Abstract
Idelalisib is a potent and selective phosphatidylinositol 3-kinase-δ inhibitor, which is a first-in-class agent to be approved for the treatment of relapsed chronic lymphocytic leukaemia, follicular B cell non-Hodgkin’s lymphoma and small lymphocytic lymphoma. In dose-ranging studies, idelalisib exposure increased in a less than dose-proportional manner, likely because of solubility-limited absorption. The approved starting dose of 150 mg twice daily was supported by extensive exposure–response evaluations, with dose reduction to 100 mg twice daily being allowed for specific toxicities. Idelalisib may be administered without regard to food on the basis of the absence of clinically relevant food effects, and was accordingly dosed in primary efficacy/safety studies. Idelalisib is metabolized primarily via aldehyde oxidase (AO) and, to a lesser extent, via cytochrome P450 (CYP) 3A. Coadministration with the strong CYP3A inhibitor ketoconazole 400 mg once daily resulted in a ~79 % increase in the idelalisib area under the plasma concentration–time curve (AUC). Administration with the potent inducer rifampin resulted in a 75 % decrease in idelalisib exposure (AUC) and, as such, coadministration with strong inducers should be avoided. GS-563117 is an inactive primary circulating metabolite of idelalisib formed mainly via AO. Unlike idelalisib, GS-563117 is a mechanism-based inhibitor of CYP3A. Accordingly, idelalisib 150 mg twice-daily dosing increases the midazolam AUC 5.4-fold. Clinically, idelalisib is not an inhibitor of the transporters P-glycoprotein, breast cancer resistance protein, organic anion–transporting polypeptide (OATP) 1B1 or OAPT1B3. In a population pharmacokinetic model, no meaningful impact on idelalisib pharmacokinetics was noted for any of the covariates tested. Idelalisib exposure was ~60 % higher with moderate/severe hepatic impairment; no relevant changes were observed with severe renal impairment. This article reviews a comprehensive pharmacology programme, including drug–drug interaction studies and mechanistic and special population studies, which has allowed a thorough understanding of idelalisib clinical pharmacokinetics and their impact on clinical safety and efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Idelalisib, a selective phosphatidylinositol 3-kinase-δ inhibitor, demonstrates substantial efficacy in patients with indolent non-Hodgkin’s lymphoma or chronic lymphocytic leukaemia following 150 mg twice-daily oral administration. |
Idelalisib dosing in cancer patients is supported by extensive pharmacokinetic–pharmacodynamic (efficacy and safety) assessments. |
Idelalisib has well-characterized pharmacokinetics with low inter-subject variability, also evaluated in subjects with organ impairment. |
Idelalisib is metabolized primarily via aldehyde oxidase and, to a lesser extent, via cytochrome P450 (CYP) 3A. Coadministration with strong CYP3A inducers could lead to lower idelalisib exposure. |
The idelalisib metabolite GS-563117 inhibits CYP3A and may increase systemic exposure of co-administered CYP3A substrates. |
Idelalisib has clear dosing recommendations with regard to drug–drug interactions. |
1 Introduction
Phosphatidylinositol 3-kinases (PI3Ks) are enzymes that regulate several cellular functions [1–4]. Activation of PI3K generates phospholipid second messengers at the cell membrane; these recruit and activate multiple intracellular enzymes, which are regulators of cell proliferation, survival and motility. PI3K signalling is mediated by four catalytic isoforms of the p110 subunit of the enzyme—α, β, γ and δ [4–6].
PI3Kδ is critical for multiple signalling pathways that are hyperactive in B cell malignancies. Inhibition of PI3Kδ modulates B cell receptor signalling, as well as signalling through cytokine and chemokine receptors and integrin-αβ1. These signalling pathways act via downstream enzymes (most importantly, serine/threonine protein kinase B [Akt]) to regulate proliferation, apoptosis, motility, homing and retention of malignant B cells in lymphoid tissues and bone marrow compartments [7–10].
Idelalisib (formerly CAL-101) is a PI3Kδ inhibitor and a highly selective competitive inhibitor of adenosine-5′-triphosphate (ATP) binding to the catalytic subunit of PI3Kδ. In lymphoid cell lines derived from B cell malignancies and primary tumour samples from patients, idelalisib blocked PI3Kδ–Akt signalling, resulting in inhibition of proliferation and induction of apoptosis [11–13]. Recently, idelalisib was approved (as Zydelig®) for treatment of relapsed chronic lymphocytic leukaemia (CLL) in combination with rituximab, for relapsed follicular B cell non-Hodgkin’s lymphoma and for relapsed small lymphocytic lymphoma (SLL). Clinical efficacy has been demonstrated using idelalisib as monotherapy for treatment of previously treated indolent non-Hodgkin’s lymphoma (iNHL), where the response rate was 57 % (71 of 125 patients) and the median duration of response (DOR) was 12.5 months [14]. Additionally, in a randomized, placebo-controlled, phase 3 study, patients with relapsed CLL who received idelalisib as a combination therapy with rituximab had an improved overall response rate (ORR; 81 versus 13 %) and improved overall survival at 12 months (92 versus 80 %), compared with patients who received rituximab alone [15]. Idelalisib has also demonstrated efficacy in CLL subpopulations with adverse prognostic factors, including those with del(17p) [16]. In a long-term follow-up phase 1 study of CLL patients with relapsed or refractory disease and various adverse prognostic factors, including del(17p) and/or TP53 mutation (30 %), immunoglobulin heavy chain variable (IGHV) unmutated status (82 %) or NOTCH1 mutation (28 %), idelalisib in combination with chemo- and immuno-chemotherapies demonstrated potent activity, with a median time to response of 1.9 months, and strong efficacy, with an ORR of 82 % and a Kaplan–Meier estimate of progression-free survival (PFS) of 57 % (95 % confidence interval [CI] 43–72) at 24 months [17]. Ongoing clinical studies of idelalisib include evaluation of idelalisib plus ofatumumab (versus ofatumumab alone) and idelalisib plus bendamustine and rituximab (versus bendamustine plus rituximab) in previously treated CLL patients, and idelalisib plus rituximab (versus rituximab alone) and idelalisib plus bendamustine and rituximab (versus bendamustine plus rituximab) in previously treated iNHL patients. This review focuses on the clinical pharmacology of idelalisib, including its drug interaction profile, pharmacokinetics in special populations, exposure–efficacy and exposure–safety relationships, and an evaluation of covariates affecting pharmacokinetics and pharmacodynamics.
2 Preclinical Pharmacology
2.1 Chemical and Physical Properties
Idelalisib (formerly GS-1101, CAL-101; chemical name: 5-fluoro-3-phenyl-2-[(1S)-1-(9H purin-6-ylamino)propyl]quinazolin-4(3H)-one) is a low molecular weight (415.4 g/mol) agent with a molecular formula of C22H18FN7O and a chemical structure shown in Fig. 1.
Structural modelling evaluation has demonstrated that idelalisib, a propeller-shaped inhibitor, binds to the ATP-binding pocket of PI3Kδ, consistent with findings from biochemical studies; enzyme-binding studies have shown that idelalisib is a reversible inhibitor. When compared with the binding of ATP, the purine group of idelalisib forms hydrogen bonds to the hinge region. In crystal structure evaluation of PI3Kδ in complex with idelalisib, the electron density of idelalisib showed that there was no covalent bond between the inhibitor and the protein. The reversibility of idelalisib binding was also demonstrated using surface plasmon resonance studies, with saturable, dose-response interaction with p110δ, a very fast on-rate and a moderate off-rate. The response signal in these studies returned to the baseline level during the dissociation phase, indicating complete dissociation, in contrast with the control agent (wortmannin), which was irreversibly bound to p110δ [18].
Idelalisib (Zydelig®) is marketed as an immediate-release tablet formulation for oral administration, which incorporates an anhydrous, crystalline form of idelalisib. Idelalisib has three ionizable moieties (with acid dissociation constant [pKa] values of 1.6, 3.4 and 9.8), and its solubility is highest at acidic and basic extremes over the pH range of 1–12. Because idelalisib exhibits low water solubility and moderate to high permeability, it is considered a Biopharmaceutics Classification System (BCS) class 2 compound.
2.2 Pharmacodynamic Properties
PI3Kδ is critical for multiple signalling pathways that are hyperactive in B cell malignancies. Inhibition of PI3Kδ modulates B cell receptor–mediated signalling and cytokine, chemokine and integrin receptor-mediated signalling. These pathways, using downstream enzymes (notably Akt), regulate proliferation, apoptosis, motility, homing and retention of malignant B cells in lymphoid tissues and bone marrow. Thus, by inhibiting PI3Kδ-mediated signalling, idelalisib inhibits proliferation, survival, homing, motility and retention in the tumour microenvironment in various B cell malignancies [19–21]. Biochemical enzyme assays evaluating the potency and selectivity of idelalisib indicate that the half-maximal inhibitory concentration [IC50] (19 nM) of idelalisib for PI3Kδ is substantially lower than those for PI3Kα (8600 nM), PI3Kβ (4000 nM) and PI3Kγ (2100 nM) [13]; additionally, no significant activity was seen with various other kinases or receptors, including G-protein-coupled receptors, ion channels, receptor tyrosine kinases, steroid receptors and transporters [22]. In PI3Kδ-dependent cell-based assays, the idelalisib half-maximal effective concentration [EC50] was 6 nM in purified human B lymphocytes; in a human whole-blood PI3Kδ-dependent assay, the EC50 and EC90 were 39 and 301 nM, respectively. In vitro experiments in cell lines derived from B cell malignancies and in primary tumour samples of follicular B cell non-Hodgkin’s lymphoma, mantle cell lymphoma (MCL) and CLL from adult patients showed that idelalisib causes a dose-dependent reduction in Akt phosphorylation driven by B cell growth, survival and chemotaxis-promoting signals. PI3Kδ inhibition by idelalisib resulted in inhibition of proliferation, induction of apoptosis in CLL cells and B cell lymphoma cell lines, and inhibition of chemotaxis towards chemokine (C-X-C motif) ligand (CXCL) 12 and CXCL13 chemokines, demonstrating the potential for idelalisib to suppress growth and survival of malignant cells [22, 23].
2.3 Preclinical Pharmacokinetics
Single-dose escalation of idelalisib administered orally to rats resulted in a less than dose-proportional increase in the plasma concentration. Following oral administration of [14C]idelalisib to rats and dogs, radioactivity was widely distributed and declined steadily, with most tissues having undetectable levels by 72 h post-dose. In bile duct–cannulated rats and dogs, ≥69 % of radioactivity was recovered in bile and urine, indicating high absorption in vivo. Systemic clearance after oral administration (CL/F) was moderate to high, and the volume of distribution was greater than the volume of total body water (data on file).
3 Clinical Pharmacology
3.1 Single-Dose and Steady-State Pharmacokinetics
Following single oral doses of 17, 50, 125, 250 and 400 mg administered to healthy subjects under fasted conditions, the mean idelalisib (percentage coefficient of variation [%CV]) maximum plasma concentration (C max) values were 348 (36), 762 (40), 1460 (21), 2390 (38) and 3350 (32) ng/mL, respectively, achieved 1.0–1.3 h post-dose, and the corresponding area under the concentration–time curve (AUC) extrapolated to infinity (AUC∞) values were 1090 (47), 2880 (39), 5000 (30), 13,000 (18) and 17,700 (21) ng·h/mL, respectively [24]. The idelalisib AUC∞ and C max were less than dose proportional, with approximately 16- and 10-fold increases, respectively, over a 24-fold dose range of 17–400 mg. The mean (%CV) AUC∞ and C max of GS-563117, the major metabolite, were 23,900 (55) ng·h/mL and 2070 (37) ng/mL, respectively, following administration of idelalisib 150 mg, and slightly less than dose proportional (AUC∞ ~2.4 times higher and C max ~1.8 times higher for the 400 mg versus 150 mg idelalisib doses [2.6-fold]). Upon multiple-dose fasted administration (twice daily for 7 days) at doses of 50, 100 and 200 mg in healthy subjects, the idelalisib steady-state AUC during the dosage interval (AUCτ) values (3480 [31], 7830 [20] and 9120 [36] ng·h/mL, respectively) and C max values (765 [29], 1850 [16] and 1790 [34] ng/mL, respectively) were less than dose proportional, with approximate 2.6- and 2.3-fold increases, respectively, over a 4-fold dose range, consistent with findings from the single-dose evaluation.
In subjects with haematological malignancies, steady-state idelalisib exposure was evaluated following multiple-dose administration of idelalisib (twice daily or once daily) over a dose range of 50–350 mg, based on population pharmacokinetic modelling. Consistent with data in healthy subjects, the multiple-dose C max, trough plasma concentration (C trough) and AUCτ values increased in a less than dose-proportional manner, with only modest increases in exposure observed above the dose level of 150 mg twice daily. Of note, compared with 150 mg twice daily, dosing at 350 mg twice daily provided approximately 54, 48 and 54 % higher values for the idelalisib C max, C trough and AUCτ, respectively. The small, incremental differences in idelalisib exposure at doses substantially greater than 150 mg twice daily were consistent with the comparable clinical efficacy data (lymph node response [LNR]) at these doses (150–350 mg twice daily) in the dose-ranging study. Overall, these findings, along with the exposure–response analyses, were helpful in the selection of the 150 mg twice-daily dose for subsequent clinical evaluation in iNHL and CLL [25].
Population pharmacokinetic analyses following multiple dose administration of idelalisib in subjects with haematological malignancies were performed using intensive and/or sparse sampling [26]. The final population model that best described idelalisib pharmacokinetics was a 2-compartment model with a first-order absorption rate constant and absorption lag time. The typical CL/F was estimated to be 14.9 L/h, the typical distributional inter-compartmental clearance (Q/F) was 11.8 L/h, the apparent volume of distribution in the central compartment (V c/F) was 22.7 L and the apparent volume of distribution in the peripheral compartment (V p/F) was 73.0 L. Idelalisib was absorbed at a typical rate of 0.48 h−1 with a lag time of 0.25 h. Idelalisib 150 mg twice daily provided typical steady-state C max, AUCτ and C trough values of 1770 ng/mL, 10,100 ng·h/mL and 318 ng/mL, respectively, and typical time to reach C max (t max) and elimination half-life (t ½) values of 1.5 and 8.2 h, respectively. A nonlinear relationship was observed between dose and relative bioavailability, consistent with the dose-ranging data in healthy subjects described earlier. The pharmacokinetic parameters of idelalisib are presented in Table 1; the final model characterized the idelalisib data well, with reasonable precision for estimated parameters as well as descriptions of inter-subject and intra-subject variabilities.
The plasma pharmacokinetics of the major metabolite GS-563117 were described by a two-compartment model with first-order absorption, first-order elimination from the central compartment and a lag time. The typical systemic clearance was 4.4 L/h, the distributional clearance was 1.3 L/h, the volume of the central compartment was 7.5 L, the volume of the peripheral compartment was 16.1 L/h and the typical absorption rate constant was 0.08 h−1. The estimated population elimination half-life for a typical patient was 11.6 h.
3.2 Absorption
Following oral administration, idelalisib was rapidly absorbed, reaching C max at roughly 0.5–1.5 h under fasted conditions, and expectedly displayed slower absorption with food (reaching C max 2.0–4.0 h post-dose) [27]. Idelalisib exposure was approximately dose proportional at doses of up to 100 mg and, as noted earlier, less than dose proportional at doses above 150 mg, likely driven by solubility-limited dissolution [24]. Idelalisib displayed high forward permeability (mean apparent permeability coefficient [P app] 4.6 × 10−6–17 × 10−6 cm/s; apical to basolateral [AB]) across human Caco-2 cell monolayers. The reverse permeability (basolateral to apical [BA]) exceeded the forward permeability, resulting in a BA-to-AB ratio of 12.2 (at 1 µM), indicating secretory transport. The permeability increased in a concentration-dependent manner, resulting in a lower efflux ratio at higher concentrations (2.8 at 50 μM), likely because of saturation of efflux (secretory) transport (data on file). On the basis of the anticipated local gastrointestinal concentration following a therapeutic oral dose, after accounting for solubility (~480 μM in simulated intestinal fluid at pH 6.5), the observed in vitro secretory transport is unlikely to be clinically relevant. This is consistent with clinical evidence, including the lack of a greater than dose-proportional increase in systemic exposure over the dose range evaluated and a minimal/modest effect of a P-glycoprotein (P-gp) inhibitor such as ketoconazole on the idelalisib C max (~26 % increase). The plasma exposure of idelalisib was unaffected by acid-reducing agents, as detailed further in the Sect.‘3.7’.
Following single-dose administration with a high-fat meal (evaluated in order to maximally perturb absorption with food) versus fasted conditions, peak plasma concentrations were delayed (4 versus 1–2 h). However, no relevant changes in C max and a slight increase in AUC∞ (36 %) were noted [24]. The dose-ranging study was administered under fasted conditions, and subsequent clinical studies in patients entailed dosing without any restrictions regarding food. Overall, the small changes observed in idelalisib exposure with food are not considered to be clinically relevant. Accordingly, idelalisib may be administered without regard to food.
3.3 Distribution
The plasma protein binding of idelalisib and GS-563117 in humans was high and approximately 93–94 and 99 %, respectively, on the basis of equilibrium dialysis experiments. The blood-to-plasma ratio of radioactivity after a single oral dose of idelalisib 150 mg (containing tracer amounts of [14C]idelalisib) was approximately 0.52, indicating that idelalisib and its metabolites were predominantly distributed to plasma relative to the cellular components of the blood [27]. These results were consistent with those from an in vitro blood distribution study (whole-blood-to-plasma ratio 0.68 for idelalisib; data on file). The distribution of idelalisib into compartments other than plasma (e.g. cerebrospinal fluid, genital tract secretions, breast milk) has not been clinically evaluated.
3.4 Metabolism and Elimination
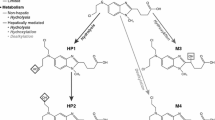
The biotransformation of idelalisib was primarily via oxidation by aldehyde oxidase (AO) to its major circulating plasma metabolite, GS-563117. Other metabolic pathways involved to a lesser extent include oxidation by cytochrome P450 (CYP) 3A and glucuronidation by uridine 5′-diphospho-glucuronosyltransferase (UGT) 1A4 [28]. Oxidative defluorination, methylation, dealkylation and glutathione conjugation were also noted in a human mass balance study [27]. Systemic exposure of GS-563117 is 2- to 4-fold higher than that of idelalisib; however, GS-563117 is inactive against PI3Kδ (data on file). The elimination of GS-563117 is mediated via CYP3A, with a smaller component of glucuronidation, mediated via UGT1A4 (data on file).
Following single-dose oral administration of [14C]idelalisib in healthy subjects [27], recovery of the radioactive dose was mainly from the faeces (78.0 %), compared with urine (14.4 %), suggesting primarily hepatobiliary excretion of idelalisib. The combined faecal and urinary recovery accounted for 92.4 ± 1.67 % of the entire administered radioactive dose. Quantifiable levels of radioactivity in whole blood and plasma were observed for up to 48 and 72 h, respectively; in plasma, the only two circulating species were idelalisib (38 %) and GS-563117 (62 %).
In urine, total radioactivity consisted primarily of idelalisib (23 %) and GS-563117 (49 %), and several trace metabolites. In faeces, radioactivity was accounted for mainly by idelalisib (~12 %, representing potentially a combination of unabsorbed and/or biliary-secreted drug) and GS-563117 (44 %), and other trace oxidation products.
3.5 Influence of Demographic Factors and Patient/Disease Characteristics
The effect of demographic or disease-relevant factors on idelalisib pharmacokinetics and inter-individual variability was evaluated on the basis of population pharmacokinetics from healthy subjects and patient studies using a stepwise forward addition and backward deletion model selection strategy. The correlation between the pharmacokinetic parameters and the covariates was initially explored graphically, followed by linear regression (continuous covariates) and analysis of variance (ANOVA) testing (categorical covariates) using sequential addition to determine those that provided a significant, scientifically plausible improvement in the objective function. The ensuing stepwise forward addition process identified and added the most significant covariate first to the model, with remaining covariates added to evaluate potential further model improvement (based on a statistically significant decrease in the minimum objective function). All significant covariates were included to form a full model. The last step was a stepwise backward elimination process, starting with the full model, and removal of each covariate individually; the covariate with the smallest change in the minimum objective function was removed from the model, and the process was repeated. All covariates retained in the final model were significant. The covariates evaluated included age, sex, race, health status (cancer patient versus healthy subject), body weight, type of cancer (iNHL, CLL, other), background treatment (rituximab usage), treatment status (frontline versus relapsed or refractory), baseline creatinine clearance (CLCR), alanine aminotransferase (ALT) and aspartate transaminase (AST). On the basis of this extensive evaluation, no relevant relationship with idelalisib pharmacokinetics was noted for most covariates.
On the basis of population pharmacokinetic analyses (N = 736), 55 % of subjects (N = 406) were aged <65 years, 33 % of subjects (N = 239) were aged 65–75 years and 12 % of subjects (N = 91) were aged >75 years. Age did not have an effect on idelalisib exposure among subjects in these three age groups. In addition, age did not show an effect on GS-563117 exposure. In the pooled phase 1 and phase 2 population, ~30 % of subjects were female and 87 % of subjects were Caucasian; sex or race did not have any relevant effect on idelalisib pharmacokinetics [29]. Similarly, idelalisib pharmacokinetics were similar across subjects with iNHL, CLL or other types of haematological cancers, and were unaffected by rituximab co-use or line of treatment (early versus relapsed or refractory). A weak relationship was observed between body weight and idelalisib clearance: relative to the median body weight, the 5th to 95th percentiles were ≤10 % different for idelalisib clearance. These differences are not considered to be clinically relevant.
3.6 Pharmacokinetics in Special Populations
3.6.1 Hepatic Impairment
The pharmacokinetics of idelalisib and GS-563117 were evaluated in noncancer subjects with moderate (Child–Pugh–Turcotte [CPT] class B) or severe (CPT class C) hepatic impairment following a single oral dose of idelalisib 150 mg; a cohort of healthy control subjects with normal hepatic function, matched for age, sex, and body mass index (BMI), were also enrolled and idelalisib/GS-563117 pharmacokinetics evaluated [30].
The idelalisib C max was generally comparable in subjects with moderate or severe hepatic impairment relative to matched healthy control subjects, while the mean AUC∞ was higher (by 58–60 %). GS-563117 exposure (AUC∞ and C max) was lower in subjects with moderate or severe hepatic impairment relative to matched healthy control subjects, suggesting lower formation of GS-563117 potentially due to lesser expression of AO (an enzyme highly expressed in the liver and primarily responsible for idelalisib metabolism to GS-563117) in the setting of hepatic impairment [31, 32].
The observed differences were not considered to be clinically relevant, on the basis of the overall exposure–response relationships for idelalisib and GS-563117. Exploratory analyses indicated no clinically relevant correlations between idelalisib or GS-563117 exposure versus CPT scores for subjects with moderate or severe hepatic impairment. Overall, a single oral dose of idelalisib at 150 mg was well tolerated in subjects with moderate or severe hepatic impairment and in healthy control subjects, with headache being the most commonly reported adverse event (AE), which resolved without treatment. The majority of AEs and laboratory abnormalities were grade 1 or 2. No clinically meaningful changes in vital signs or safety electrocardiograms were observed during the study. Severe hepatotoxicity, defined as any elevation in AST or ALT exceeding five times the upper limit of normal, was observed in 14 % of cancer patients receiving chronic idelalisib dosing; such findings usually occurred within the first 12 weeks of treatment and were generally asymptomatic and reversible with dose interruption. Because chronic dosing data are not available in patients with malignancy and severe hepatic impairment at baseline, it is particularly important to monitor those patients closely for any signs of AEs. Dose modifications for idelalisib are not considered necessary.
3.6.2 Renal Impairment
The pharmacokinetics of idelalisib and GS-563117 were evaluated in noncancer subjects with severe renal impairment (CLCR 15–29 mL/min) who were not on dialysis, following a single oral dose of idelalisib 150 mg. As with the hepatic impairment study, a dedicated cohort of healthy control subjects, matched for age, sex and BMI with normal renal function (CLCR ≥80 mL/min) were enrolled and idelalisib/GS-563117 pharmacokinetics evaluated [33].
In comparison with the healthy matched controls, idelalisib exposure was slightly increased (27 % for AUC), while C max was unaffected. Similar corresponding changes were observed for GS-563117. These results were consistent with the finding that renal excretion of idelalisib and GS-563117 was a minor pathway for the elimination of both drugs (~15 % of the administered dose was eliminated in urine, primarily as GS-563117 and idelalisib in the mass balance study). The minor changes observed in idelalisib and GS-563117 exposure in subjects with severe renal impairment were not considered to be clinically meaningful, supported by the overall exposure–response relationships for both agents. These results were also consistent with the idelalisib population pharmacokinetic analyses, which did not identify baseline CLCR as a clinically relevant covariate. Accordingly, dose adjustments for idelalisib are not considered necessary in subjects with severe (or moderate or mild) renal impairment.
3.7 Drug Interactions
The elimination of idelalisib is predominantly mediated by hepatic metabolism, and the role of renal excretion is minor. The oxidative metabolism of idelalisib is primarily mediated via AO and, to a lesser extent, by CYP3A to form GS-563117, a metabolite that has no activity against PI3Kδ. Following oral administration of idelalisib, the only moieties detectable in plasma were idelalisib and GS-563117. On the basis of in vitro assessment (data on file) at clinically relevant concentrations, neither idelalisib nor GS-563117 are inhibitors of the metabolizing enzymes CYP1A2, CYP2B6, CYP2C9, CYP2C19 or CYP2D6 (IC50 >60 µM) or of the uptake of the secretory transporters breast cancer resistance protein (BCRP), organic anion transporter 1 (OAT1), OAT3 or organic cation transporter 2 (OCT2). In contrast to idelalisib, GS-563117 is a time-dependent inhibitor of CYP3A (IC50 5.1 µM; inhibition constant [K i] 0.2 µM; rate of enzyme inactivation [k inact] 0.033 min−1). Idelalisib is an inhibitor of P-gp (IC50 7.7 µM), organic anion–transporting polypeptide (OATP) 1B1 (IC50 10.1 µM) and OATP1B3 (IC50 7.0 µM). GS-563117 is an inhibitor of OATP1B1 (IC50 26.1 µM) and OATP1B3 (IC50 36.1 µM). Given the involvement of CYP3A in idelalisib metabolism, the potential for a strong inducer or inhibitor of CYP3A to affect idelalisib pharmacokinetics was evaluated. Additionally, the pharmacokinetics of sensitive substrates of CYP3A, P-gp, OATP1B1 or OATP1B3 upon coadministation with idelalisib were also evaluated. Idelalisib pharmacokinetics following a single oral dose were evaluated with or without coadministration of ketoconazole (a potent CYP3A inhibitor) 400 mg once daily for 4 days in healthy subjects [24]. The idelalisib C max and AUC∞ increased 26 and 79 %, respectively, after coadministration of idelalisib with ketoconazole for 4 days in comparison with dosing of idelalisib alone. The modest/moderate increase in idelalisib exposure caused by potent CYP3A inhibition is not considered to be of clinical relevance, on the basis of overall exposure–response evaluation and in accordance with in vitro data demonstrating that idelalisib is not a sensitive CYP3A substrate. Patients taking CYP3A inhibitors should be monitored for signs of idelalisib toxicity. Another clinical study evaluated the effect of idelalisib and/or GS-563117 as a perpetrator of interactions, specifically CYP3A (using the probe substrate midazolam) and the drug transporters P-gp (using the probe substrate digoxin), OATP1B1 and OATP1B3 (using the probe substrate rosuvastatin), and idelalisib and GS-563117 as victims of induction (using the probe agent rifampin) [34]. Multiple dosing of idelalisib was performed to achieve steady-state/clinically relevant exposure of idelalisib and GS-563117 to assess the impacts on digoxin, rosuvastatin and midazolam pharmacokinetics.
The pharmacokinetics of digoxin, an agent routinely used in clinical drug–drug interaction studies as a probe P-gp substrate [35], was unaffected upon coadministration of digoxin with idelalisib 150 mg twice daily, suggesting no clinically relevant inhibition of P-gp by idelalisib. Accordingly, no dose adjustment is necessary when idelalisib is coadministered with substrates of P-gp. As expected, idelalisib pharmacokinetics were not impacted by digoxin. Coadministration of rosuvastatin with idelalisib resulted in exposure (AUC and C max) similar to that of rosuvastatin, a sensitive BCRP, OATP1B1 and OATP1B3 substrate, indicating the lack of clinically relevant inhibition of these transporters by idelalisib and GS-563117. Thus, no dose adjustment is needed when substrates of BCRP or OATP1B1/OATP1B3 are coadministered with idelalisib.
In contrast to digoxin and rosuvastatin, coadministration of midazolam with idelalisib resulted in substantial increases in midazolam exposure relative to midazolam dosing alone, with increases of 138, 355 and 437 % for the midazolam C max, AUC from time zero to the time of the last measurable concentration (AUClast) and AUC∞, respectively. Correspondingly, 1′-OH-midazolam exposure was decreased. These findings were consistent with the mechanism-based inhibition of CYP3A by GS-563117, given that idelalisib itself is not a clinically relevant inhibitor of CYP3A (IC50 ≥44 µM). The observed changes in the midazolam C max, AUC and plasma half-life suggest changes in the bioavailability (increased) and systemic clearance (decreased) of midazolam when it was coadministered with idelalisib, driven by GS-563117. Exposure to concomitant medications metabolized via CYP3A is expected to increase upon coadministration with idelalisib.
Because of the lack of relevant in vitro inhibitory effects of idelalisib and GS-563117 on other CYP enzymes, the exposure to agents in general that are primarily metabolized via non-CYP3A pathways is not expected to be meaningfully impacted by idelalisib coadministration.
The in vitro enzymology findings on idelalisib metabolism, driven mainly via oxidation by AO to form GS-563117, with smaller contributions by CYP3A and UGT1A4, are consistent with clinical findings. The modest effect of ketoconazole, a strong CYP3A inhibitor and a weak AO inhibitor, on idelalisib exposure support a smaller role of CYP3A (versus AO) in idelalisib metabolism. Accordingly, CYP3A inhibition by GS-563117 is not expected to meaningfully affect idelalisib plasma exposure. On the other hand, coadministration of idelalisib with rifampin caused a substantial decrease in idelalisib and GS-563117 exposure relative to the exposure with idelalisib administration alone. The idelalisib C max and AUC∞ showed mean decreases of 58 and 75 %, respectively, and the corresponding values for GS-563117 were 68 and 89 %, respectively. CYP3A activity has been shown to be substantially increased (13-fold) by rifampin at the plasma concentrations observed (10 µM), whereas AO is not considered to be inducible [36]. Therefore, idelalisib oxidation through CYP3A may have been substantially increased and resulted in a significant decrease in idelalisib exposure. Increases in glucuronidation may have also occurred. GS-563117 metabolism is primarily mediated via CYP3A and, accordingly, a larger reduction in GS-563117 exposure was noted. On the basis of the results from a mass balance study, there was no evidence of non-CYP3A-mediated metabolism of GS-563117. Overall, the effect of CYP3A induction may have a bigger impact on GS-563117 clearance than on idelalisib clearance.
On the basis of the pH-dependent solubility of idelalisib (decreasing solubility over a pH range of 1–6) and the solubility-limited absorption of idelalisib as described earlier, a clinical evaluation of the effect of acid-reducing agents on the pharmacokinetics of idelalisib was performed on the basis of co-use of histamine 2 receptor antagonists (H2RAs), proton pump inhibitors (PPIs) and antacids in the long-term patient studies [37]. Subjects were counted as receiving concomitant acid-reducing agents if these were administered during any of the idelalisib pharmacokinetic sampling times (analysis using idelalisib steady-state observed pre-dose concentration [C predose] values), regardless of the duration of acid-reducer use (i.e. taken once or chronically). Subjects were counted as not receiving an acid-reducing agent if there was no overlapping period of pharmacokinetic sampling with concomitant use of acid-reducing agents. In a second analysis performed using population pharmacokinetics–estimated C trough, C max and AUC values, subjects were counted as receiving concomitant acid-reducing agents only if such agents were administered ≥50 % of the time, coinciding with idelalisib pharmacokinetic sampling. Subjects were counted as not receiving an acid-reducing agent if there was no overlapping period of pharmacokinetic sampling with concomitant use of acid-reducing agents. The geometric least-squares mean ratio and 90 % CI of idelalisib exposure parameters between subjects with and those without use of acid-reducing agents was determined. Using the C predose data, the geometric mean ratios (%) and associated 90 % CIs were 111 (86–143) and 80 (59–109) in iNHL and CLL patients, respectively. The corresponding population pharmacokinetics-based values in iNHL and CLL patients were 104 (94–116) and 89 (78–101), respectively, for AUC, 106 (88–128) and 95 (76–119), respectively, for C max and 103 (92–116) and 80 (69–92), respectively, for C trough. Collectively, these data demonstrate the lack of clinically relevant effects of acid-reducing agents on idelalisib pharmacokinetics.
3.8 Exposure–Response
On the basis of dose ranging and subsequent phase 2 evaluation in iNHL patients, idelalisib 150 mg twice daily was shown to provide strong and durable efficacy and a favourable safety profile. This dose was selected on the basis of a study (study 101-02) evaluating idelalisib doses of 50, 100, 150, 200 or 350 mg twice daily or 150 or 300 mg once daily administered as monotherapy to subjects with relapsed or refractory haematological malignancies. As noted earlier, idelalisib exposure increased in a less than dose-proportional manner; the different dosing frequencies (i.e. once daily versus twice daily) varied the shape of the concentration–time curves and the associated pharmacokinetic parameters used in exposure–response analyses. Tumour responses, assessed by changes in tumour size (the sum of the products of the largest perpendicular diameters [SPD]), were evaluated in subjects receiving these treatments. Predicted idelalisib exposure based on population pharmacokinetic modelling was evaluated against the SPD to assess the exposure–efficacy relationship [25].
Figure 2a shows the box plot of SPD responses, stratified by quartile, of idelalisib C trough values for iNHL subjects in study 101-02. In this pharmacokinetic–pharmacodynamic analysis over a wide dose/exposure range, the median SPD response increased over the quartiles of the idelalisib C trough values and reached a plateau at/above the second quartile (Q2). Idelalisib 150 mg twice daily provided exposure (C trough 349 ng/mL) encompassed by the third exposure quartile (Q3; C trough range 280–405 ng/mL), corresponding to this plateau of high exposure–response and well above the EC90 for PI3Kδ inhibition (~125 ng/mL or 301 nM). At the same total daily dose, idelalisib twice-daily treatment provided higher C trough values as expected versus once-daily dosing, while the AUC from 0 to 24 h (AUC0–24h) values were comparable (with median C trough values of 349 and 167 ng/mL and AUC0–24h values of 19,600 and 16,300 ng·h/mL for idelalisib 150 mg twice daily and 300 mg once daily, respectively). Comparison of the SPD response in the twice-daily versus once-daily regimens indicated that efficacy was best associated with idelalisib C trough values. Accordingly, 150 mg twice daily was chosen for further evaluation in clinical studies (including study 101-09) and to provide mean C trough values above the EC90 for PI3Kδ inhibition and achievement of robust efficacy.
a Lymph node response (% change from baseline) across quartiles of idelalisib trough plasma concentration (C trough) values in the dose-ranging study 101-02 in patients with indolent non-Hodgkin’s lymphoma (iNHL) over a 7-fold dose range. b Lymph node response ( % change from baseline) across quartiles of idelalisib C trough values in study 101-09 in iNHL patients receiving 150 mg twice daily. Q1 quartile 1, Q3 quartile 3
In the dose-ranging study 101-02, the exposure–response relationship (the SPD response versus the quartile of the idelalisib C trough) in CLL subjects was similar to that observed in iNHL subjects. Overall, idelalisib exposure across all quartiles provided SPD reductions of >50 %. The median SPD response (reduction) increased over the quartiles of the idelalisib C trough and reached a plateau at Q3. Idelalisib 150 mg twice daily provided exposure (C trough 349 ng/mL) in CLL subjects encompassed by Q3 (C trough range in Q3 295–437 ng/mL), corresponding to the plateau phase of exposure–response, as observed with iNHL subjects.
Following the selection of the idelalisib 150 mg twice-daily dose, the efficacy of idelalisib given as monotherapy at 150 mg twice daily to subjects with iNHL refractory to rituximab and alkylating agents was further evaluated in the pivotal phase 2 study 101-09. The primary objective of this study was to evaluate tumour regression, as assessed by an independent review committee (IRC) on the basis of the ORR. Additional efficacy endpoints based on IRC assessments, such as DOR, PFS, LNR and SPD, were also evaluated. Exposure–efficacy analyses were conducted to assess the relationship between idelalisib exposure (derived from population pharmacokinetic modelling) and various efficacy endpoints.
Figure 2b shows the idelalisib C trough versus SPD relationship from study 101-09, indicating similar SPD changes across the observed exposure range. Figure 3 shows the box plot of the relationship between the idelalisib C trough and the best overall response status (BOR), and also stratified by categories of response. These analyses indicated that the median idelalisib C trough was similar between responders (complete response [CR] or partial response [PR]) and nonresponders (stable disease [SD] or progressive disease [PD]), and across the four response categories. These results indicated a lack of relationship between idelalisib exposure at 150 mg twice daily and BOR. Additional logistic regression analysis further indicated a lack of relationship between idelalisib plasma exposure and response status (responder versus nonresponder), as shown in Table 2.
Overall, exposure–efficacy analysis in study 101-09 following treatment with idelalisib 150 mg twice daily confirmed the lack of a relationship between idelalisib plasma exposure and various efficacy endpoints, indicating that the 150 mg twice-daily regimen produced robust and consistent therapeutic effects across the exposure range observed. Relative to the median idelalisib C trough for the study 101-09 population, the C trough midpoints in the lowest quartile (Q1) and the highest quartile (Q4) were 155 and 553 ng/mL, respectively, and the within-quartile ranges for Q1 and Q4 were 44–203 ng/mL and 437–1658 ng/mL, respectively. As such, the exposure–response analyses represented a wide range of idelalisib C trough values, representing 53 and 188 % excursions from the median at the midpoints of Q1 and Q4, respectively. These analyses indicated the absence of exposure–response relationships over an exposure range that accounted for the potential modest effects of intrinsic/extrinsic factors on idelalisib pharmacokinetics, which were not clinically relevant. In summary, the overall analyses indicated the absence of exposure–efficacy relationships and support the idelalisib 150 mg twice-daily dose.
Idelalisib 150 mg twice daily was also evaluated as combination therapy in subjects with relapsed CLL. Exposure–efficacy analyses were conducted to assess the relationship between idelalisib exposure (derived from population pharmacokinetic modelling) in the phase 3 study GS-US-312-0116 (idelalisib plus rituximab versus rituximab alone) and in selected cohorts from study 101-07 (where idelalisib was administered at 150 mg twice daily in combination with the anti-CD20 agents rituximab or ofatumumab) and various efficacy endpoints, as noted earlier. The relationships between the idelalisib C trough and BOR, and also across categories of response, were evaluated. These analyses indicated that the median idelalisib C trough was similar in responders (CR or PR) and nonresponders (SD or PD) and across the response categories, indicating a lack of relationship between idelalisib exposure at 150 mg twice daily and BOR. Additional logistic regression analysis further indicated a lack of relationship between idelalisib plasma exposure and response status (responder versus nonresponder).
Idelalisib exposure–efficacy analysis using data from studies 101-07 and GS-US-312-0116 following idelalisib 150 mg twice-daily treatment confirmed a lack of a relationship between idelalisib exposure and various efficacy endpoints, indicating that idelalisib 150 mg twice daily in combination with anti-CD20 agents (rituximab or ofatumumab) produced robust and consistent therapeutic effects in subjects with relapsed CLL across the exposure range observed. The median (Q1, Q3) idelalisib average daily dose in study 312-0116 over the treatment duration (including interruptions) was 150 mg (145, 150 mg) twice daily (i.e. a median of 300 mg daily), indicating the lack of a meaningful role of dose reductions and/or interruptions. Overall, these results were consistent with the results from exposure–efficacy analyses in iNHL patients.
Pharmacokinetic–pharmacodynamic analyses of the idelalisib/GS-563117 exposure–safety relationships were performed in subjects with haematological malignancies (including iNHL and CLL) who received idelalisib as monotherapy in phase 1 and 2 studies (101-02 and 101-09), using idelalisib/GS-563117 exposure values derived from population pharmacokinetic modelling versus safety parameters, which included laboratory abnormalities of AST and ALT, and grade 3 AEs including neutropenia, diarrhoea, skin rash and infection.
In study 101-02, over the wide range of dose/exposure evaluated, no consistent exposure–driven trends (similar across quartiles of idelalisib AUC0–24h and C max) were observed in the incidence rates for grade 3 or 4 AST and ALT elevations. These data, together with the dose/exposure–efficacy analyses, supported the selection of 150 mg twice daily as the intended dosing regimen for further clinical development, including study 101-09 in iNHL patients. Idelalisib exposure–safety relationships were further evaluated for subjects receiving 150 mg twice-daily treatment in studies 101-02 and 101-09. The relationship between idelalisib C max and the incidence of AEs (the percentage of subjects with a specific AE) is shown in Fig. 4. The idelalisib C max was comparable whether grade 3 or 4 AEs, neutropenia, diarrhoea, skin rash or infection were present or absent. Similar results were observed when AEs were evaluated against the steady-state AUC0–24h. Further, the incidence rates for grade 3 or 4 AST and ALT elevations for subjects who received 150 mg twice daily in both studies 101-02 and 101-09 were similar across the quartiles of the idelalisib AUC0–24h and C max, confirming the lack of an exposure–safety relationship. A logistic regression analysis indicated the lack of a relationship between idelalisib exposure and occurrence of infection. Taken together, there were no relationships between idelalisib exposure and the selected safety endpoints. Idelalisib exposure–safety relationships were further evaluated in subjects with CLL receiving idelalisib 150 mg twice daily in combination with rituximab or ofatumumab. The relationships between the idelalisib C max and the incidence of AEs (the percentage of subjects with a given AE) were evaluated; the idelalisib C max was comparable regardless of the incidence (present versus absent) of any grade 3 or 4 AEs or the incidence of grade 3 or 4 neutropenia, diarrhoea, skin rash, infection or pneumonia; similar findings were observed using the steady-state AUC0–24h. Further, the incidence rates for grade 3 or 4 AST and ALT elevations for subjects who received idelalisib 150 mg twice daily were comparable across the quartiles of the AUC0–24h and C max, confirming the lack of an exposure–safety relationship. A logistic regression analysis indicated the lack of a relationship between idelalisib exposure and occurrence of infection or pneumonia. Taken together, there were no relationships between idelalisib or GS-563117 exposure and the selected safety endpoints in iNHL or CLL patients.
4 Conclusions
Idelalisib is the first PI3Kδ inhibitor approved for the treatment of iNHL and CLL. The clinical pharmacokinetics of idelalisib have been extensively characterized in patients. Idelalisib displays low pharmacokinetic variability and has a well-understood drug interaction profile. There are no clinically relevant covariates that affect idelalisib pharmacokinetics. Exposure–response analyses support the 150 mg twice-daily dose of idelalisib with respect to both efficacy and safety.
References
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–9.
Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5(12):921–9.
Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196(6):753–63.
Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317–30.
Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30(4):194–204.
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–41.
Bernal A, Pastore RD, Asgary Z, Keller SA, Cesarman E, Liou HC, et al. Survival of leukemic B cells promoted by engagement of the antigen receptor. Blood. 2001;98(10):3050–7.
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275(5300):661–5.
Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204.
Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121(7):1424–32.
Herman SE, Lapalombella R, Gordon AL, Ramanunni A, Blum KA, Jones J, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117(16):4323–7.
Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–12.
Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–4.
Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–18.
Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007.
Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Second interim analysis of phase 3 study of idelalisib plus rituximab for relapsed CLL: efficacy analysis in patient subpopulations with del(17p) and other adverse prognostic factors. Blood. 2014;124(21):330.
Barrientos JC, Coutre SE, Vos S, Wagner-Johnston ND, Flinn IW, Sharman JP, et al. Long-term follow-up of a phase 1 trial of idelalisib (Zydelig®) in combination with bendamustine (B), bendamustine/rituximab (BR), fludarabine (F), chlorambucil (Chl), or chlorambucil/rituximab (ChlR) in patients with relapsed or refractory chronic lymphocytic leukemia (CLL). Blood. 2014;124(21):3343.
Somoza JR, Koditek D, Villasenor AG, Novikov N, Wong MH, Liclican A, et al. Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase delta. J Biol Chem. 2015;290(13):8439–46.
Herman SE, Johnson AJ. Molecular pathways: targeting phosphoinositide 3-kinase p110-delta in chronic lymphocytic leukemia. Clin Cancer Res. 2012;18(15):4013–8.
Seda V, Mraz M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol. 2015;94(3):193–205.
Vanhaesebroeck B, Khwaja A. PI3Kdelta inhibition hits a sensitive spot in B cell malignancies. Cancer Cell. 2014;25(3):269–71.
Yang Q, Modi P, Ramanathan S, Quéva C, Gandhi V. Idelalisib for the treatment of B-cell malignancies. Expert Opinion Orphan Drugs. 2015;3(1):109–23.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–7.
Webb HK, Chen H, Yu AS, Peterman S, Holes L, Lannutti B, et al. Clinical pharmacokinetics of CAL-101, a p110δ isoform-selective PI3K inhibitor, following single- and multiple-dose administration in healthy volunteers and patients with hematological malignancies. ASH Annual Meeting Abstracts. 2010;116(21):1774.
Jin F, Zhou H, Fang L, Li X, Newcomb T, Dansey R, et al. Exposure–response of idelalisib, a novel PI3Kδ inhibitor, in the treatment of hematologic malignancies. Blood. 2013;122(21):5054.
Jin F, Gao Y, Zhou H. Population pharmacokinetic modeling of idelalisib, a novel PI3Kδ inhibitor, in healthy subjects and patients with hematologic malignancies. J Pharmacokinet Pharmacodyn. 2014;41:S69.
Jin F, Robeson M, Zhou H, Kwan E, Ramanathan S. Pharmacokinetics, metabolism and excretion of idelalisib. Blood. 2013;122(21):5570.
Chen H, Evarts J, Webb H, Ulrich R. Biotransformation of GS-1101 (CAL-101), a potent and selective inhibitor of PI3K delta for the treatment of patients with hematologic malignancies. FASEB J. 2012;26:850.
Jin F, Zhou H, Gao Y, Li X, Newcomb T, Ramanathan S. Effect of intrinsic and extrinsic factors on pharmacokinetics of idelalisib, a novel PI3Kδ inhibitor, in patients with hematologic malignancies. Clin Pharmacol Ther. 2015;97(Suppl 1):S84.
Jin F, Robeson M, Zhou H, Hisoire G, Ramanathan S. The pharmacokinetics and safety of idelalisib in subjects with moderate or severe hepatic impairment. J Clin Pharmacol. 2015. doi:10.1002/jcph.504 (Epub 2015 Mar 27).
Garattini E, Terao M. The role of aldehyde oxidase in drug metabolism. Expert Opin Drug Metab Toxicol. 2012;8(4):487–503.
Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet. 2006;21(5):357–74.
Jin F, Robeson M, Zhou H, Hisoire G, Ramanathan S. The pharmacokinetics and safety of idelalisib in subjects with severe renal impairment. Blood. 2013;122(21):5572.
Jin F, Robeson M, Zhou H, Moyer C, Wilbert S, Murray B, et al. Clinical drug interaction profile of idelalisib in healthy subjects. J Clin Pharmacol. 2015. doi:10.1002/jcph.495 (Epub 2015 Mar 11).
Fenner KS, Troutman MD, Kempshall S, Cook JA, Ware JA, Smith DA, et al. Drug–drug interactions mediated through P-glycoprotein: clinical relevance and in vitro–in vivo correlation using digoxin as a probe drug. Clin Pharmacol Ther. 2009;85(2):173–81.
Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos. 2007;35(10):1853–9.
Jin F, Sharma S, Zhou H, Gao Y, Li X, Newcomb T, et al. Effect of acid reducing agents on the pharmacokinetics of idelalisib, a novel PI3Kδ inhibitor, in patients with hematologic malignancies. Clin Pharmacol Ther. 2015;97(Suppl 1):S83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Srinivasan Ramanathan, Feng Jin, Shringi Sharma and Brian P. Kearney are employees of Gilead Sciences, Inc. (Foster City, CA, USA), contributed significantly to the design, conduct, analyses and interpretation of data, and were involved in the preparation, review and approval of this article. Gilead Sciences, Inc., provided funding for the research presented in this article. The idelalisib project team and individual study team members contributed towards the conduct and management of clinical trials described here.
Rights and permissions
About this article
Cite this article
Ramanathan, S., Jin, F., Sharma, S. et al. Clinical Pharmacokinetic and Pharmacodynamic Profile of Idelalisib. Clin Pharmacokinet 55, 33–45 (2016). https://doi.org/10.1007/s40262-015-0304-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-015-0304-0