-rater inconsistencies within and between healthcare professional groups. Such inconsistencies can be partly attributed to lack of clarity in the tools and scenarios used for validation, shortage of time for proper case reading and coding, and different assessor viewpoints.
The inconsistency of coding between raters prevents individual evaluation. Many studies used an expert panel; however, no strict criteria govern the selection of experts. With regard to medical research, Jones and Hunter [165] defined the term ‘expert’ to be “clinicians practicing in the field under consideration”. According to this definition, suitable experts for studies such as those proposed in this paper include pharmacists and medical practitioners. It has been recommended that experts should be selected based on their appropriateness for the study in terms of experience in the therapeutic area, reputation, geographic representation, practice type and specialty, heterogeneity in treatment patterns, and willingness to participate in the study [11, 171]. Wright et al. [172] demonstrated that community pharmacists, hospital pharmacists, general practitioners, and specialist physicians attribute significantly different values when undertaking these assessments.
4.5 Properties of Ideal Tools for Assessing the Potential Significance of a PI
Currently, there are no formal guidelines or standardization of methodology concerning methods of assessing the potential significance of PIs. Given the results of this review, we suggest some desirable pragmatic psychometric and theoretical properties, as follows.
4.5.1 Theoretical Properties
-
1.
Tools should be developed based on (1) comprehensive theoretical models, (2) a systematic literature review of available evidence that reflects the whole range of impacts of a PI, (3) an evaluation of existing tools, and (4) input from healthcare professionals.
-
2.
Tools should be able to demonstrate that the benefits outweigh the costs in a given patient, healthcare system, and society at the level of each PI.
-
3.
An evaluation from a multi-impact perspective, rather than simply focusing on clinical impact, should be used to enhance understanding of the comprehensive effect of PIs. For example, a tool integrating clinical, humanistic, economic, and process-related impacts and the probability of these impacts.
-
4.
The views of patients, healthcare providers, institutions, payers, and society should be considered.
4.5.2 Psychometric Properties
-
1.
Tools should be validated prior to use.
-
2.
Along with information on the clinical case, experts should be provided with a literature review, coding instructions, and examples. Indices for agreement/validity/reliability should be conform to the current guidelines [173].
-
3.
The guideline proposed for the use of experts in pharmacoeconomic studies [174] is suitable for this type of study: description of consensus techniques (e.g., Delphi process, Nominal Group Technique, expert panels); justification in using such methods; description of selection of experts; provision of a definition of consensus in advance of the execution of a study; information provided to panelists in advance must be as objective and as comprehensive as possible; and modification of the tool as appropriate, with input from independent experts or a pilot test; appropriate presentation and interpretation of findings.
4.5.3 Pragmatic Properties
-
1.
Tools must be brief, not time-consuming, and acceptable to evaluators.
-
2.
Tools should be well-defined.
-
3.
Tools must be well-structured and flexible enough to adapt to meet specific needs (e.g., multi-dimensional tool, possibility of modification of terminology of economic impact is based on different perspectives or modification of number of levels; independence between dimensions).
-
4.
Tools should have an open, numeric, and hierarchical structure (with main dimensions, main levels of each dimensions, and an open structure to include the option ‘non-determinable’).
-
5.
Same definitions, terminology, and grading systems for both the potential significance of a PI and the actual severity of consequence of MEs/ADEs/ADRs.
4.6 Assessment of Quality of Tools
Researchers and clinicians may have different needs in relation to a tool for assessing the potential significance of PIs. Due to the wide range of tools used in the literature, researchers need to consider developing a basis of comparison between tools. Therefore, we tried to assess the quality of each tool in included studies using ten criteria to assist in comparing tools across studies (see ESM 4). According to these criteria, the tools with the highest scores were those by Caleo et al. [56] and Hick et al. [81] (seven scores), Eadon [45], Overhage and Lukes [12], Kopp et al. [109], Virani and Crown [87], Lee and McPherson [100], and Lewinski et al. [24] (six scores). No tool could be found that met all of our above criteria. It appears that further research in this field is necessary.
5 Conclusion
Various structures and contents of tools for the evaluation of impacts of PIs were highlighted, as well as suggestions for an optimal evaluation method. The majority of tools focused primarily on assessing clinical aspects and failed to detect other impacts. Our summary was hindered by variations in tools and assessment processes. Limited and varied validity and reliability brought into question the level of evidence for the evaluation of the potential significance of PIs for justification of added value of PIs. The development of tools with optimal theoretical, pragmatic, and psychometric properties and their integration into pharmacist’s daily practice through rational assessment processes (e.g., peer review) and standardized documentation systems (e.g., information technology tools) are needed.
References
Cipolle RJ, Strand L, Morley P, Cipolle R. Pharmaceutical care practice: the clinician’s guide. 2nd ed. New York: McGraw-Hill Companies; 2004.
Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47(3):533–43.
Rothschild JM, Churchill W, Erickson A, Munz K, Schuur JD, Salzberg CA, et al. Medication errors recovered by emergency department pharmacists. Ann Emerg Med. 2010;55:513–21.
Strand LM, Cipolle RJ, Morley PC. Documenting the clinical pharmacist’s activities: back to basics. Drug Intell Clin Pharm. 1988;22(1):63–7.
Vessal G. Detection of prescription errors by a unit-based clinical pharmacist in a nephrology ward. Pharm World Sci. 2010;32:59–65.
Dean Franklin B, Vincent C, Schachter M, Barber N. The incidence of prescribing errors in hospital inpatients: an overview of the research methods. Drug Saf. 2005;28(10):891–900.
Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070–7.
Schmidt IK, Claesson CB, Westerholm B, Nilsson LG. Physician and staff assessments of drug interventions and outcomes in Swedish nursing homes. Ann Pharmacother. 1998;32(1):27–32.
McLennan DN, Dooley MJ, Brien JE. Beneficial clinical outcomes resulting from pharmacist interventions. J Oncol Pharm Pract. 1999;5(4):184–9.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655.
Stafford AC, Bindoff IK, Tenni PC, Peterson GM, Doran CM. A methodological framework for estimating the clinical and economic value of community pharmacists’ clinical interventions using expert opinion. J Clin Pharm Ther. 2011;37(4):378–85.
Overhage JM, Lukes A. Practical, reliable, comprehensive method for characterizing pharmacists’ clinical activities. Am J Health Syst Pharm. 1999;56(23):2444–50.
Holdford DA, Smith S. Improving the quality of outcomes research involving pharmaceutical services. Am J Health Syst Pharm. 1997;54(12):1434–42.
Quélennec B. Assessment of the potential impact of errors found through medication reconciliation [Evaluation de l’impact potentiel des erreurs récupérées par conciliation des traitements médicamenteux] [Thesis ]: Université de Strasbourg; 2011.
Donabedian A. The quality of medical care. Science. 1978;200(4344):856–64.
Kozma CM, Reeder CE, Schulz RM. Economic, clinical, and humanistic outcomes: a planning model for pharmacoeconomic research. Clin Ther. 1993;15(6):1121–32.
National Patient Safety Agency. A risk matrix for risk managers. 2008. Available from: http://www.npsa.nhs.uk/nrls/improvingpatientsafety/patient-safety-tools-and-guidance/risk-assessment-guides/risk-matrix-for-risk-managers/. Cited 19 Oct 2015.
US Department of Veterans Affairs. Safety Assessment Code (SAC) Matrix; 2013. http://www.patientsafety.va.gov/professionals/publications/matrix.asp. Accessed 9 Sept 2015.
Society of Hospital Pharmacists of Australia. SHPA standards of practice for clinical pharmacy. J Pharm Pract Res. 2005;35(2):122–46.
Hawkey CJ, Hodgson S, Norman A, Daneshmend TK, Garner ST. Effect of reactive pharmacy intervention on quality of hospital prescribing. BMJ. 1990;300(6730):986–90.
Dooley MJ, Allen KM, Doecke CJ, Galbraith KJ, Taylor GR, Bright J, et al. A prospective multicentre study of pharmacist initiated changes to drug therapy and patient management in acute care government funded hospitals. Br J Clin Pharmacol. 2004;57(4):513–21.
Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–9.
Elliott RA, Woodward MC. Assessment of risk associated with medication-related problems in elderly outpatients. J Pharm Pract Res. 2009;39:109–13.
Lewinski D, Wind S, Belgardt C, Plate V. Prevalence and safety-relevance of drug-related problems in German community pharmacies. Pharmacoepidemiol Drug Saf. 2010;19(2):141–9.
Schumock GT. Methods to assess the economic outcomes of clinical pharmacy services. Pharmacotherapy. 2000;20(10 Pt 2):243S–52S.
Benrimoj SI, Langford JH, Berry G, Collins D, Lauchlan R, Stewart K, et al. Economic impact of increased clinical intervention rates in community pharmacy. A randomised trial of the effect of education and a professional allowance. Pharmacoeconomics. 2000;18(5):459–68.
Nesbit TW, Shermock KM, Bobek MB, Capozzi DL, Flores PA, Leonard MC, et al. Implementation and pharmacoeconomic analysis of a clinical staff pharmacist practice model. Am J Health Syst Pharm. 2001;58(9):784–90.
Krahenbuhl JM, Kremer B, Guignard B, Bugnon O. Practical evaluation of the drug-related problem management process in Swiss community pharmacies. Pharm World Sci. 2008;30(6):777–86.
Alderman CP. A prospective analysis of clinical pharmacy interventions on an acute psychiatric inpatient unit. J Clin Pharm Ther. 1997;22(1):27–31.
Briceland LL, Kane MP, Hamilton RA. Evaluation of patient-care interventions by Pharm.D. clerkship students. Am J Hosp Pharm. 1992;49(5):1130–2.
Williams M, Peterson GM, Tenni PC, Bindoff IK, Stafford AC. DOCUMENT: a system for classifying drug-related problems in community pharmacy. Int Journal Clin Pharm. 2012;34:43–52.
Carmines EG, Zeller RA. Reliability and validity assessment. London: Sage Publications; 1979.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0: The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Accessed 9 Sept 2015.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Iafrate RP, Smith PD. Documenting medication errors averted by pharmacists. Am J Hosp Pharm. 1986;43:1672.
Folli HL, Poole RL, Benitz WE, Russo JC. Medication error prevention by clinical pharmacists in two children’s hospitals. Pediatrics. 1987;79(5):718–22.
Blum KV, Abel SR, Urbanski CJ, Pierce JM. Medication error prevention by pharmacists. Am J Hosp Pharm. 1988;45(9):1902–3.
Hatoum HT, Hutchinson RA, Witte KW, Newby GP. Evaluation of the contribution of clinical pharmacists: inpatient care and cost reduction. Drug Intell Clin Pharm. 1988;22(3):252–9.
Hatoum HT, Hutchinson RA, Elliott LR, Kendzierski DL. Physicians’ review of significant interventions by clinical pharmacists in inpatient care. Drug Intell Clin Pharm. 1988;22(12):980–2.
Neville RG, Robertson F, Livingstone S, Crombie IK. A classification of prescription errors. J R Coll Gen Pract. 1989;39:110–2.
Bayliff CD, Einarson TR. Physician assessment of pharmacists’ interventions—a method of estimating cost avoidance and determining quality assurance. Can J Hosp Pharm. 1990;43(4):167–71, 95.
Lesar TS, Briceland LL, Delcoure K, Parmalee JC, Masta-Gornic V, Pohl H. Medication prescribing errors in a teaching hospital. JAMA. 1990;263(17):2329–34.
Mueller BA, Abel SR. Impact of college of pharmacy-based educational services within the hospital. Drug Intell Clin Pharm. 1990;24(4):422–5.
Western Australian Clinical Pharmacists Group. Recording clinical pharmacist interventions: is there a better way? Austr J Hosp Pharm. 1991;21(3):158–62.
Eadon H. Assessing the quality of ward pharmacist’s intervention. Int J Pharm Pract. 1992;1:145–7.
Ho L, Brown GR, Millin B. Characterization of errors detected during central order review. Can J Hosp Pharm. 1992;45(5):193–7.
Lipton HL, Bero LA, Bird JA, McPhee SJ. The impact of clinical pharmacists’ consultations on physicians’ geriatric drug prescribing. A randomized controlled trial. Med Care. 1992;30(7):646–58.
Rupp MT. Value of community pharmacists’ interventions to correct prescribing errors. Ann Pharmacother. 1992;26(12):1580–4.
Rupp MT, DeYoung M, Schondelmeyer SW. Prescribing problems and pharmacist interventions in community practice. Med Care. 1992;30(10):926–40.
Strong DK, Tsang GW. Focus and impact of pharmacists’ interventions. Can J Hosp Pharm. 1993;46(3):101–8.
Tang I, Vrahnos D, Hatoum H, Lau A. Effectiveness of clinical pharmacist interventions in a hemodialysis unit. Clin Ther. 1993;15(2):459–64.
Mason RN, Pugh CB, Boyer SB, Stiening KK. Computerized documentation of pharmacists’ interventions. Am J Hosp Pharm. 1994;51(17):2131–8.
Slaughter RL, Erickson SR, Thomson PA. Clinical interventions provided by doctor of pharmacy students. Ann Pharmacother. 1994;28(5):665–70.
Chisholm MA, Pittman DG, Longley JM, Mullis SR. Implementation of pharmaceutical care in acute medical cardiovascular patients. Hosp Pharm. 1995;30(7):572–4, 7–8.
Wang Chin JM, Muller RJ, Lucarelli CD. A pharmacy intervention program: recognizing pharmacy’s contribution to improving patient care. Hosp Pharm. 1995;30(2):120, 3–6, 9–30.
Caleo SUE, Benrimoj S, Collins D, Lauchlan R, Stewart KAY. Clinical evaluation of community pharmacists’ interventions. Int J Pharm Pract. 1996;4(4):221–7.
Kettle J, Downie G, Palin A, Chesson R. Pharmaceutical care activities within a mental health team. Pharm J. 1996;257:814–6.
Wernick A, Possidente CJ, Keller EG, Gilroy G. Enhancing continuity of care through pharmacist review of discharge medications. Hosp Pharm. 1996;31:672–6.
Chedru V, Juste M. Medical evaluation of the clinical impact of pharmacist interventions [Evaluation médicale de l’impact clinique des interventions pharmaceutiques]. J Pharm Clin. 1997;16(4):254–8.
Chisholm MA, Hawkins DW, Taylor AT. Providing pharmaceutical care: are pharmacy students beneficial to patients? Hosp Pharm. 1997;32(3):370–5.
Cousins D, Gerrett D, Luscombe D. Reliability and validity of hospital pharmacists’ clinical intervention data. Am J Health Syst Pharm. 1997;54(14):1596–603.
Grabe DW, Low CL, Bailie GR, Eisele G. Evaluation of drug-related problems in an outpatient hemodialysis unit and the impact of a clinical pharmacist. Clin Nephrol. 1997;47(2):117–21.
Lesar TS, Lomaestro BM, Pohl H. Medication-prescribing errors in a teaching hospital. A 9-year experience. Arch Intern Med. 1997;157(14):1569–76.
Lesar TS, Briceland L, Stein DS. Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–7.
Lucas C, Glare PA, Sykes JV. Contribution of a liaison clinical pharmacist to an inpatient palliative care unit. Palliat Med. 1997;11(3):209–16.
Dennehy C, Kroon L, Byrne M, Koda-Kimble M. Increase in number and diversity of clinical interventions by PharmD. Students over a clerkship rotation. Am J Pharm Educ. 1998;62:373–9.
Smythe MA, Shah PP, Spiteri TL, Lucarotti RL, Begle RL. Pharmaceutical care in medical progressive care patients. Ann Pharmacother. 1998;32(3):294–9.
Weidle P, Bradley L, Gallina J, Daniel C, Thorn N, Siegel L. Pharmaceutical care intervention documentation program and related cost savings at a University Hospital. Hosp Pharm. 1998;34(1):43–52.
Dean BS, Barber ND. A validated, reliable method of scoring the severity of medication errors. Am J Health Syst Pharm. 1999;56(1):57–62.
Hawksworth GM, Corlett AJ, Wright DJ, Chrystyn H. Clinical pharmacy interventions by community pharmacists during the dispensing process. Br J Clin Pharmacol. 1999;47:695–700.
Nathan A, Goodyer L, Lovejoy A, Rashid A. ‘Brown bag’ medication reviews as a means of optimizing patients’ use of medication and of identifying potential clinical problems. Fam Pract. 1999;16(3):278–82.
Possidente CJ, Bailie GR, Hood VL. Disruptions in drug therapy in long-term dialysis patients who require hospitalization. Am J Health Syst Pharm. 1999;56(19):1961–4.
Krass I, Smith C. Impact of medication regimen reviews performed by community pharmacists for ambulatory patients through liaison with general medical practitioners. Int J Pharm Pract. 2000;8:111–20.
Lustig A. Medication error prevention by pharmacists—an Israeli solution. Pharm World Sci. 2000;22(1):21–5.
Price RN, Rogers A. Intervention monitoring on admissions wards. Hosp Pharm. 2000;7:81–4.
Reddick JB, Murphy JE. Evaluating the clinical interventions of students during clerkships using a cognitive services claim form. Am J Pharm Educ. 1999;64:38–43.
Taylor CT, Church CO, Byrd DC. Documentation of clinical interventions by pharmacy faculty, residents, and students. Ann Pharmacother. 2000;34(7–8):843–7.
Alderman CP, Farmer C. A brief analysis of clinical pharmacy interventions undertaken in an Australian teaching hospital. J Qual Clin Pract. 2001;21(4):99–103.
Ewan MA, Greene RJ. Evaluation of mental health care interventions made by three community pharmacists—a pilot study. Int J Pharm Pract. 2001;9:225–34.
Guignon AM, Grain F, Allenet B, Brudieu E, Barjhoux C, Bosson J-L, et al. Evaluation de l’impact clinique des opinions pharmaceutiques dans un service de médecine spécialisée. J Pharm Clin. 2001;20(2):118–23.
Hick HL, Deady PE, Wright DJ, Silcock J. The impact of the pharmacist on an elective general surgery pre-admission clinic. Pharm World Sci. 2001;23(2):65–9.
Needham DS, Wong IC, Campion PD. Evaluation of the effectiveness of UK community pharmacists’ interventions in community palliative care. Palliat Med. 2002;16(3):219–25.
Taxis K, Dean B, Barber N. The validation of an existing method of scoring the severity of medication administration errors for use in Germany. Pharm World Sci. 2002;24:236–9.
Van den Bemt PM, Postma MJ, van Roon EN, Chow MC, Fijn R, Brouwers JR. Cost-benefit analysis of the detection of prescribing errors by hospital pharmacy staff. Drug Saf. 2002;25:135–43.
Dale A, Copeland R, Barton R. Prescribing errors on medical wards and the impact of clinical pharmacists. Int J Pharm Pract. 2003:19–24.
Davydov L, Caliendo GC, Smith LG, Mehl B. Analysis of clinical intervention documentation by dispensing pharmacists in a teaching hospital. Hosp Pharm. 2003;38(4):346–50.
Virani A, Crown N. The Impact of a Clinical Pharmacist on Patient and Economic Outcomes in a Child and Adolescent Mental Health Unit. Can J Hosp Pharm. 2003;56:158–62.
Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164(7):785–92.
Buurma H, De Smet PA, Leufkens HG, Egberts AC. Evaluation of the clinical value of pharmacists’ modifications of prescription errors. Br J Clin Pharmacol. 2004;58:503–11.
Gray S, Woolfrey S, Copeland R, Gill D, Dennett G. Evaluating the potential impact of community pharmacy interventions on patient care in Northumberland. Qual Prim Care. 2004;12:47–51.
Prowse A, Scott D. An evaluation of a clinic-based pharmacists and a medicines home delivery service from a transplant outpatient department. Pharm J. 2004;272:547–51.
Denneboom W, Dautzenberg MG, Grol R, De Smet PA. User-related pharmaceutical care problems and factors affecting them: the importance of clinical relevance. J Clin Pharm Ther. 2005;30(3):215–23.
Fertleman M, Barnett N, Patel T. Improving medication management for patients: the effect of a pharmacist on post-admission ward rounds. Qual Saf Health Care. 2005;14(3):207–11.
Ling JM, Mike LA, Rubin J, Abraham P, Howe A, Patka J, et al. Documentation of pharmacist interventions in the emergency department. Am J Health Syst Pharm. 2005;62(17):1793–7.
Nickerson A, MacKinnon NJ, Roberts N, Saulnier L. Drug-therapy problems, inconsistencies and omissions identified during a medication reconciliation and seamless care service. Healthc Q. 2005;8 Spec No:65–72.
Serrano Fabiá A, Cavero Rodrigo E, Albert Marí A, Almenar Cubells D, Jiménez Torres NV. Pharmaceutical validation as a process of improving the quality of antineoplastic treatment. J Oncol Pharm Pract. 2005;11(2):45–50.
Blix HS, Viktil KK, Moger TA, Reikvam A. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci. 2006;28:152–8.
Bosma L, Jansman FG, Franken AM, Harting JW, Van den Bemt PM. Evaluation of pharmacist clinical interventions in a Dutch hospital setting. Pharm World Sci. 2008;30:31–8.
Grangeasse L, Chaigneau L, Medjoub M, Larosa F, Limat S. Computerized and protocolled prescription of chemotherapy: residual iatrogenic risk and pharmacist interventions [Prescription informatisée et protocolisée des chimiothérapies: risque iatrogène résiduel et interventions pharmaceutiques]. J Pharm Clin. 2006;25:33–8.
Lee J, McPherson ML. Outcomes of recommendations by hospice pharmacists. Am J Health Syst Pharm. 2006;63:2235–9.
Pham DQ. Evaluating the impact of clinical interventions by PharmD students on internal medicine clerkships: the results of a 3 year study. Ann Pharmacother. 2006;40(9):1541–5.
Spinewine A, Dhillon S, Mallet L, Tulkens PM, Wilmotte L, Swine C. Implementation of ward-based clinical pharmacy services in Belgium—description of the impact on a geriatric unit. Ann Pharmacother. 2006;40:720–8.
Stubbs J, Haw C, Taylor D. Prescription errors in psychiatry—a multi-centre study. J Psychopharmacol. 2006;20(4):553–61.
Viktil KK, Blix HS, Moger TA, Reikvam A. Interview of patients by pharmacists contributes significantly to the identification of drug-related problems. Pharmacoepidemiol Drug Saf. 2006;15:667–74.
Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15(2):122–6.
Bayley KB, Savitz LA, Maddalone T, Stoner SE, Hunt JS, Wells R. Evaluation of patient care interventions and recommendations by a transitional care pharmacist. Ther Clin Risk Manag. 2007;3:695–703.
Estellat C, Colombet I, Vautier S, Huault-Quentel J, Durieux P, Sabatier B. Impact of pharmacy validation in a computerized physician order entry context. Int J Qual Health Care. 2007;19(5):317–25.
Knudsen P, Herborg H, Mortensen AR, Knudsen M, Hellebek A. Preventing medication errors in community pharmacy: frequency and seriousness of medication errors. Qual Saf Health Care. 2007;16:291–6.
Kopp BJ, Mrsan M, Erstad BL, Duby JJ. Cost implications of and potential adverse events prevented by interventions of a critical care pharmacist. Am J Health Syst Pharm. 2007;64(23):2483–7.
Kwan Y, Fernandes OA, Nagge JJ, Wong GG, Huh JH, Hurn DA, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034–40.
Lindblad A, Alleyne A, Howorko J. Development and Initial Evaluation of a Software-Based Clinical Workload Measurement System for Pharmacists. Can J Hosp Pharm. 2007;60(5):295–301.
Nguyen A, Yu K, Shakib S, Doecke CJ, Boyce M, March G, et al. Classification of findings in the home medicines reviews of post-discharge patients at risk of medication misadventure. J Pharm Pract Res. 2007;37(2):111–4.
Struck P, Pharmacist S, Pedersen KH, Moodley P. A pilot study of pharmacist-initiated interventions in drug therapy in an Australian paediatric hospital. EJHP Sci. 2007;13:105–12.
Lalonde L, Lampron AM, Vanier MC, Levasseur P, Khaddag R, Chaar N. Effectiveness of a medication discharge plan for transitions of care from hospital to outpatient settings. Am J Health Syst Pharm. 2008;65(15):1451–7.
Midlov P, Holmdahl L, Eriksson T, Bergkvist A, Ljungberg B, Widner H, et al. Medication report reduces number of medication errors when elderly patients are discharged from hospital. Pharm World Sci. 2008;30(1):92–8.
Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–22.
Wang HY, Chan AL, Chen MT, Liao CH, Tian YF. Effects of pharmaceutical care intervention by clinical pharmacists in renal transplant clinics. Transplant Proc. 2008;40(7):2319–23.
Wong JD, Bajcar JM, Wong GG, Alibhai SM, Huh JH, Cesta A, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373–9.
Coffey M, Mack L, Streitenberger K, Bishara T, De Faveri L, Matlow A. Prevalence and clinical significance of medication discrepancies at pediatric hospital admission. Acad Pediatr. 2009;9(5):360–5 (e1).
Granas AG, Berg C, Hjellvik V, Haukereid C, Kronstad A, Blix HS, et al. Evaluating categorisation and clinical relevance of drug-related problems in medication reviews. Pharm World Sci. 2010;32:394–403.
Lövgren S, Clark RA, Angley M, Ponniah AP, Colley D, Shakib S. Timeliness and clinical impact of hospital-initiated medication reviews. J Pharm Pract Res. 2009;39(4):269–73.
Vasileff HM, Whitten LE, Pink JA, Goldsworthy SJ, Angley MT. The effect on medication errors of pharmacists charting medication in an emergency department. Pharm World Sci. 2009;31(3):373–9.
Westerlund T, Marklund B. Assessment of the clinical and economic outcomes of pharmacy interventions in drug-related problems. J Clin Pharm Ther. 2009;34(3):319–27.
Abdel-Qader DH, Harper L, Cantrill JA, Tully MP. Pharmacists’ interventions in prescribing errors at hospital discharge: an observational study in the context of an electronic prescribing system in a UK teaching hospital. Drug Saf. 2010;33:1027–44.
Climenté-Marti M, Garcia-Manon ER, Artero-Mora A, Jimenez-Torres NV. Potential risk of medication discrepancies and reconciliation errors at admission and discharge from an inpatient medical service. Ann Pharmacother. 2010;44(11):1747–54.
Eichenberger PM, Kahmann IV. Foppe van Mil JW, Hersberger KE. Classification of drug-related problems with new prescriptions using a modified PCNE classification system. Pharm World Sci. 2010;32:362–72.
Gisev N, Bell JS, O’Reilly CL, Rosen A, Chen TF. An expert panel assessment of comprehensive medication reviews for clients of community mental health teams. Soc Psychiatry Psychiatr Epidemiol. 2010;45(11):1071–9.
Gleason KM, McDaniel MR, Feinglass J, Baker DW, Lindquist L, Liss D, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–7.
Goarin C, Mugnier N. Clinical pharmacist activity in an oncology and haematology unit significantly improves and secures patient care. Pharm Hosp Clin. 2011;46:e24–32.
Haavik S, Soeviknes S, Erdal H, Kjonniksen I, Guttormsen A, Granas AG. Prescriptions from general practitioners and in hospital physicians requiring pharmacists’ interventions. Pharmacoepidemiol Drug Saf. 2011;20(1):50–6.
Knez L, Laaksonen R, Duggan C. Evaluation of clinical interventions made by pharmacists in chemotherapy preparation. Radiol Oncol. 2010;44:249–56.
Lee JY, Leblanc K, Fernandes OA, Huh JH, Wong GG, Hamandi B, et al. Medication reconciliation during internal hospital transfer and impact of computerized prescriber order entry. Ann Pharmacother. 2010;44(12):1887–95.
Nerich V, Limat S, Demarchi M, Borg C, Rohrlich PS, Deconinck E, et al. Computerized physician order entry of injectable antineoplastic drugs: an epidemiologic study of prescribing medication errors. Int J Med Inform. 2010;79:699–706.
Niquille A, Bugnon O. Relationship between drug-related problems and health outcomes: a cross-sectional study among cardiovascular patients. Pharm World Sci. 2010;32:512–9.
Abu-Ramaileh AM, Shane R, Churchill W, Steffenhagen A, Patka J, Rothschild JM. Evaluating and classifying pharmacists’ quality interventions in the emergency department. Am J Health Syst Pharm. 2011;68:2271–5.
Bourne RS, Dorward BJ. Clinical pharmacist interventions on a UK neurosurgical critical care unit: a 2-week service evaluation. Int J Clin Pharm. 2011;33(5):755–8.
Castelino RL, Sathvik BS, Parthasarathi G, Gurudev KC, Shetty MS, Narahari MG. Prevalence of medication-related problems among patients with renal compromise in an Indian hospital. J Clin Pharm Ther. 2011;36(4):481–7.
Patanwala AE, Hays DP, Sanders AB, Erstad BL. Severity and probability of harm of medication errors intercepted by an emergency department pharmacist. Int J Pharm Pract. 2011;19:358–62.
Patanwala AE, Sanders AB, Thomas MC, Acquisto NM, Weant KA, Baker SN, et al. A prospective, multicenter study of pharmacist activities resulting in medication error interception in the emergency department. Ann Emerg Med. 2011;59(5):369–73.
Perera PN, Guy MC, Sweaney AM, Boesen KP. Evaluation of prescriber responses to pharmacist recommendations communicated by fax in a Medication Therapy Management Program (MTMP). J Manag Care Pharm. 2011;17(5):345–54.
Schröder S, Martus P, Odin P, Schaefer M. Drug-related problems in Parkinson’s disease: the role of community pharmacists in primary care. Int J Clin Pharm. 2011;33(4):674–82.
Villanyi D, Fok M, Wong RY. Medication reconciliation: identifying medication discrepancies in acutely ill hospitalized older adults. Am J Geriatr Pharmacother. 2011;9(5):339–44.
Williams M, Peterson GM, Tenni PC, Bindoff IK, Curtain C, Hughes J, et al. Drug-related problems detected in Australian community pharmacies: the PROMISe Trial (English). Ann Pharmacother. 2011;45(9):1067–76.
Bondesson A, Holmdahl L, Midlov P, Hoglund P, Andersson E, Eriksson T. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int J Clin Pharm. 2012;34(2):272–6.
Cesarz JL, Steffenhagen AL, Svenson J, Hamedani AG. Emergency Department discharge prescription interventions by emergency medicine pharmacists. Ann Emerg Med. 2013;61(2):209–14 (e1).
Chua SS, Kok LC, Yusof FA, Tang GH, Lee SW, Efendie B, et al. Pharmaceutical care issues identified by pharmacists in patients with diabetes, hypertension or hyperlipidaemia in primary care settings. BMC Health Serv Res. 2012;12:388.
Elliott RA, Martinac G, Campbell S, Thorn J, Woodward MC. Pharmacist-led medication review to identify medication-related problems in older people referred to an Aged Care Assessment Team: a randomized comparative study. Drugs Aging. 2012;29(7):593–605.
Fernandez-Llamazares CM, Calleja-Hernández M-Á, Manrique-Rodríguez S, Pérez-Sanz C, Durán-García E, Sanjurjo-Sáez M. Prescribing errors intercepted by clinical pharmacists in paediatrics and obstetrics in a tertiary hospital in Spain. Eur J Clin Pharmacol. 2012;68:1339–45.
Fernández-Llamazares CM, Manrique-Rodríguez S, Pérez-Sanz C, Durán-García ME, Sanjurjo-Sáez M, Calleja-Hernández MA. Validation of a method for recording pharmaceutical interventions. J Clin Pharm Ther. 2012;37:459–63.
Harrison JJ, Wang J, Cervenko J, Jackson L, Munyal D, Hamandi B, et al. Pilot study of a pharmaceutical care intervention in an outpatient lung transplant clinic. Clin Transplant. 2012;26(2):E149–57.
Khalili H, Karimzadeh I, Mirzabeigi P, Dashti-Khavidaki S. Evaluation of clinical pharmacist’s interventions in an infectious diseases ward and impact on patient’s direct medication cost. Eur J Intern Med. 2012;24(3):227–33.
Kwint HF, Faber A, Gussekloo J, Bouvy ML. The contribution of patient interviews to the identification of drug-related problems in home medication review. J Clin Pharm Ther. 2012;37(6):674–80.
Midlov P, Bahrani L, Seyfali M, Hoglund P, Rickhag E, Eriksson T. The effect of medication reconciliation in elderly patients at hospital discharge. Int J Clin Pharm. 2012;34(1):113–9.
Rashed AN, Neubert A, Tomlin S, Jackman J, Alhamdan H, AlShaikh A, et al. Epidemiology and potential associated risk factors of drug-related problems in hospitalised children in the United Kingdom and Saudi Arabia. Eur J Clin Pharmacol. 2012;68(12):1657–66.
Mekonnen AB, Yesuf EA, Odegard PS, Wega SS. Implementing ward based clinical pharmacy services in an Ethiopian University Hospital. Pharm Pract (Granada). 2013;11(1):51–7.
Quélennec B, Beretz L, Paya D, Blickle JF, Gourieux B, Andres E, et al. Potential clinical impact of medication discrepancies at hospital admission. Eur J Intern Med. 2013;24(6):530–5.
Somers A, Robays H, De Paepe P, Van Maele G, Perehudoff K, Petrovic M. Evaluation of clinical pharmacist recommendations in the geriatric ward of a Belgian university hospital. Clin Interv Aging. 2013;8:703–9.
Refolo P, Minacori R, Mele V, Sacchini D, Spagnolo AG. Patient-reported outcomes (PROs): the significance of using humanistic measures in clinical trial and clinical practice. Eur Rev Med Pharmacol Sci. 2012;16(10):1319–23.
Farris KB, Kirking DM. Assessing the quality of pharmaceutical care. II. Application of concepts of quality assessment from medical care. Ann Pharmacother. 1993;27(2):215–23.
Dean B, Schachter M, Vincent C, Barber N. Prescribing errors in hospital inpatients: their incidence and clinical significance. Qual Saf Health Care. 2002;11:340–4.
Spinewine A, Dean B. Measuring the impact of medicines information services on patient care: methodological considerations. Pharm World Sci. 2002;24:177–81.
Midlands Health Network. Clinical pharmacy in general practice—a review of the first nine months. 2012. Available from https://www.midlandshn.health.nz/uploads/clinical-pharmacy-in-general-practice-review-web.pdf. Accessed 19 Nov 2015.
Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267–70.
National Coordinating Council for Medication Error Reporting and Prevention. NCC MERP index for categorizing medication errors. 2001. http://www.nccmerp.org/types-medication-errors. Accessed 9 Sept 2015.
Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–80.
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34.
Kunac DL, Reith DM, Kennedy J, Austin NC, Williams SM. Inter- and intra-rater reliability for classification of medication related events in paediatric inpatients. Qual Saf Health Care. 2006;15(3):196–201.
Walshe K. Adverse events in health care: issues in measurement. Qual Health Care. 2000;9(1):47–52.
Williams SD, Ashcroft DM. Medication errors: how reliable are the severity ratings reported to the national reporting and learning system? Int J Qual Health Care. 2009;21:316–20.
Forrey RA, Pedersen CA, Schneider PJ. Interrater agreement with a standard scheme for classifying medication errors. Am J Health Syst Pharm. 2007;64(2):175–81.
Evans C. The use of consensus methods and expert panels in pharmacoeconomic studies. Practical applications and methodological shortcomings. Pharmacoeconomics. 1997;12(2 Pt 1):121–9.
Wright DJ, Aykroyd RG, Chrystyn H. Rating clinical pharmacy interventions by clinical panels: which health professionals should be included? Br J Clin Pharmacol. 1998;46:278P–305P.
Gisev N, Bell JS, Chen TF. Interrater agreement and interrater reliability: key concepts, approaches, and applications. Res Social Adm Pharm. 2013;9(3):330–8.
Evans C, Crawford B. Expert judgement in pharmacoeconomic studies. Guidance and future use. Pharmacoeconomics. 2000;17(6):545–53.
Acknowledgments
The authors wish to thank Dr. Alison Foote (Grenoble Clinical Research Centre) for critically reading and editing the manuscript; the librarians of the Grenoble Faculty of Medicine and Pharmacy; and Monique Boucquin from CDIP, Hospices Civils de Lyon for the literature search.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Thi-Ha Vo, Bruno Charpiat, Claire Catoire, Michel Juste, Renaud Roubille, François-Xavier Rose, Sébastien Chanoine, Jean-Luc Bosson, Ornella Conort, Benoît Allenet, and Pierrick Bedouch declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
40264_2015_370_MOESM5_ESM.pdf
Supplementary material 5: Indicators used in existing tools for assessment of potential significance of PIs (PDF 277 kb)
Rights and permissions
About this article
Cite this article
Vo, TH., Charpiat, B., Catoire, C. et al. Tools for Assessing Potential Significance of Pharmacist Interventions: A Systematic Review. Drug Saf 39, 131–146 (2016). https://doi.org/10.1007/s40264-015-0370-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0370-0