Abstract
Intravenous 18F-labelled florbetaben ([18F]florbetaben) [Neuraceq™] is a polyethylene glycol stilbene derivative that is approved in the USA, EU and South Korea for positron emission tomography (PET) imaging of the brain. It is used to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease and other causes of cognitive impairment. In vitro, [18F]florbetaben has high affinity and selectivity for β-amyloid. It has a short PET scan time (15–20 min). Visual assessment of regional and whole brain [18F]florbetaben PET images detected brain β-amyloid with high sensitivity and specificity, with good inter-reader agreement, in a phase III study in patients with various levels of cognitive function when compared with postmortem histopathological assessment. The whole brain visual assessment displayed high positive and negative predictive values, enabling amyloid pathology to be reliably detected or excluded. Quantitative PET analyses were generally consistent with the visual assessments. [18F]florbetaben was generally well tolerated in clinical trials. All adverse reactions in [18F]florbetaben recipients were mild to moderate in severity and the most common were injection-site-related (erythema, irritation and pain). There were no serious adverse reactions related to [18F]florbetaben. In summary, [18F]florbetaben is a highly accurate β-amyloid PET tracer that has the potential to support the clinical diagnosis of Alzheimer’s disease and other causes of cognitive decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High diagnostic accuracy (as verified by postmortem histopathology), with good inter-reader agreement, in detecting/excluding brain β-amyloid in patients with various levels of cognitive function |
High predictive values for reliably detecting or excluding amyloid pathology |
Generally well tolerated; the most common adverse reactions are injection-site-related |
Electronic training is established |
Short PET scan time of 15–20 min |
Long decay half-life permits centralized production and remote distribution of the final product, with increased patient access to β-amyloid imaging |
1 Introduction
Accumulation of β-amyloid peptide in the cerebral cortex is postulated to initiate a cascade of events that finally may lead to dementia in Alzheimer’s disease (AD) [1]. Thus, brain β-amyloid plaques are one of the pathological hallmarks of AD [2, 3]. Of note, β-amyloid deposition may also be present in patients with other neurological conditions, such as dementia with Lewy bodies (DLB), as well as in some cognitively normal older individuals who may or may not progress to AD [4, 5].
In vivo β-amyloid imaging with positron emission tomography (PET) is a validated diagnostic tool for detecting fibrillar β-amyloid, particularly neuritic plaques and amyloid angiopathy [2]. This noninvasive tool allows estimation of brain β-amyloid burden during the life time of patients as opposed to postmortem detection as performed historically, thus providing an opportunity for early diagnosis and potential therapeutic interventions. It can also support differential diagnosis of dementias [4, 5]. The most widely used β-amyloid PET tracer, 11C-labelled Pittsburgh compound-B [6] accurately detects β-amyloid [4, 5]. However, the short radioactive decay half-life of 11C (20 min) means that the use of this tracer is restricted to centres with an on-site cyclotron and 11C radiochemistry expertise. Therefore, β-amyloid PET 18F-labelled tracers, with a longer decay half-life of 110 min, have been developed (florbetaben, florbetapir, flutemetamol) or are being evaluated (flutafuranol) [7, 8]. All these tracers share some common structural and pharmacological features with [11C]Pittsburgh-compound-B [7]. The longer decay half-life of 18F tracers means that production can be centralized, with remote distribution of the final product to numerous PET imaging sites, thus reducing production costs and increasing access to β-amyloid imaging [8].
[18F]florbetaben (Neuraceq™) is an intravenously administered 18F-polyethylene glycol stilbene derivative [9]. This article reviews the diagnostic efficacy and tolerability of [18F]florbetaben for PET imaging of the brain to detect/exclude β-amyloid in patients with AD and other dementias, and briefly summarizes its pharmacological properties.
2 Mechanism of Action of [18F]Florbetaben
[18F]florbetaben binds to β-amyloid plaques in the brain [9]. The 18F isotope decays to a stable 18O by emitting a positron followed by photonic annihilation radiation, which is detected by a PET scanner [10]. In vitro, [18F]florbetaben showed nanomolar binding affinity to synthetic β-amyloid fibrils [10, 11] and to β-amyloid plaques in human AD brain homogenates [inhibition constant (K i) 6.7 nmol/L] [9]. A high- and a low-affinity binding site (K i 1.0 and 65 nmol/L) have been identified for unlabelled florbetaben in frontal cortex homogenates of human AD brain [12].
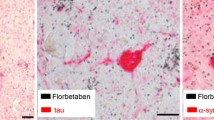
In vitro, florbetaben was highly selective for β-amyloid over tau and α-synuclein proteins [13]. [18F]florbetaben bound to β-amyloid in multiple cortical brain regions in postmortem human AD brain tissue sections, and [19F]florbetaben did not bind to α-synuclein or tau in frontal cortices of patients with DLB and frontotemporal dementia, respectively [13]. Binding of [18F]florbetaben to β-amyloid plaques in human AD brain sections correlated with immunohistochemical and Bielschowsky silver stains (Sect. 4) [14].
Using [18F]florbetaben PET imaging, brain β-amyloid accumulation was monitored in an animal model of AD [15]. In mice overexpressing Swedish mutant β-amyloid precursor protein, cortical [18F]florbetaben uptake significantly (p ≤ 0.01) increased from baseline at 16 and 20 months of age, while there was no change in wild-type mice [15]. [18F]florbetaben PET scan also allowed monitoring and predicting the response to a γ-secretase modulator in this model [16]. There was a significant (p < 0.001) correlation between PET results and postmortem histochemical analyses (β-amyloid plaque load and density, and insoluble β-amyloid42 levels) at the end of 6 month treatment [16].
3 Pharmacokinetic Properties of [18F]Florbetaben
The pharmacokinetics of [18F]florbetaben were evaluated in several early phase clinical studies in patients with AD or frontotemporal lobar degeneration and in healthy volunteers [17–20]. Following intravenous bolus injection of [18F]florbetaben 300 MBq, the maximum plasma concentration of total 18F radioactivity was reached within the first 1–2 min after the end of injection and then declined rapidly, with 2–3 % of the injected dose/L evident in arterial plasma 10 min post-injection [10, 17]. The uptake of radioactivity in the brain was rapid, reaching ~6 % of the injected radioactivity at 10 min post-injection [10, 14] and declining steadily thereafter (2 % at 2 h post-injection) [17]. [18F]florbetaben is highly bound to plasma proteins (≥98.5 %) [10, 14].
In patients with AD and in healthy volunteers, [18F]florbetaben was rapidly metabolized to a major radioactive polar metabolite fraction and a minor N-desmethyl derivative of [18F]florbetaben [18, 19]. The polar metabolites of [18F]florbetaben are the principle source of 18F in circulation during the 45–130 min imaging window [14]. In vitro studies showed that [18F]florbetaben is metabolized predominantly by cytochrome P450 (CYP) enzymes CYP4F2 and CYP2J2 and, at concentrations present in vivo, it did not inhibit CYP enzymes [10, 14]. As [18F]florbetaben is administered as a single small dose resulting in a low plasma concentration, no clinically relevant drug interactions are expected [17].
Data from patients with AD show that [18F]florbetaben is eliminated from plasma primarily via the hepatobiliary system, with a mean biological half-life of ~1 h; no radioactivity could be measured in blood at ~4 h post-injection [10, 14]. Approximately, 26–36 % of the injected radioactivity was excreted in urine up to 12 h post-injection, almost all as polar metabolites of [18F]florbetaben [18]. 18F has a physical half-life of 110 min; 98.93 and 99.99 % of its activity is decayed at 12 and 24 h post-injection [10].
The pharmacokinetics of a single dose of [18F]florbetaben 300 MBq of low or high mass dose (≤5 and 50–55 μg) did not differ to a clinically relevant extent between German and Japanese healthy volunteers and the effect of the dose mass was minimal [18]. The effect of age, gender and renal or hepatic impairment on the pharmacokinetics of [18F]florbetaben has not been formally studied [10, 17].
4 Diagnostic Efficacy of [18F]Florbetaben
4.1 Early-Phase Trials
The potential for [18F]florbetaben PET imaging to discriminate AD patients from non-AD patients or healthy volunteers was shown in early-phase clinical studies, using clinical diagnosis as the surrogate standard-of-truth (SoT) [8, 20–23]. For instance, in a phase II trial in which participants received a single intravenous injection of [18F]florbetaben 300 MBq, visual assessment of PET images by blinded experts discriminated patients with probable AD (n = 81) from healthy controls (n = 69) with high sensitivity (80 %) and specificity (91 %) [22]. Similarly, five inexperienced readers who assessed [18F]florbetaben PET images from 116 AD patients and 120 healthy volunteers after undertaking an electronic web-training programme were able to discriminate between the two groups with 69–81 % sensitivity and 82–95 % specificity (presented as an abstract [23]), with good inter-reader agreement (κ 0.81) [11].
Cortical uptake of [18F]florbetaben after a 300 MBq injection was high in a large proportion of patients with a clinical diagnosis of AD (29 of 30) or mild cognitive impairment (MCI) (12 of 20) [20]. Conversely, high cortical uptake was seen in less than one-third of healthy volunteers (5 of 32) or patients with a clinical diagnosis of DLB (2 of 7), vascular dementia (1 of 4) or frontotemporal lobar degeneration (1 of 11) and in no Parkinson’s disease patients (0 of 5) [20]. Uptake was generally extensive in the frontal and posterior cingulate/precuneus cortex and slightly less in the lateral temporal and parietal cortex [8, 20], with relative sparing of primary sensorimotor [8, 20], occipital [8] and mesial temporal [8] cortices. There was no appreciable uptake in the cerebellar cortex [8, 20, 21].
These findings were generally supported by quantitative assessments of [18F]florbetaben uptake, expressing standardized uptake values (SUV) for predefined brain regions as SUV ratios (SUVRs), with the cerebellar cortex as the reference region [8, 20–22]. SUVRs in the cortical regions and striatum of AD patients were generally significantly (p ≤ 0.05) higher than in healthy volunteers [8, 20–22], with the greatest effect size in the lateral temporal cortex [8], frontal cortex [21] or posterior cingulate [22]. Regional SUVRs distinguished AD patients from healthy volunteers with 85 % sensitivity and 91 % specificity [22]. Patients with AD [8, 20–22] or MCI [20] had significantly (p ≤ 0.05) higher neocortical SUVRs (i.e. composite SUVRs, as defined in Table 1) than healthy volunteers.
The neocortical SUVR for [18F]florbetaben 300 MBq significantly (p < 0.0001) correlated with that of [11C]Pittsburgh-compound-B 370 MBq in AD patients (n = 10) and healthy controls (n = 10) [24]. In patients, the neocortical SUVR was significantly (p < 0.001) lower with [18F]florbetaben than with [11C]Pittsburgh-compound-B (2.06 vs. 2.42), although the effect size for distinguishing them from healthy controls did not markedly differ between the tracers (3.0 and 3.3, respectively) [24].
PET imaging using [18F]florbetaben (mean dose 312 MBq) also enabled whole-brain visual assessment of β-amyloid in patients with Down syndrome (n = 39) in a phase II trial, discriminating them from healthy volunteers (n = 70) with 46 % sensitivity and 100 % specificity [25]. [18F]florbetaben uptake significantly (p < 0.0001) increased with age; 90, 53 and 7 % of scans in patients aged ≥50, 45–49 and 40–45 years, respectively, were visually assessed as positive for β-amyloid [26]. There was a significant (p = 0.0007) correlation between visual and quantitative assessments [26].
A prospective, longitudinal study in 45 patients with MCI showed that [18F]florbetaben PET imaging may help to identify patients who will progress to AD [27]. At baseline, 24 patients were positive and 21 were negative for [18F]florbetaben (i.e. neocortical SUVR ≥1.45 and <1.45, respectively). At 2 years, 75 and 9.5 % of the respective groups had progressed to clinically diagnosed AD based on a blinded assessment, yielding positive and negative predictive values of 75.0 and 90.5 %, and predictive accuracy of 82.8 %. After adjusting for both amnestic MCI and hippocampal atrophy (which are both predictive of progression to AD), [18F]florbetaben PET-positivity at baseline still predicted progression to AD at 2 years (hazard ratio 6.9; p = 0.03). Generally similar results were also seen at 4 years, although clinical diagnosis of AD at this timepoint was based on unblinded assessment [27].
Based on dosimetry and dose simulation studies, a [18F]florbetaben dose of 300 MBq was selected for further investigation as it provided optimal PET image quality with the permissible radioactivity dose [17, 28]. In healthy Caucasian adults, the effective dose resulting from the 300 MBq dose was 5.8 mSv (~8 mSv when combined with CT scan) and the estimated mean absorbed radiation dose per MBq was the highest for gall bladder (137 µGy), followed by urinary bladder (70 µGy), liver (39 µGy), upper and lower large intestine (38 and 35 µGy) and small intestine (31 µGy) [10, 14, 29]. In healthy Japanese adults, the effective dose following the 300 MBq dose was 8.08 mSv [29]. The organ and whole body effective doses were substantially higher with [18F]florbetaben than with [11C]Pittsburgh-compound-B because of the difference in radioactive half-lives [30].
4.2 Phase III Trials
This section focuses on the diagnostic efficacy of [18F]florbetaben PET imaging in detecting/excluding neuritic β-amyloid deposits in the brains of adults with varying degrees of cognitive function, as evaluated in a pivotal, nonrandomized, open-label, multinational phase III study, which used postmortem brain histopathology as the SoT [17, 31]. Limited data from a phase III non-interventional pooled read study (presented as an abstract [32]) are briefly discussed (Sect. 4.2.3).
In the pivotal phase III study [31], end-of-life patients (aged 48–98 years) with a clinical diagnosis of AD (n = 139), DLB (n = 5), other dementias (n = 31), no dementia (n = 32, mainly oncological disorders) or cognitively-normal healthy volunteers (aged 22–38 years; n = 11) were included, representing individuals with a low to high probability of cerebral β-amyloid deposition. Exclusion criteria included severe cerebral macrovascular disease or brain tumours and severe cardiovascular instability requiring intensive care surveillance or therapy [31].
All participants received a single intravenous bolus injection of [18F]florbetaben 300 MBq and underwent PET imaging 90–110 min post-injection [31]. Three-dimensional volumetric T1-weighted brain magnetic resonance imaging (MRI) was acquired within the previous 6 months of, or simultaneously with, PET imaging [17, 31]. The PET results were compared against the SoT for six predefined brain regions [regions of interest (ROI)] and for the whole brain [31]. Methods used for the assessment of β-amyloid in PET images and postmortem brains are summarized in Table 1. A typical example of [18F]florbetaben-positive and -negative scans of four brain regions used for the whole brain visual assessment is shown in Fig. 1. The primary study objective was to establish the sensitivity and specificity of the visual assessment of [18F]florbetaben PET images in detecting β-amyloid in the six brain regions compared with the SoT [17].
Typical transverse [18F]florbetaben positron emission tomography slices showing normal and abnormal (negative and positive for β-amyloid plaques, respectively) scans [42]. In the cerebellum, a contrast between white matter (arrows) and grey matter is seen in both positive and negative scans, with tracer uptake also seen in the scalp and posterior sagittal sinus (arrow head). In other regions, the normal scan shows lack of tracer uptake in the outer rim (dashed lines) because of lower uptake in the grey matter, spiculated appearance of the white matter (short arrows in the lateral temporal, frontal and parietal lobes), a hypointense hole (circle) and readily identifiable midline between the parietal lobes (long arrow); the abnormal scan shows smooth appearance of the outer rim of the brain (dashed lines) because of increased tracer uptake in the grey matter, a filled-up hole (circle) in an area posterior to the splenium (short arrow) and thin midline between the parietal lobes (long arrow)
4.2.1 Regional Analysis
[18F]florbetaben PET imaging detected β-amyloid in precisely the same anatomical brain regions confirmed to have β-amyloid plaques by postmortem histopathology, thus providing target validation for [18F]florbetaben [31]. The visual reading of the PET images showed high sensitivity and specificity against the SoT in four of the five assessed cerebral regions known to show β-amyloid pathology in AD brains (such as frontal cortex, anterior cingulate, posterior cingulate/precuneus) (primary endpoint; Table 2) [31]. The sensitivity was 57 % for the hippocampus/parahippocampal gyrus region, which contains grey and white matter in a relatively small volume and is often atrophic, thus rendering it difficult to assess visually or quantitatively in the PET images [31]. The overall sensitivity and specificity of the ROI analysis was 77 and 94 % (Table 2), with good agreement between the three blinded readers for all brain ROIs (κ 0.66) [31].
Quantitative ROI assessments were generally consistent with the visual assessment (Table 2) [31]. Regional SUVRs were significantly (p ≤ 0.01) higher in samples with histopathologically-confirmed β-amyloid than without β-amyloid for the middle frontal gyrus (1.30 vs. 0.95), occipital cortex (1.34 vs. 1.10), anterior cingulate cortex (1.36 vs. 0.96) and posterior cingulate cortex/precuneus (1.49 vs. 1.06), with no significant difference between the samples in the hippocampus/parahippocampal gyrus (0.99 vs. 0.92). SUVRs did not differ significantly between samples with different types of β-amyloid plaques [neuritic and diffuse (co-occurring) vs. diffuse] [31].
4.2.2 Whole-Brain Analysis
The whole-brain visual assessment of [18F]florbetaben PET images (without MRI coregistration) showed good diagnostic efficacy in detecting/excluding cerebral neuritic β-amyloid plaques [31]. The sensitivity and specificity of the assessment was 98 and 89 % against the SoT (Table 2), with good agreement between the three in-person-trained, blinded readers (κ 0.90). The negative and positive predictive values for [18F]florbetaben PET were 96.0 % (95 % CI 88.3–100) and 93.9 % (87.2–100), respectively [31].
Consistent with the visual reading, quantitative whole-brain assessment (i.e. composite SUVR) also detected β-amyloid with high sensitivity and specificity (Table 2). The composite SUVR was significantly (p < 0.0001) higher in brains with histopathologically-confirmed β-amyloid than those without β-amyloid (1.71 vs. 1.24) [31].
4.2.3 Other Analyses
The electronic training tool for the visual assessment of [18F]florbetaben PET images was associated with reliable diagnostic performance in additional clinical trial analyses [14, 32] in which five blinded readers assessed the images after receiving the training. For 82 end-of-life patients participating in the phase III trial [31], the median sensitivity and specificity was 96 and 77 % for visual assessment of brain β-amyloid against the postmortem histopathology assessment [14]. In the pooled read study in which [18F]florbetaben PET images from 460 patients participating in phase I, II and III trials were assessed, there was good agreement between the five readers [κ 0.787 (95 % CI 0.750–0.824)] [32].
5 Tolerability of [18F]Florbetaben
Intravenous bolus injections of [18F]florbetaben used for brain PET imaging were generally well tolerated in patients with AD, other dementias, no dementia and healthy volunteers in clinical trials [8, 10, 14, 20–22, 24, 26, 27]. In an integrated analysis of 872 patients receiving 978 administrations of [18F]florbetaben and 12 participants receiving vehicle only, no serious adverse reactions related to [18F]florbetaben were reported [14]. All adverse reactions reported in [18F]florbetaben recipients were transient in nature and mild to moderate in severity, and the most common (incidence ≥0.5 %) were injection-site pain (3.9 % of patients), injection-site erythema (1.7 %) and injection-site irritation (1.2 %) [14]. There was no overall difference in the tolerability of [18F]florbetaben between participants aged ≥65 or ≥75 years and younger participants [14]. With repeat yearly administration of [18F]florbetaben, the tolerability profile did not differ after the first, second or third administration [10].
In a phase II study of [18F]florbetaben, the following five adverse events (two in patients with AD and three in healthy volunteers) were considered to be related to the radiotracer: fatigue, feeling hot, increased blood pressure, haematoma and shaking of both hands [22].
[18F]florbetaben was not associated with any clinically relevant changes from baseline in laboratory parameters for blood, urine, vital signs and ECG [20, 22, 26, 27].
6 Dosage and Administration of [18F]Florbetaben
In the USA [14] and EU [10], [18F]florbetaben is indicated for PET imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for AD and other causes of cognitive decline. [18F]florbetaben is also approved in South Korea [33]. PET imaging with this tracer should be used as an adjunct to other diagnostic evaluations [10, 14].
The recommended dose of [18F]florbetaben is 300 MBq (maximum 360 MBq and minimum 240 MBq at the time of administration [10]), administered as a slow (1 mL/6 s) intravenous bolus injection in a total volume of up to 10 mL, followed by a saline flush of ~10 mL [10, 14]. No dose adjustment based on age is recommended; in patients with renal or hepatic impairment, the radioactivity to be administered should be carefully considered, since increased radiation exposure is possible in these patients [10]. [18F]florbetaben PET images should be acquired over 15–20 min (starting at 45–130 min [14] or ~90 min [10] after the injection) and should be interpreted only by readers trained in interpreting these images. The images are categorized as β-amyloid negative or β-amyloid positive based on the whole-brain visual assessment (Table 1; Fig. 1) [10, 14].
A positive [18F]florbetaben PET scan by itself does not establish a diagnosis of AD or other cognitive disorders, as β-amyloid neuritic plaques may be present in asymptomatic elderly individuals and some patients with neurodegenerative dementias [10, 14]. A negative scan indicates sparse or no β-amyloid neuritic plaques (which is inconsistent with a neuropathological diagnosis of AD at the time of PET acquisition) and reduces the likelihood that a patient’s cognitive impairment is because of AD. The use of [18F]florbetaben is not established for predicting the development of dementia or any other neurological conditions, or for monitoring responses to therapies [10, 14].
Local prescribing information for [18F]florbetaben should be consulted for detailed information, including radiation safety and dosimetry, PET image acquisition guidelines, interpretation of the images, warnings and precautions, and use in special patient populations.
7 Current Status of [18F]Florbetaben in β-Amyloid PET Imaging in Cognitive Impairment
The International Working Group (IWG) for New Research Criteria for the Diagnosis of Alzheimer’s Disease [2] and the US National Institute on Aging and the Alzheimer’s Association [3, 34–36] include biomarkers of β-amyloid accumulation and/or neuronal injury in the diagnosis of AD, although the relative role of biomarkers varies between these guidelines. Both guidelines cover the full range of AD stages (preclinical, prodromal or MCI due to AD, dementia) and recognize that AD pathophysiology (detected using biomarkers) precedes the onset of clinical symptoms. An IWG diagnosis of AD (any stage) requires the presence of an appropriate clinical phenotype and a pathophysiological biomarker [either increased tracer retention on amyloid PET or decreased β-amyloid1–42 together with increased total or phosphorylated tau (i.e. a high ratio of tau to β-amyloid1–42) in the cerebrospinal fluid] [2].
The Diagnostic and Statistical Manual of Mental Disorders (fifth edition) recognizes major or mild neurocognitive disorder due to AD as clinical entities and considers that β-amyloid PET scans may have diagnostic value for these conditions [37]. The US Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association consensually recommend that the β-amyloid PET imaging is appropriate for individuals with all of the following characteristics: objective evidence of cognitive impairment; possible AD but the diagnosis is uncertain after a comprehensive evaluation by a dementia expert; and, β-amyloid test result would increase the diagnostic certainty and alter the patient management plan [38].
[18F]florbetaben has high affinity and selectivity for β-amyloid plaques (Sect. 2). Following intravenous injection of [18F]florbetaben, the initial uptake of radioactivity in the brain is rapid followed by a steady decline (Sect. 3). In patients with AD, [18F]florbetaben showed extensive cortical uptake in multiple cerebral regions, with no appreciable binding in the cerebellar cortex (Sect. 4.1).
[18F]florbetaben PET imaging showed good diagnostic performance in detecting/excluding neuritic β-amyloid in the brains of adults with various levels of cognitive function (patients with AD, other dementias or no dementia, or cognitively-normal healthy volunteers) in a phase III trial (Sect. 4.2). For the majority of the cerebral regions assessed, visual assessment of the PET images showed high sensitivity and specificity versus postmortem histopathology assessment, with a high level of inter-reader agreement. Furthermore, whole-brain visual analysis without using MRI coregistration (performed to better reflect the proposed use of [18F]florbetaben in clinical settings) showed similar results, with high positive and negative predictive values. As with 18F PET tracers in general, [18F]florbetaben has a short PET scan time of 15–20 min. An electronic training programme for inexperienced [18F]florbetaben PET readers produced reliable results (Sect. 4.2.3). These findings are supported by early phase studies (Sect. 4.1), with a survey conducted as an add-on to a phase II trial indicating that [18F]florbetaben PET imaging is associated with increased physician confidence in initially diagnosing AD, which may positively influence patient management [39]. Intravenous [18F]florbetaben was generally well tolerated in clinical trials, with no radiotracer-related serious adverse reactions (Sect. 5).
When compared directly, [18F]florbetaben and [11C]Pittsburgh-compound-B did not differ markedly in distinguishing patients with AD from healthy volunteers (Sect. 4.1). Two other 18F-labelled PET tracers (florbetapir and flutemetamol) are approved for visualizing brain β-amyloid. Like [18F]florbetaben, these agents have high affinity and selectivity for β-amyloid, are validated by postmortem findings and readily distinguish subjects with high brain β-amyloid burden from those with little or no β-amyloid burden [7, 40]. In the absence of head-to-head trials comparing 18F-labelled PET tracers, a standardized scale (named, Centiloid) for quantifying β-amyloid PET data has been developed, so that uptake of various tracers can be directly compared [41].
In conclusion, [18F]florbetaben is a highly accurate and generally well tolerated tracer for detecting/excluding β-amyloid neuritic plaques in the brains of patients with cognitive impairment because of AD or other causes. The high accuracy is reflected in good inter-reader agreement and high predictive values. Of note, the high negative predictive value indicates that a negative [18F]florbetaben scan can reliably exclude AD pathology. The tracer also has the advantage of a short PET scan time and electronic training for inexperienced visual readers. Well designed trials of [18F]florbetaben in patients with early stages of dementia would be of interest.
Data selection sources:
Relevant medical literature (including published and unpublished data) on [18F]florbetaben was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 8 June 2015], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Florbetaben, NeuraCeq, positron emission tomography, PET, imaging, tracer, scan, amyloid, 18F, F18
Study selection: Studies in patients with cognitive impairment because of Alzheimer’s disease and other causes who received [18F]florbetaben positron emission tomography scan. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–6.
Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–29.
Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–62.
Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21(1):117–28.
Rowe CC, Villemagne VL. Brain amyloid imaging. J Nucl Med. 2011;52(11):1733–40.
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19.
Mathis CA, Mason NS, Lopresti BJ, et al. Development of positron emission tomography beta-amyloid plaque imaging agents. Semin Nucl Med. 2012;42(6):423–32.
Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7(2):129–35.
Zhang W, Oya S, Kung MP, et al. F-18 polyethyleneglycol stilbenes as PET imaging agents targeting Aβ aggregates in the brain. Nucl Med Biol. 2005;32(8):799–809.
European Medicines Agency. Neuraceq 300 MBq/mL solution for injection: summary of product characteristics. 2014. http://www.ema.europa.eu. Accessed 13 Mar 2015.
Sabri O, Seibyl J, Rowe C, et al. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3(1):13–26.
Ni R, Gillberg PG, Bergfors A, et al. Amyloid tracers detect multiple binding sites in Alzheimer’s disease brain tissue. Brain. 2013;136(7):2217–27.
Fodero-Tavoletti MT, Brockschnieder D, Villemagne VL, et al. In vitro characterization of [18F]-florbetaben, an Aβ imaging radiotracer. Nucl Med Biol. 2012;39(7):1042–8.
Piramal Imaging. Neuraceq (florbetaben F 18 injection): US prescribing information. 2014. http://www.neuraceq.com. Accessed 13 Mar 2015.
Rominger A, Brendel M, Burgold S, et al. Longitudinal assessment of cerebral β-amyloid deposition in mice overexpressing Swedish mutant β-amyloid precursor protein using 18F-florbetaben PET. J Nucl Med. 2013;54(7):1127–34.
Brendel M, Jaworska A, Herms J, et al. Amyloid-PET predicts inhibition of de novo plaque formation upon chronic gamma-secretase modulator treatment. Mol Psychiatry. 2015;. doi:10.1038/mp.2015.74.
European Medicines Agency. Neuraceq [florbetaben (18F)]: assessment report for an initial marketing authorisation application. 2013. http://www.ema.europa.eu. Accessed 13 Mar 2015.
Senda M, Sasaki M, Yamane T, et al. Ethnic comparison of pharmacokinetics of 18F-florbetaben, a PET tracer for beta-amyloid imaging, in healthy Caucasian and Japanese subjects. Eur J Nucl Med Mol Imaging. 2015;42(1):89–96.
Patt M, Schildan A, Barthel H, et al. Metabolite analysis of [18F]florbetaben (BAY 94-9172) in human subjects: a substudy within a proof of mechanism clinical trial. J Radioanal Nucl Chem. 2010;284(3):557–62.
Villemagne VL, Ong K, Mulligan RS, et al. Amyloid imaging with 18F-florbetaben in Alzheimer disease and other dementias. J Nucl Med. 2011;52(8):1210–7.
Barthel H, Luthardt J, Becker G, et al. Individualized quantification of brain β-amyloid burden: results of a proof of mechanism phase 0 florbetaben PET trial in patients with Alzheimer’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2011;38(9):1702–14.
Barthel H, Gertz HJ, Dresel S, et al. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10(5):424–35.
Sabri O, Seibyl J, Ishihara K, et al. Close to clinical routine phase 2 trial on florbetaben PET imaging of β-amyloid (Aβ) in Alzheimer’s disease (AD) [abstract no. 299]. J Nucl Med. 2013;54(Suppl 2).
Villemagne VL, Mulligan RS, Pejoska S, et al. Comparison of 11C-PiB and 18F-florbetaben for Aβ imaging in ageing and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39(6):983–9.
US National Institutes of Health. ClinicalTrials.gov. 2014. http://www.clinicaltrials.gov/. Accessed 13 Mar 2015.
Jennings D, Seibyl J, Sabbagh M, et al. Age dependence of brain β-amyloid deposition in Down syndrome: an [18F]florbetaben PET study. Neurology. 2015;84(5):500–7.
Ong KT, Villemagne VL, Bahar-Fuchs A, et al. Aβ imaging with 18F-florbetaben in prodromal Alzheimer’s disease: a prospective outcome study. J Neurol Neurosurg Psychiatry. 2015;86(4):431–6.
US Food and Drug Administration. Neuraceq (florbetaben F 18 injection): clinical pharmacology and biopharmaceutics review(s). 2014. http://www.fda.gov/. Accessed 13 Mar 2015.
Sattler B, Barthel H, Patt M, et al. Radiation exposure by [F18]florbetaben, a new PET tracer for detection of cerebral β-amyloid in Caucasian and Asian healthy volunteers [abstract no. OP432]. Eur J Nucl Med Mol Imaging. 2010;37(Suppl 2):S274.
O’Keefe GJ, Saunder TH, Ng S, et al. Radiation dosimetry of β-amyloid tracers 11C-PiB and 18F-BAY94-9172. J Nucl Med. 2009;50(2):309–15.
Sabri O, Sabbagh MN, Seibyl J, et al. Florbetaben PET imaging to detect amyloid plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 2015. doi:10.1016/j.jalz.2015.02.004.
Seibyl J, Barthel H, Stephens A, et al. Reliability, reproducibility and efficacy of the 18F florbetaben β-amyloid PET scan visual assessment method as trained via a computer-based instructional tool [abstract no. 300]. J Nucl Med. 2013;54(Suppl 2).
Piramal Imaging SA. Piramal Imaging SA and Ci-Co Healthcare announce commercial approval of Neuraceq™ in Korea [media release]. 2 Jun 2015. http://www.piramal.com.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92.
Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9.
American Psychiatric Association. Neurocognitive disorders. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9(1):e-1–16.
Schipke CG, Peters O, Heuser I, et al. Impact of beta-amyloid-specific florbetaben PET imaging on confidence in early diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;33(6):416–22.
Saidlitz P, Voisin T, Vellas B, et al. Amyloid imaging in Alzheimer’s disease: a literature review. J Nutr Health Aging. 2014;18(7):723–40.
Klunk WE, Koeppe RA, Price JC, et al. The Centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11(1):1–15 e1–4.
Data on file, Piramal Imaging. 2015.
Disclosure
The preparation of this review was not supported by any external funding. Yahiya Y. Syed and Emma Deeks are salaried employees of Adis/Springer. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author(s) on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: H. Barthel, Department of Nuclear Medicine, Leipzig University Hospital, Leipzig, Germany; K. Ong, Department of Nuclear Medicine, Austin Hospital, Melbourne, Australia; R. Vandenberghe, Laboratory for Cognitive Neurology, Catholic University Leuven, Leuven, Belgium.
Rights and permissions
About this article
Cite this article
Syed, Y.Y., Deeks, E. [18F]Florbetaben: A Review in β-Amyloid PET Imaging in Cognitive Impairment. CNS Drugs 29, 605–613 (2015). https://doi.org/10.1007/s40263-015-0258-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-015-0258-7