Abstract
Monoclonal antibodies (mAbs) constitute a therapeutically and economically important drug class with increasing use in both adult and paediatric patients. The rather complex pharmacokinetic and pharmacodynamic properties of mAbs have been extensively reviewed in adults. In children, however, limited information is currently available. This paper aims to comprehensively review published pharmacokinetic and pharmacokinetic–pharmacodynamic studies of mAbs in children. The current status of mAbs in the USA and in Europe is outlined, including a critical discussion of the dosing strategies of approved mAbs. The pharmacokinetic properties of mAbs in children are exhaustively summarised along with comparisons to reports in adults: for each pharmacokinetic process, we discuss the general principles and mechanisms of the pharmacokinetic/pharmacodynamic characteristics of mAbs, as well as key growth and maturational processes in children that might impact these characteristics. Throughout this review, considerable knowledge gaps are identified, especially regarding children-specific properties that influence pharmacokinetics, pharmacodynamics and immunogenicity. Furthermore, the large heterogeneity in the presentation of pharmacokinetic/pharmacodynamic data limited clinical inferences in many aspects of paediatric mAb therapy. Overall, further studies are needed to fully understand the impact of body size and maturational changes on drug exposure and response. To maximise future knowledge gain, we propose a ‘Guideline for Best Practice’ on how to report pharmacokinetic and pharmacokinetic–pharmacodynamic results from mAb studies in children which also facilitates comparisons. Finally, we advocate the use of more sophisticated modelling strategies (population analysis, physiology-based approaches) to appropriately characterise pharmacokinetic–pharmacodynamic relationships of mAbs and, thus, allow for a more rational use of mAb in the paediatric population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Careful review of the current knowledge of the pharmacokinetic and pharmacokinetic–pharmacodynamic characteristics of monoclonal antibodies (mAbs) in children has identified a considerable knowledge gap, especially with respect to mechanistic insight regarding the impact of physiological and developmental aspects. |
We provide a ‘Guideline for Best Practice’ on how to report study results in order to gain maximum knowledge and enable comparison, e.g. across studies, across the mAb drug class, and with adult characteristics. |
More sophisticated modelling approaches (population analysis, physiology based) are needed to appropriately characterise the complex pharmacokinetics/pharmacodynamics of mAbs in children, preferably also considering body size and maturation processes. |
1 Introduction
Therapeutic monoclonal antibodies (mAbs) have gained large attention over recent decades given their desirable features, such as high potency and limited off-target toxicity. mAbs now constitute a therapeutically and economically important drug class, evident by the growing number of mAbs on the market and in development [1]. An increased use of mAbs has also been seen in the paediatric population, especially in the areas of inflammatory diseases, organ transplantation and oncology [2].
mAbs are large proteins with a structure similar to endogenous immunoglobulins. They comprise two domains: (i) the variable antigen-binding region (Fab), responsible for the specificity to the target antigen; and (ii) the constant region (Fc), triggering immune responses through interaction with Fc receptors (FcR) [3]. Immunoglobulins are grouped into five classes according to their structure: IgA, IgD, IgE, IgG and IgM. IgG is the predominant type representing approximately 80 % of endogenous immunoglobulins in serum, and the only subtype currently represented in mAbs. mAbs are also commonly classified based on their genetic origin: murine, chimeric, humanised or fully human; all of which are in therapeutic use. More details about the structure of mAbs and the different types can be found in Dirks and Meibohm [3] and Keizer et al. [4].
The pharmacokinetics and pharmacodynamics of mAbs are often complex and mutually influenced, i.e. molecules bind to their target with high affinity and to a significant extent, such that the interaction may have an impact on the pharmacokinetics of the drug [5]. These processes have been extensively reviewed in adults [3, 4, 6–9] but the information available in children is limited. Paediatric patients represent a special population: as children grow and develop, body size and composition change alongside with maturation processes. These changes may modify absorption, distribution, elimination and response to the drug [10–12].
This paper aims to critically review available pharmacokinetic and pharmacokinetic–pharmacodynamic characteristics of mAbs in children. For this purpose, mAbs (approved and in development) have been identified utilising multiple sources: the online drug databases of the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) [13, 14], as well as paediatric investigation plans (PIPs) and post-marketing Pediatric Research Equity Acts (PREAs) from the two agencies, respectively. Thereafter, an exhaustive literature search of pharmacokinetic and pharmacokinetic–pharmacodynamic studies was undertaken: a PubMed search was performed using the international non-proprietary names (INNs) of the mAbs or ‘monoclonal antibody(ies)’ combined with keywords such as ‘pharmacokinetic*’, ‘pharmacodynamic*’, ‘concentrations of …’, ‘neonate(s)’, ‘infant(s)’, ‘child(ren)’, ‘paediatric/pediatric’ and/or ‘adolescent(s)’. In addition, Google Scholar was used to identify cited abstracts or publications not indexed in PubMed. Studies were included regardless of the legal status of the mAb, i.e. approved, in development, off-label use or withdrawn from the market; mAb fragments were not included.
The first sections of this review concentrate on the mAbs in the paediatric population in the USA and Europe. To round-off, immunogenicity is discussed with respect to its impact on paediatric mAb therapy. Throughout the review, future perspectives in the clinical use and development of mAbs in paediatric patients are highlighted.
2 Current Status of Monoclonal Antibodies in the Paediatric Population
2.1 Approved mAbs
At present (April 2014), approximately one-third of all mAbs approved for adults in the European and/or US market are also approved for paediatric use. In addition, one mAb (palivizumab) has been approved solely for paediatric use given its therapeutic indication. In total, 11 mAbs are licensed for use in the paediatric population (nine in the USA and eight in Europe) [13, 14]. No murine mAb is currently marketed for paediatric use, probably due to increasing interest in humanised and fully human mAbs. The approved paediatric indication(s), their age ranges and dosing regimens can be found in Table 1.
Palivizumab is approved for the youngest children, starting at 35 weeks of gestational age, followed by daclizumab which is approved for children ≥11 months. The majority of the mAbs are, however, approved for older children. The indication and age range are frequently the same in Europe and the USA but in a few cases (e.g. adalimumab) one, or both, aspect(s) differ. For these mAbs it can be observed that for the same indication they are approved for younger children by the EMA than by the FDA.
Oral administration is not feasible for mAbs in general. Hence, they are administered intravenously (n = 6), subcutaneously (n = 4) or intramuscularly (n = 1). The route of administration in the paediatric population corresponds to that in adults, except for tocilizumab being only available for intravenous administration in children (no explanation available on the label).
2.2 Off-Label Use
Off-label use constitutes a common practice in paediatric patients [15, 16] and the mAb drug class is no exception. An increased off-label use of mAb therapies has been observed for a wide variety of indications, mostly as second-line treatment [2]. Rituximab, as one example of a widely used mAb without any approved indication in the paediatric population, has been administered in a spectrum of diseases including haematological and renal disorders [17–19]. There are also many examples of mAbs with one or several approved paediatric indication(s) being used for other indications [20–23]. Although these reports in general point towards beneficial effects, they should be interpreted carefully as most of them only include small numbers of patients and lack control groups [18]. Additionally, the dosing strategies vary considerably between the studies, e.g. a body size-normalised adult dose [24] or identical to an approved paediatric indication. As is discussed in the sections below, these approaches might be particularly precarious when dealing with mAbs, since pharmacokinetic/pharmacodynamic aspects may differ between adults and children [12] as well as between indications.
2.3 mAbs in Development
Safety and efficacy studies in the paediatric population are more challenging than in adults, not only due to physiological differences and changes during childhood, but also from ethical and practical considerations [25]. Consequently, fewer studies are in general carried out in this group. In recent years, however, requirements to conduct clinical trials in children have increased. All European applications for new marketing authorisation now have to include a PIP describing study results covering all paediatric age groups and necessary age-appropriate formulations [26], although a waiver may be granted, e.g. if the indication is irrelevant for the paediatric population. The FDA has so far established PREAs but will soon introduce the Pediatric Study Plan (PSP) [27], an equivalent to the PIP.
Currently, 38 mAbs are registered with a (non-waived) PIP and/or PREA (Table 2). Some have already been approved in adults (n = 14) and are aiming to extend the age range, while others are still in clinical development (n = 24). Although some studies plan to include infants or neonates, the majority are planned for children ≥2 years. Thus, the to date limited pharmacokinetic and pharmacokinetic–pharmacodynamic information in children is certainly about to increase.
3 Dosing Strategies of Approved mAbs
Dose selection strategies for mAbs approved for children include (i) a fixed dose for the approved age range; (ii) fixed doses for different body weight (BW) intervals; and (iii) linear dose scaling by body surface area (BSA, i.e. mg/m2) or by BW (i.e. mg/kg). There are a few examples that combine the two last strategies, e.g. tocilizumab, which is dosed 10 mg/kg to patients <30 kg and 8 mg/kg to patients ≥30 kg [28]. Very little information is available regarding the rationale behind the chosen posology. If the dosing regimen in adults was based on BW or BSA (i.e. mg/kg or mg/m2), the same regimen was commonly used in children. Otherwise, dose reduction based on BW or BSA was performed; whether a pharmacokinetic and/or pharmacodynamic scaling approach was applied beforehand or not was only mentioned for raxibacumab (mAb against anthrax). Raxibacumab has not been tested in children (due to ethical reasons) and the approval is based solely on adult data [14]. However, no further information regarding the used (covariate) model was provided.
In a paediatric population BW considerably varies, suggesting that dose adjustment according to body size may be a reasonable approach. The strategy of simply adjusting dose or pharmacokinetic parameters linearly according to BW or BSA is, however, frequently questioned [29–31] (see [29, 32] for details on scaling principles). The linear BW model under-predicts drug clearance (CL) while the BSA model over-predicts CL in children when compared with allometric scaling [31]. In addition, several equations for calculation of BSA exist [33–35]; equations developed only on adults [35] should be applied carefully given the anatomical differences compared with children. Moreover, relevant maturation processes that might affect the pharmacokinetics of mAbs may not be directly related to body size, as is the case for small molecules (e.g. hepatic enzymes or kidney function). Further discussion on these aspects is provided in Sect. 4. Dosing regimens linearly based on BW or BSA may also have drawbacks from a convenience perspective in terms of drug preparation, drug administration and risk of medication errors [30]. As long as information technology (IT)-related solutions are not available to guide dose selection in daily clinical practice, a fixed dosing regimen for a given BW range may represent a more pragmatic alternative. Furthermore, it is important to remember that when adjusting dosing regimens based on these scaling principles, the effective and safe plasma concentration range is assumed to be the same across ages, which might not always be the case. Since mAbs possess unique pharmacokinetic–pharmacodynamic characteristics, a good understanding of the physiological differences between adults and children, affecting not only pharmacokinetic but also pharmacodynamic processes, as well as the relationship between the two (in both populations), is needed to achieve optimal dosing paradigms.
4 Pharmacokinetics
The pharmacokinetic characteristics of mAbs in adults have been extensively reviewed [4, 6–8, 36]; however, little has been accomplished in the paediatric arena [37]. In the subsequent sections, the characteristics of the different pharmacokinetic processes (absorption, distribution, elimination) are summarised and critically discussed. Each subsection starts with a brief summary of the general principles and mechanisms, followed by a discussion of the biological aspects in children that influence the pharmacokinetic processes. Furthermore, the pharmacokinetic parameters in children are comprehensively summarised and compared with reports in adults. For this purpose, a thorough literature search was undertaken (see Sect. 1), identifying a total number of 54 studies (understood as different pharmacokinetic analyses) covering the full paediatric age range from pre-term to 18 years (Fig. 1). For most of the mAbs, a large gap in the information in pharmacokinetic understanding was observed for neonates and infants, consistent with the observations of the approved age ranges of the mAbs.
Post-natal age of paediatric patients included in the pharmacokinetic studies or, if applicable, the respective subpopulations, as reported. The numbers on the y-axis refer to the number assigned in the ‘Study no.’ column of Tables 3 and 5. The colours indicate the type of pharmacokinetic analysis performed, points indicate the mean, dashed lines indicate ±1 standard deviation from the mean, triangles indicate the median, solid lines indicate the range and dot-dashed lines indicate the interquartile range. EDCA exploratory drug concentration analysis, Ind. CMT individual compartmental analysis, NCA non-compartmental analyses, PK pharmacokinetic, Pop. CMT population compartmental analysis
Overall, the level of complexity varied among the reviewed data analyses. More than two-thirds of the studies only reported (i) distributions of measured drug concentrations at a certain point in time (37 %)—these studies will hereafter be referred to as ‘exploratory drug concentration analysis’ (EDCA); or (ii) non-compartmental analysis (NCA) (33 %). A considerably smaller proportion of studies performed compartmental analysis, either at an individual (Ind. CMT) or population (Pop. CMT) level (8 or 22 %, respectively). The type of reported pharmacokinetic parameters naturally differed depending on the type of performed analysis. For that reason, the results have been summarised in two sets of tables. For EDCA and NCA, the key characteristics of the studies with respect to dosing regimen, age and BW ranges, indication and type of pharmacokinetic analysis are outlined in Table 3 and the extracted pharmacokinetic parameters in Table 4. Although the area under the plasma concentration–time curve (AUC), the maximum concentration (C max) and minimum concentration (C min) will not be explicitly compared across studies due to their dose-dependent nature, they have been included in Table 4 to provide information about ranges of observed drug concentrations/exposure. Information available only in plots has been digitalised (using WebPlotDigitizer: http://arohatgi.info/WebPlotDigitizer/app/) and included in Table 4. Likewise, study characteristics and parameter estimates have been systematically collated for the Ind. CMT and Pop. CMT in Tables 5 and 6, respectively. To all these four tables, a ‘Study no.’ column has been added to enable cross-referencing of studies and groups in Tables 3 and 5 to the corresponding parameters in Tables 4 and 6 (these ‘study numbers’ are also used in Figs. 1, 2 and 3).
Estimate and variability between individuals of V d/V tot values (x-axis) within and across monoclonal antibodies. The numbers on the y-axis refer to the number assigned in the ‘Study no.’ column of Tables 3 and 5. First panel: V d/V tot in mL/kg; second panel: V d/V tot in mL/m2; third panel: V d/V tot in mL (non-scaled value); fourth panel: V d/V tot in mL (scaled to ‘reference adult’). The colours show (i) the type of pharmacokinetic analysis; and (ii) blue shades represent the values/units reported in the original publications, whereas values with red shades have been transformed/normalised using the reported body weight or body surface area (see Sect. 4.2.3 for full explanation). Points indicate the mean, dashed lines indicate the 95 % CI calculated based on reported standard deviation and the mean, triangles indicate the median, solid lines indicate the range, diamonds indicate the estimate for typical individual (Pop. CMT), dot-dashed lines indicate the 95 % CI calculated based on reported between-subject variability, crosses indicate the mean or median EBEs (Pop. CMT), and dotted lines indicate the range of EBEs or 95 % CI calculated based on the coefficient of variation of EBEs. CI confidence interval, EBEs empirical Bayes estimates, Ind. CMT individual compartmental analysis, NCA non-compartmental analyses, Pop. CMT population compartmental analysis, V d volume of distribution, V tot total volume
Estimate and variability between individuals of CL values (x-axis) within and across monoclonal antibodies. The numbers on the y-axis refer to the number assigned in the ‘Study no.’ column of Tables 3 and 5. First panel: CL in mL/day/kg; second panel: CL in mL/day/m2; third panel: CL in mL/day (non-scaled value); fourth panel: CL in mL/day (scaled to ‘reference adult’). The different colours show the type of pharmacokinetic analysis. No transformation based on body weight was performed on CL values; hence, they are referred to as ‘original’ (see explanation in Sect. 4.3.3). Points indicate the mean, dashed lines indicate the 95 % CI calculated based on reported standard deviation and the mean, triangles indicate the median, solid lines indicate the range, diamonds indicate the estimate for typical individual (Pop. CMT), dot-dashed lines indicate the 95 % CI calculated based on reported between subject variability, crosses indicate the mean or median EBEs (Pop. CMT) and dotted lines indicate the range of EBEs. CI confidence interval, CL clearance, EBEs empirical Bayes estimates, Ind. CMT individual compartmental analyses, MMF mycophenolate mofetil, NCA non-compartmental analyses, Pop. CMT population compartmental analysis, w with, w/o without
The number of studies per mAb was found to vary highly; ten studies were found for infliximab whereas for many mAbs only one study was published. Similarly, the number of individuals per pharmacokinetic analysis showed a large range from three to 4,316. As is evident from Tables 4 and 6, only a small fraction of the reviewed studies provided a complete summary of the pharmacokinetic characteristics; this is not only due to the analysis complexity but parameters also seem to have been omitted. As a result of the varying analysis complexity, and many studies being more clinically orientated, the parameters have been described in a rather heterogeneous fashion, e.g. as an estimate for the studied population, or scaled to a BW of 70 kg. This heterogeneity was handled differently depending on the pharmacokinetic parameter and is, thus, explained in each of the following subsections.
4.1 Absorption
4.1.1 General Principles and Mechanisms
Oral administration of mAbs is generally limited by low permeability and degradation throughout the gastrointestinal tract [9]. mAbs are, thus, administered intravenously, subcutaneously or intramuscularly. The mechanisms of absorption after subcutaneous and intramuscular administration are not yet fully understood [38]. Based on the molecular properties of mAbs and animal studies of subcutaneously administered biotherapeutics (excluding mAbs), convective transport via the lymphatic system is considered to be the primary pathway for subcutaneous absorption, meaning that they move together with fluids [38]. No information was found regarding intramuscular administration specifically. For small molecules, however, intramuscular administration has been described as risky, especially for small children since the blood flow to muscle and muscle mass may vary considerably [10].
Factors identified to influence absorption and bioavailability (F) of biotherapeutics (not including mAbs) include (i) site of administration; (ii) physiological factors (e.g. blood and lymph flow, catabolism at the site of injection and physical activity); and (iii) formulation-related factors (e.g. pH and injection volume) [38]. The impact of these factors on absorption of mAbs still needs to be elucidated considering also their inherent Fc structure and the potential influence of FcRs on absorption. Studies in rats suggest that the neonatal FcR (FcRn; see Sect. 4.3.1 for further details) plays an important role in the absorption of mAbs [39, 40]. It is also unclear whether physiological changes during growth (e.g. differences in blood and/or lymph flow) will affect the extent and/or rate of absorption in children.
4.1.2 Summary of Absorption-Related Parameters
Few of the retrieved studies characterised mAb absorption. One reason for the limited information is, naturally, that many mAbs were not extravascularly administered. Another reason may be a sparse data situation, commonly seen in late-stage clinical trials and data from daily practice, potentially preventing adequate estimation of absorption-associated parameters. Indeed, for omalizumab the absorption rate constant (k a) after subcutaneous administration was obtained from adult healthy volunteers and fixed to that value during further pharmacokinetic model development [41].
After intramuscular administration, the estimate of k a was 1.0 day−1 (palivizumab); this is a higher value, thus indicating quicker absorption than in adults (0.37 day−1) [42]. The population in this study was very young (pre-term to <2 years). Whether the differences in k a depended on, for example, differences in blood flow to muscle or other physiological differences, remains to be elucidated. The median/mean time to C max (t max) after subcutaneous administration (by NCA) was rather similar across studies, ranging from 1.43 to 2.29 days (n groups = 4). However, the within-mAb variability was large, e.g. ranging from 1.63 to 6.86 days for canakinumab [80, 81]. One study reports a t max after intramuscular administration, which was in agreement with the report after subcutaneous administration (2 days) [106].
Regarding F, two studies reported F after subcutaneous (23.4 and 53–71 %) [116, 121] and one after intramuscular (69 %) administration [42]. These values seem consistent with results for adults after subcutaneous administration (49.1–74.7 % [3]), although it is difficult to draw clinical conclusions based on this small number of studies. Overall, to date, evidence is too limited to infer whether absorption characteristics are similar in children and adults; more studies as well as more sophisticated data analysis methods are required (see below).
4.2 Distribution
4.2.1 General Principles and Mechanisms
The transfer of mAbs across cell membranes is limited due to their large molecular mass and hydrophilic character. Hence, the volume of distribution (V d) values in adults are in general reported to be small and mAbs are presumed to be restricted to vascular and extracellular fluid (ECF) [3, 4, 6, 7]. mAbs may reach the intracellular space by pinocytosis or via receptor-mediated endocytosis (RME, i.e. internalisation of the mAb–target/mAb–FcR complex) [43]. Within the cell, it either undergoes degradation or is recycled to the ECF or blood by the FcRn (discussed further in Sect. 4.3.1). Distribution into the brain is constrained by low transport across the blood–brain barrier (BBB). IgG molecules in general are effluxed from the brain, a mechanism potentially mediated also by the FcRn [44]. To our knowledge, there is so far no information on the influence of the FcRn (expression/efficacy) on the overall mAb distribution but, considering the potential effects on absorption, one could hypothesise that it would also affect distribution.
mAb concentration–time profiles after intravenous administration commonly follow a bi-exponential decay, often described by a two-compartment disposition model [4]. After subcutaneous administration, however, the profiles typically display mono-exponential decline as a result of slow absorption, masking the first (relatively) rapid distribution phase [4]. As mentioned, convection is believed to be the primary mechanism of transport [3, 4, 6, 7, 9]. The rate of transport is hence determined by the rate of fluid movement and the sieving effect by paracellular pores [6], and mAbs show, in general, slow transfer between central and peripheral compartments [3]. In summary, the overall distribution of mAbs may be affected by the rate of extravasation/pore size, rate/flow/proportion of ECF, the degree of binding in tissue, RME [6, 9] and, potentially, expression/efficacy of FcRn.
4.2.2 Developmental Aspects
During growth and development, children undergo changes in, for example, body composition, membrane permeability and plasma protein concentrations [10]. The relative total body water decreases from 80–90 % to approximately 60 % over the first 5 months, and thereafter remains relatively constant [45]. The main contributing factor is a decrease in ECF, which is approximately 45 % at birth, 26 % at 1 year and 18 % when an adult [45]. It is reasonable to believe that these changes influence V d, especially in children <1 year, since mAbs are considered to be distributed mainly in the vascular and ECF space, although further research is needed to explore to what extent.
Children <1 year have reduced concentrations of albumin, globulins and α1-acid glycoprotein and, hence, a reduced total plasma protein concentration (neonates: 59 g/L vs. adults: 72 g/L) [10]. Even though a reduced plasma protein concentration and other known factors (e.g. higher BBB permeability) may play an important role in the distribution of small molecules [10], it will probably be of less clinical importance for mAbs due to their molecular properties, as previously discussed.
4.2.3 Summary of Distribution-Related Parameters
Consistent with observations in adults, the mAb concentration–time profiles in the paediatric population have predominantly been described using two-compartment disposition models, except for omalizumab which was modelled with a one-compartment model (subcutaneously administered; see also Sect. 4.2.1). A one-compartment model was also used in one out of four infliximab studies [46]; a difference potentially due to the sparse data situation.
A large diversity in the pharmacokinetic analysis approaches and reported parameter units was observed, which complicated comparison of V d values across studies, as well as their comparison with reports in adults. The comparison was further challenged by the fact that some studies reporting a two-compartment model did not report the estimate for the peripheral volume. As a first approach to enable comparison between NCA and Ind./Pop. CMT parameters, the central (V 1) and peripheral (V 2) volumes were summed to a total volume (V tot) for studies in which both were reported. In the next step, the reported parameters were transformed as follows:
-
for V d reported in volume (mL, L) for the studied population, the mean/median estimate was normalised by the reported mean/median BW (for studies in which both were reported) and then scaled linearly to 70 kg (‘reference adult’);
-
for V d reported in volume/kg BW (e.g. mL/kg), the estimate was scaled linearly to 70 kg;
-
for V d reported in volume/BSA (e.g. L/m2), the estimate was scaled linearly to 1.73 m2 (also considered ‘reference adult’); and
-
V d reported in values scaled to a reference adult (70 or 80 kg) were left untransformed.
A graphical overview of the original and transformed V d values for the different mAbs is provided in Fig. 2. The conjugated mAb gemtuzumab ozogamicin showed rather atypical parameter values compared with the other mAbs, potentially due to its different structural nature, and was therefore excluded from the summary. V d/V tot was reported in a total of 25 studies of 14 different mAbs (12 NCAs, 13 Ind./Pop. CMTs). By excluding studies not reporting BW, 19 studies were retained (9 NCA, 10 Ind./Pop. CMT). In some cases, parameters were reported for several groups within one study (e.g. based on dose or age); all groups were considered. The median (range) of V d/V tot scaled to a reference adult was 6,310 (3,220–19,900) mL (n groups = 32) (Fig. 2, right panel), in agreement with physiological ECF and plasma volume values. In adults, median (range) estimates of V 1 and V 2 have been reported to be 3,100 (2,400–5,500) mL and 2,800 (1,300–6,800) mL, respectively [3]; which would give a comparable V tot of 5,900 (3,700–12,300) mL, suggesting that V d/V tot scales reasonably well with BW. Regarding V d/V tot and age, no trend could be observed; the highest V d was observed for pagibaximab in pre-term neonates [63, 85] but a value in the lower range (3,710 mL; scaled value) was observed for infliximab in Kawasaki disease (<1 year) [23], indicating that a large variability can be expected.
Eight studies identified factors (covariates) influencing V d, related to population characteristics (e.g. BW, ethnicity) or the investigated drug (e.g. formulation strength, administration route). As in adults [3], BW was most frequently identified, being significant in all studies performing covariate analysis [41, 42, 50, 58, 60, 116–118]. Two studies, both covering a wide age range, included an age effect on top of BW [59, 60], indicating that there might be maturation processes that need to be considered. In summary, V d seems to scale reasonably well with BW. Nevertheless, the variability in the reviewed studies was high, which indicates that the relation to body size should be explored further before it is considered as a factor in dosing regimens.
Seven studies reported an estimate of inter-compartmental clearance (Q) or transfer rate constants [transfer rate constant (first-order) from the central (1) to peripheral (2) compartment (k 12)/from the peripheral (2) to central (1) compartment (k 21)]. To enable comparison, k 12/k 21 were transformed to Q (e.g. Q = k 12·V 1); no transformation based on BW was performed for this parameter (see discussion in Sect. 4.3.3). The median (range) of Q reported as a BW-normalised value was 14.6 (3.52–334) mL/day/kg (n groups = 6). One study reported a Q value allometrically scaled to 70 kg (879 mL/day) [42]. Finally, unscaled estimates of Q were reported for three studies: 72 [119], 148 [118] and 658 mL/day [116]. Given this sparse and heterogeneously reported data, it is difficult to infer whether these results are in total comparable with adult values [median (range): 789 (154–53,300) mL/day) [3]].
4.3 Elimination
4.3.1 General Principles and Mechanisms
Due to the physicochemical properties of mAbs, elimination mechanisms that are of main importance for small molecules, such as hepatic enzymatic metabolism and renal excretion, are of minor importance. The elimination of mAbs is instead considered to comprise two pathways: one IgG non-specific and one target-mediated pathway [8]. The non-specific pathway refers to intra-cellular proteolysis, equivalent to the degradation of endogenous IgG. This pathway is highly influenced by the FcRn, which binds to IgGs (endogenous as well as mAbs), directing them from the intracellular space back to the blood or ECF and, thus, protecting them from degradation. The FcRn is, hence, responsible for the long half-life of IgG molecules [47]. The vascular endothelial cells or reticuloendothelial system seem to be the main site for FcRn salvage, but FcRn is also expressed in other cells [47]. The FcRn salvage pathway is usually not saturated in the therapeutic concentration ranges and/or by physiological variations of endogenous IgG [3, 8]. The affinity between FcRn and the (Fc part of) IgG is species specific; the lower affinity of human FcRn and mouse/rat-derived mAbs typically results in a shorter half-life for these mAbs. The second, target-mediated pathway (often saturable) refers to the degradation mAbs may undergo after binding to their specific target(s), commonly resulting in a faster CL of the mAb-target complex than the unbound mAb. The increased elimination rate may be caused by either direct internalisation of the drug-target complex (for mAbs with membrane-bound targets) or internalisation upon binding to FcγRs, which are expressed by various cells of the immune system. To what extent this pathway affects the total CL depends on aspects such as target localisation (important for mAbs with a membrane-bound, internalising target), target expression and binding affinity [3, 48].
Other disease-specific (non-target-mediated) attributes might also affect the elimination of mAbs. In inflammatory bowel diseases, for example, a low serum albumin concentration has been found to predict higher CL [49, 50]. This relationship might be explained by a general protein loss in the damaged ‘leaky’ gut [51, 52]. Similarly, a relationship between protein loss and CL has been identified in patients with proteinuria [53], suggesting that, for example, albumin acts as a surrogate marker of this additional ‘CL route’ in these diseases.
4.3.2 Developmental Aspects
The impact of growth and maturation on the elimination pathways has not been well-characterised. For small molecules, maturation of metabolic enzymes (e.g. the cytochrome P450 system) and kidney function are known aspects influencing pharmacokinetics in children [11]. Use of age (e.g. gestational or post-natal) has been suggested to account for these processes, as a surrogate marker of maturation [32, 54]. Since mAbs neither undergo renal excretion nor classical hepatic enzymatic metabolism to a notable extent, these principles may not be directly transferrable to this drug class. There might, however, be other maturation processes that need to be considered. Indeed, Robbie et al. described a maturation process of palivizumab CL related to post-menstrual age (PMA) [42] (further discussed below); however, the cause of this maturation was not described. Factors affecting concentrations of endogenous IgG might provide some hints, given the molecular similarity. At birth, the concentrations of all IgG isotypes are low as a result of an impaired IgG production [55]. IgG1 concentrations stabilise by the age of 5 years, whereas other subclasses may not reach adult levels until adolescence [56]. Low IgG concentrations during childhood could also be a result of increased catabolism or excretion. This is, however, most frequently observed in certain diseases [57].
To our knowledge there is no information regarding the development (expression, efficacy, IgG affinity, etc.) of the FcRn in the various cell types in children compared with in adults and how these potential differences could affect CL of mAbs. As mentioned above, mAb CL can also be affected by disease-specific factors (target expression/availability or non-target related). If the disease progression/state differs between children and adults this may translate into differences in CL. In conclusion, the influence and clinical implication of maturation processes on mAb CL is still unclear and needs further investigation.
4.3.3 Summary of Clearance-Related Parameters
The median (range) terminal half-life was 11.9 (3.85–29.7) days (n studies = 23, n groups = 34). In general, a large variability between individuals was seen; palivizumab showed the most extreme example with a median (range) of 22.4 (9.9–56.7) days [86]. The variability was too large to evaluate differences considering the genetic origin of mAbs, as inferred in adults; the ranges of central measure were 4.8–29, 3.85–24.4 and 6.63–23.7 days for chimeric, humanised and fully human mAbs, respectively.
Similar to the situation for V d, a large diversity concerning analysis approaches and reported parameter units was observed for CL. In order to harmonise the units of CL values, strong assumptions would be required with respect to both scaling approaches and handling of missing information, making across-study comparisons of CL unfeasible (this also applies for Q). Hence, unlike for V d, no parameter transformation was performed and the estimates were summarised with respect to their reported units. After excluding two studies that reported implausible units (see Table 4) and the conjugated mAb gemtuzumab ozogamicin due to its different nature, CL values from a total of 27 studies of 14 different mAbs were available (Fig. 3). Some studies reported CL for several groups; all groups were included in the following summary [presented as median (range)]; note that one of the studies reported two of the options:
-
Eleven studies reported CL in volume/time for the studied population: 199 (7.68–2,300) mL/day (n groups = 16, Fig. 3, panel 3);
-
Nine studies reported a BW-normalised CL: 4.25 (2.64–6.00) mL/day/kg (n groups = 12, Fig. 3, panel 1);
-
One study reported a BSA-normalised CL: 492 (360–1,370) mL/day/m2 (n groups = 6, Fig. 3, panel 2); and
-
Seven studies reported CL in volume/time scaled to a ‘reference adult’: 528 (198–1,280) mL/day (n groups = 13, Fig. 3, panel 4):
-
Six of these scaled to a BW of 70 or 80 kg (three used a power model with an exponent of 0.75, two estimated the exponent being close to 1, and one did not report the used covariate model); and
-
One of them scaled linearly to a BSA of 1.73 m2.
-
The estimates scaled to a reference adult covered the range of linear CL pathway for adults (200–500 mL/day) reported in Dirks and Meibohm [3], predominantly showing values in the upper range or higher. This result is not particularly surprising since the CL reported for children in this review typically represents the total CL, without differentiation of potential linear and non-linear elimination pathways, whereas CL of mAbs in adults frequently is shown to also have a non-linear/dose-dependent part [3], resulting in a higher total CL. When looking at total CL of molecules with saturable CL mechanisms, a non-linear elimination pathway relating to target availability will be observed at saturation. At lower concentrations, a mixture of both linear and non-linear elimination will occur. Which pathway will dominate will depend on the closeness to saturation. The fact that only total CL typically has been estimated in children might partially explain the different covariate models used to scale CL with BW (i.e. linear scaling, exponent of power function, etc.).
In fact, a trend towards dose dependency in the pharmacokinetics could be observed in children when several dose concentrations were available in one study (e.g. for cetuximab) [93]. However, of the 54 reviewed studies, only three reported non-linear and/or target-mediated elimination [41, 58, 116]. These studies benefited from the use of a population analysis approach on pooled clinical trial data from children and adults. The limited number of identified non-linear CL processes in the paediatric studies may arise from (i) true exclusively linear elimination process(es); (ii) the studied dose range not being wide enough; (iii) complexity of the pharmacokinetic analysis; (iv) a sparse data situation; (v) a small number of individuals; or (vi) non-linearity not being considered during model development. Further research is urgently needed to better characterise CL mechanisms in children, including characterisation of target availability in case of saturable elimination pathways, and how to more adequately scale the pathways from adults to children in these more complex relationships.
Characteristics identified as relating to CL include (i) patient-specific characteristics (e.g. BW, age or ethnicity); (ii) factors related to disease and/or disease status (e.g. chronic lung disease, albumin concentration); and (iii) anti-drug antibodies (ADAs; further discussed in Sect. 6). As for V d, BW was the most frequently introduced covariate, included in all but three of the studies performing covariate analysis (n studies = 12) [41, 42, 50, 58, 116–120]. Two of these three studies, however, reported CL for two age groups or a CL corrected by base metabolism rate (both known to correlate with BW) [59, 60]. As mentioned, BW was introduced in three of the models using a power function with an exponent of 0.75 [42, 70, 116], in agreement with allometric principles [31]. In two studies, the exponent was instead estimated to approximately 1 [41, 58], i.e. a linear model. Similarly, nine studies reported a BW-normalised CL, which is also a linear scaling model [23, 50, 59, 79, 86, 92, 94, 117, 121]. The different BW scaling models may be related to a sparse data situation, not accounting for non-linear/target-mediated pathways (see above), as well as not taking maturation processes into consideration. Robbie et al. [42] described a maturation process of palivizumab CL related to PMA, after adjusting for BW. A lower CL was estimated for younger children but the cause of this effect was not described.
Factors related to disease and/or disease state have been identified for the paediatric population, similarly to adults. This indicates that target-mediated elimination processes may play an important role for mAb CL also in the paediatric population and, hence, need to be considered more frequently.
To summarise, the elimination of mAbs is complex and may depend on body size, maturation processes and target expression/availability (pharmacodynamics), which may result in non-linear mAb CL. However, based on the limited and diversely reported data, conclusions regarding the impact of these factors on CL in the paediatric population are as yet precluded: more knowledge could have been gained if a population approach had been performed more frequently, since it allows for combined analysis of data across doses and ages (including prior knowledge). As mentioned, mAb pharmacokinetics and pharmacodynamics are inter-related and would benefit from simultaneous consideration—this can only be achieved with more sophisticated models and approaches.
5 Pharmacodynamics
The goal of any drug therapy is to translate into clinical benefit. Thus, a key aspect during drug research and development should be not only to adequately characterise the pharmacokinetics of the drug but also to establish the relationship between drug exposure and clinical response. Ideally, this relationship will be used further to design optimal dosing regimen and, hence, increase the probability of clinical success. In this section, pharmacokinetic–pharmacodynamic characteristics of studies already reviewed are systematically extracted (Table 7). To allow for a better interpretation and comprehension of the results, mAb targets and their localisation are also provided along with the clinical domain, indication, reported biomarkers and/or clinical outcome. Since the ultimate objective of this compilation was to summarise and critically evaluate pharmacokinetic–pharmacodynamic relationships, additional studies providing only safety and/or efficacy data were not considered.
Pharmacodynamic characteristics were available for 18 mAbs in 38 of the evaluated studies. The main clinical domains were immunology, oncology and infectious diseases, consistent with mAbs currently approved or in development (Fig. 4).
5.1 Target Types and Pharmacodynamic Measures/Biomarkers
Depending on the type of target and its localisation, the mAb mechanism of action may differ. Upon cell binding, mAbs can trigger lytic or coating effects, resulting in either lysis/apoptosis (e.g. rituximab) or activation/inhibition of the receptors’ biological functions (e.g. daclizumab). For soluble targets, mAbs reduce the free target concentration, and thereby decrease the target activity and/or increase target elimination [8] (e.g. omalizumab). Generally, a good agreement of reported pharmacodynamic measures (biomarkers and/or clinical outcomes) was seen within indications. In some cases, drug-specific markers/targets were also characterised, such as tumour necrosis factor-α for adalimumab or integrin α4 for natalizumab. Nevertheless, a diverse range of pharmacodynamic measures was evaluated in the different studies, as can be observed in Table 7, indicating the complexity of these diseases from a clinical point of view and a current lack of validated biomarkers. Biomarkers are biological molecules that should (i) be minimally invasive and easy to obtain; (ii) be sensitive to the drug treatment; and (iii) relate to the state/progression of the disease. Hence, biomarkers could act as a link between drug exposure and ultimate clinical response to allow for an early assessment of the pharmacological response [61]. Asthma is one of the few examples in this review for which a validated biomarker (IgE) exhibiting all mentioned properties was identified; a complex task in these clinical domains.
5.2 Pharmacokinetic–Pharmacodynamic Findings
Although 72 % of the pharmacokinetic studies also reported pharmacodynamic measures, few were able to establish significant relationships between dose/exposure and response. As previously mentioned, these results could be due to a low number of individuals/samples, the disease complexity and the high variability deduced from these aspects. Most frequently, authors were only able to conclude a trend towards clinical benefit. When looking at the type of performed pharmacokinetic analysis (in the pharmacodynamic subset), most reported an NCA or EDCA (82 %). Only seven studies (18 %) characterised the temporal concentration profiles of the drug.
Another aspect to consider is the mutual influence of pharmacokinetics and pharmacodynamics; mAbs are designed to trigger pharmacological response upon binding to their target, which might in return impact the pharmacokinetics (see Sect. 4.3.1) [62]. Simultaneous analyses of mAb concentrations and pharmacodynamic markers (target concentration/biomarkers/clinical outcome) are, thus, advantageous to fully understand the response. Given the situation, distinguished studies are discussed in the following section.
5.2.1 mAb Examples
Pagibaximab binds the lipoteichoic acid, a highly conserved macromolecule needed for staphylococcal survival. A dose-escalation study was undertaken in neonates, showing a dose-dependent antibacterial activity over the studied range (10–90 mg), which only translated into a clinical response in the highest dose group [63]. Evaluation of measured drug concentrations revealed that mAb concentrations >500 µg/mL (threshold established from in vitro studies) were not achieved in many patients. A pharmacokinetic model was developed a posteriori to optimise the dosing regimens based on the identified threshold [64]. However, in order to establish an adequate individualised dosing regimen, considering, for example, time above the threshold, the authors would also need to characterise the link between the continuous biomarker (opsonophagocytic activity) and the dichotomous clinical response. This important step towards individualised therapy has, to our knowledge, not been performed.
Basiliximab, used to prevent renal transplant rejection, is another mAb that could benefit from an exposure–response modelling approach. A significant relationship has been established between basiliximab exposure (AUC) and the suppression of interleukin-2Rα and CD25+ T lymphocytes. However, similarly to pagibaximab, no distinct relationship between biomarkers and clinical outcome has been characterised [65, 66]. Use of a population approach might enable characterisation of the relationship between basiliximab concentrations over time and suppression of, for example, CD25+ T lymphocytes, which could be used to improve dosing regimens and, thus, increase the probability of clinical benefit.
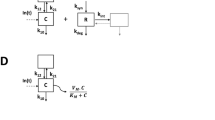
Omalizumab represents the only example for which a pharmacokinetic–pharmacodynamic model has been developed in children. Utilising a target-mediated drug disposition (TMDD) model, temporal concentration profiles of unbound and complex-bound omalizumab as well as IgE (i.e. target) were characterised [58]. In addition, a non-linear link between free IgE and response was identified (no specific differentiation was mentioned between adults and children). A target IgE suppression concentration of 14 ng/mL was suggested to achieve maximum clinical effect. In a subsequent analysis of long-term data, a feedback control mechanism of IgE on IgE production was identified [41]. Simulations, based on the TMDD model, showed that IgE concentrations could be used to guide treatment decisions. Indeed, the approved dosing regimen of omalizumab is based on IgE concentration and BW. The simulations also suggested that the therapy could be temporarily paused when IgE concentrations are low, due to the disease-modifying effect of omalizumab (reduced IgE production rate).
5.2.2 Developmental Aspects
Several studies analysing pharmacokinetics and pharmacodynamics in children aimed for the therapeutic plasma concentration established for adults (e.g. basiliximab). This assumption implies that the pharmacodynamic characteristics are the same in the two populations, which might not always be the case [12, 25]. Reasons for discrepancies between adult and paediatric patients could potentially arise from differences in target concentration/expression/availability or binding affinity, resulting from differences in disease activity and/or maturation processes. Accounting for pharmacodynamic maturation processes could in principle also explain discrepancies seen in the pharmacokinetics, given their mutual influence. However, none of the reviewed studies considered that pharmacodynamics may differ between these two patient populations. More research in this area is needed before drawing conclusions on pharmacodynamic differences across ages.
6 Immunogenicity
6.1 General Principles and Mechanisms
Although mAbs and other therapeutic proteins generally are considered rather safe and non-toxic, repeated administration often leads to generation of ADAs [67–69]—also referred to as immunogenicity.Footnote 1 Immunogenicity has been described to affect various aspects of protein therapy, such as pharmacokinetics, pharmacodynamics and safety [67–70]. The ADAs are a result of an immune response, accomplished by B and T lymphocytes and antigen-presenting cells [67]; the same system contributing to the beneficial immune effects observed after vaccinations. In mAb therapy, in contrast to vaccination, it is desirable to keep the antibody production low. Factors known to contribute to an effective vaccination are, thus, best avoided [67, 68]. These and other factors described to influence immunogenicity, include (i) drug and drug-formulation attributes (e.g. adjuvants, aggregates, genetic origin of mAb); (ii) dosing regimen (repeated dosing, administration route); (iii) concomitant medication (e.g. reduced risk with immunosuppressants); and (iv) patient-specific characteristics (e.g. genetic deficiency, disease state, human leukocyte antigen type) [67, 68, 71]. Naturally, not all of these factors can be avoided or taken into consideration. Repeated dosing, for example, is required for treatment of some chronic diseases. There might, however, be ways of optimising the therapy to minimise the risk. It has been suggested that the risk is lower when infliximab is given as maintenance therapy than when given as episodic therapy [72]. Whether the immunogenicity is truly caused by the intermittent regimen or if it is a result of other underlying factors remains to be elucidated.
At present, the impact of ADAs on pharmacokinetics and pharmacodynamics is intricate to predict, both in adults and children. Firstly, there are challenges to overcome regarding the bioanalytical assays. Many (of the older) assays are not able to reliably quantify ADAs if the therapeutic mAb is present in the sample and/or are only able to detect a specific immunoglobulin subclass [73]. Secondly, ADAs are predominately polyclonal. This implies variations between and within individuals when it comes to the type of developed antibody (immunoglobulin class, neutralising/non-neutralising), their affinity to the mAb, their abundance and the persistency of the immunisation [70, 71]. Hence, there is not only one type of ADA—not even within one patient over time. Consequently, caution should also be taken when comparing ADA data from different bioanalytical assays as they might represent different species (e.g. different semi-quantitative measures).
The important issue of immunogenicity is currently gaining attention in the field, both when it comes to the issues of the bioanalytical assays [73, 74] and to new approaches on how to model the ADA impact on pharmacokinetics and pharmacodynamics [75]. These efforts will hopefully fill some of the gaps in this area in the coming years.
6.2 Developmental Aspects
Newborns have limited ability to produce an immune response compared with older children and adults, both in quantitative (lower production of, for example, cytokines or reduced number of IgG-producing B cells) and qualitative terms (e.g. reduced ability of cytokines to induce chemotaxis, reduced function of dendritic cells) [55, 76]. During early life the immune system is fine-tuning a variety of key functions as a result of stimulation from encountered environmental signals. The response patterns learnt in this period persists into adult life; a neonate has mostly naïve T lymphocytes, whereas in adults half of the T cell population are of memory phenotype [55]. It is reasonable to believe that these differences can cause differences in the occurrence and/or impact of the ADAs on pharmacokinetics and pharmacodynamics. At present, however, the literature provides more questions than answers: will it take longer for the ADAs to develop in children than in adults? What happens during early school years when the immune system is highly active? Moreover, the challenges described in the previous section also apply for data in children. More information is urgently needed in this field.
6.3 Anti-Drug Antibody Occurrence and Impact on Pharmacokinetics and Pharmacodynamics
Twenty-eight of the 54 studies evaluating pharmacokinetics of mAbs in children also reported the occurrence of ADAs (Tables 3 and 5), eight of which reported no detectable ADAs during the study period [63, 77–86]. These studies were in general short, with a low number of doses (single dose in four out of eight). The rest reported a proportion of ADA-positive (ADA+) patients ranging from 2 to 77 %. In adults, the overall range of ADA occurrence is also large but the occurrence of each individual mAb is consistent with reports in children [3, 73, 75]. As previously mentioned, concomitant immunosuppressive medication has been described to affect ADA occurrence. Indeed, for adalimumab, a smaller proportion of ADA+ patients was reported for the group with concomitant methotrexate [87, 88], as for in adults [13].
Despite the fact that ADA occurrence was reported in more than half of the 54 studies in this review, few reported any comment on its impact on the pharmacokinetics, especially in the paediatric (sub)population. For infliximab, ADA occurrence was included as a predictor of increased CL when using adult and paediatric data [50]. The paediatric data alone was not sufficient to identify this effect. Similarly, Robbie et al. [42] used adult and paediatric data and identified a high ADA titre as an important descriptor of CL, while low titres were concluded not to be clinically relevant. These examples support that mAb CL may be increased by immunogenicity, although the ADA quantitative relationship and impact on pharmacokinetics and pharmacodynamics need to be further explored, both in children and adults.
7 Conclusions and Perspectives
Throughout this review, an exhaustive summary of pharmacokinetic and pharmacokinetic–pharmacodynamic characteristics of mAbs in children has been provided. Although a non-negligible number of studies evaluating pharmacokinetic characteristics was found, only a few examples comprehensively evaluated the pharmacokinetics/pharmacodynamics in children. Possible explanations for this situation could be an inadequate study design for pharmacokinetic analysis (e.g. sparse data) or inadequate analysis of the available data (EDCA vs. Pop. CMT). Limitations concerning the reporting of results have also been identified, complicating comparisons across studies and populations. Firstly, parameter information was reported in a very inconsistent fashion. The use of allometric scaling and normalising to a BW of 70 kg would enable easier comparison across studies as well as age groups [31]. Secondly, publication of model parameters and information regarding model-building strategies was incomplete. Publication of pharmacokinetic/pharmacodynamic models in full manuscripts rather than short abstracts, especially of internal models during drug development, could facilitate a better understanding and use of mAbs in children. All in all, these limitations constrained the ability to properly characterise pharmacokinetics with respect to body size and maturation and/or to establish relevant pharmacokinetic–pharmacodynamic relationships. Although not substantially covered in this review, drug–drug interactions also represent a poorly explored field that would benefit from further research.
The new EMA and FDA initiatives, requiring clinical drug research across age groups, will hopefully result in an increase in publically available information for this population. For future works, a ‘Guideline for Best Practice’ has been generated summarising aspects that should be considered when reporting pharmacokinetic and pharmacokinetic/pharmacodynamic studies in children (Box 1). This guideline will hopefully enable better comparisons, e.g. across studies, across the mAb drug class and with adult characteristics.
Population pharmacokinetic/pharmacodynamic modelling has proven to be an extremely valuable tool, especially in sparse data situations. It allows for (i) estimation of exposure-response relationships; (ii) quantification and explanation of variability; as well as (iii) prediction of the impact of variability and/or covariates on the final outcome. Once established, pharmacokinetic/pharmacodynamic models can thus enable a better understanding of differences between adult and paediatric patients and allow for a more rational drug use in the paediatric population, as, for example, has been shown for omalizumab. Similarly, physiologically based pharmacokinetic/pharmacodynamic models have been successfully used to adapt doses of small molecules to special populations (e.g. children). To develop these models, detailed knowledge on the impact of body composition, organ function and maturation processes on the pharmacokinetics/pharmacodynamics of mAbs is needed. In vitro data could be helpful to assess some of these aspects. Both modelling approaches could be used to optimise study designs (number of samples and individuals, and/or sample times), which is an important aspect in paediatric clinical trials.
In conclusion, limited information regarding the pharmacokinetics and, even more pronounced, pharmacokinetic–pharmacodynamic relationships of mAbs in children is available in the public domain. Further pharmacokinetic/pharmacodynamic studies are needed to allow for a more rational use of mAbs in the paediatric population, and also regarding the off-label use of mAbs in daily clinical practice. Furthermore, studies characterising maturation processes and biological differences between adults and children are needed to assess their potential impact on drug exposure and response. Wisely used, population approaches—combined with physiologically based pharmacokinetic/pharmacodynamic models or systems pharmacology approaches—can provide a global comprehension of the system.
Notes
Many other abbreviations are also used in the literature, e.g. HAMA (human anti-murine antibodies), HACA (human anti-chimeric antibodies), HAHA (human anti-human(ised) antibodies); for some mAbs, specific abbreviations have been introduced, e.g. ATI (antibodies towards infliximab).
References
Pharmaceutical Research and Manufacturers of America (PhRMA). Medicines in development. Biologics 2013 report. http://www.phrma.org/sites/default/files/pdf/biologics2013.pdf. Accessed 15 May 2014.
Belot A, Cochat P. Les biothérapies en pédiatrie [Biological therapy in pediatrics] [in French]. Arch Pediatr. 2010;17:1573–82.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59.
Keizer RJ, Huitema ADR, Schellens JHM, Beijen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507.
Levy G. Pharmacologic target-mediated drug disposition. Clin. Pharmacol Ther. 1994;56:248–52.
Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58.
Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24:23–39.
Tabrizi M, Tseng C, Roskos L. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–68.
Strolin Benedetti M, Whomsley R, Baltes EL. Differences in absorption, distribution, metabolism and excretion of xenobiotics between the paediatric and adult populations. Expert Opin Drug Metab Toxicol. 2005;1:447–71.
Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–86.
Stephenson T. How children’s responses to drugs differ from adults. Br J. Clin Pharmacol. 2005;59:670–3.
EMA. European public assessment reports [online database]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d124. Accessed 30 Apr 2014.
FDA. Drugs@FDA [online database]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?CFID=27729622&CFTOKEN=dde04172480c4c9e-D1772092-D394-B5A6-256F39805045CED6#labelinfo. Accessed 30 Apr 2014.
Cuzzolin L, Zaccaron A, Fanos V. Unlicensed and off-label uses of drugs in paediatrics: a review of the literature. Fundam Clin Pharmacol. 2003;17:125–31.
Gillick MR. Controlling off-label medication use. Ann Intern Med. 2009;150:344–7.
Prytuła A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, et al. Rituximab in refractory nephrotic syndrome. Pediatr Nephrol. 2010;25:461–8.
O’Connor K, Liddle C. Prospective data collection of off-label use of rituximab in Australian public hospitals. Intern Med J. 2013;43:863–70.
Liang Y, Zhang L, Gao J, Hu D, Ai Y. Rituximab for children with immune thrombocytopenia: a systematic review. PLoS One. 2012;7:e36698.
Anink J, Otten MH, Prince FHM, Hoppenreijs EPAH, Wulffraat NM, Swart JF, et al. Tumour necrosis factor-blocking agents in persistent oligoarticular juvenile idiopathic arthritis: results from the Dutch arthritis and biologicals in children register. Rheumatology. 2013;52:712–7.
Otten MH, Prince FHM, ten Cate R, van Rossum MAJ, Twilt M, Hoppenreijs EPAH, et al. Tumour necrosis factor (TNF)-blocking agents in juvenile psoriatic arthritis: are they effective? Ann Rheum Dis. 2011;70:337–40.
Speer ME, Fernandes CJ, Boron M, Groothuis JR. Use of palivizumab for prevention of hospitalization as a result of respiratory syncytial virus in infants with cystic fibrosis. Pediatr Infect Dis J. 2008;27:559–61.
Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008;153:833–8.
Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med. 2014;370:1268–70.
Steinbrook R. Testing medications in children. N Engl J Med. 2002;347:1462–70.
EMA, Paediatric Committee. Medicines for children. Paediatric investigation plans; 2007. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000302.jsp&mid=WC0b01ac058002d4ea. Accessed 13 May 2014.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Draft guidance for industry. Pediatric Study Plans: Content of and process for submitting. Initial pediatric study plans and amended pediatric study plans. Draft guidance. July 2013. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM360507.pdf. Accessed 13 May 2014.
Zhang X, Hsu J, Brunner H, De Benedetti F, Le Gallo C, Zuber Z, et al. Pharmacokinetics (PK) and pharmacodynamics (PD) of tocilizumab (TCZ) in polyarticular-course juvenile idiopathic arthritis (pcJIA). Clin Pharmacol Ther. 2013;93(Suppl 1):S88–9.
Johnson TN. The problems in scaling adult drug doses to children. Arch Dis Child. 2008;93:207–11.
Shi R, Derendorf H. Pediatric dosing and body size in biotherapeutics. Pharmaceutics. 2010;2:389–418.
Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098.
Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6.
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71.
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52:83–124.
Xu Z, Davis HM, Zhou H. Rational development and utilization of antibody-based therapeutic proteins in pediatrics. Pharmacol Ther. 2013;137:225–47.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14:559–70.
Kagan L, Mager DE. Mechanisms of subcutaneous absorption of rituximab in rats. Drug Metab Dispos. 2013;41:248–55.
Kuester K, Kloft C. Pharmacokinetics of monoclonal antibodies (part I and II). In: Meibohm B, editor. Pharmacokinetics and pharmacodynamics of biotech drugs: principles and case studies in drug development. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2006. p. 45–91.
Lowe PJ, Renard D. Omalizumab decreases IgE production in patients with allergic (IgE-mediated) asthma; PKPD analysis of a biomarker, total IgE. Br J Clin Pharmacol. 2011;72:306–20.
Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother. 2012;56:4927–36.
Krippendorff B-F, Kuester K, Kloft C, Huisinga W. Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn. 2009;36:239–60.
Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114:168–72.
Butte NF, Hopkinson JM, Wong WW, O’Brian Smith E, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47:578–85.
Ternant D, Mulleman D, Degenne D, Willot S, Guillaumin J-M, Watier H, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of infliximab. Ther Drug Monit. 2006;28:169–74.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25.
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51:119–35.
Fasanmade A, Adedokun O, Olson A, Strauss R, Davis H. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol. Ther. 2010;48:297–308.
Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946–64.
Kapel N, Meillet D, Favennec L, Magne D, Raichvarg D, Gobert JG. Evaluation of intestinal clearance and faecal excretion of alpha 1-antiproteinase and immunoglobulins during Crohn’s disease and ulcerative colitis. Eur J Clin Chem Clin Biochem. 1992;30:197–202.
Brandse JF, Wildenberg ME, de Bruyn JR, Wolbink G, Lowenberg M, Ponsioen CY, et al. P500 Fecal loss of infliximab as a cause of lack of response in severe inflammatory bowel disease [abstract]. J Crohns Colitis. 2013;7:S210.
Struemper H, Cai W. Population pharmacokinetics of belimumab in systemic lupus erythematosus: insights for monoclonal antibody covariate modeling from a large data set [abstract no. 2792]. PAGE 22; 11–14 Jun 2013; Glasgow. http://www.page-meeting.org/?abstract=2792. Accessed 22 Oct 2014.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76.
Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–97.
Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007;99:281–3.
Garcia-Lloret M, McGhee S, Chatila T. Immunoglobulin replacement therapy in children. Immunol Allergy Clin N Am. 2008;28:833–49.
Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol. 2009;68:61–76.
Goldman J, Davis H, Zhou H, Kearns G. Infliximab clearance in children: potential association with resting energy expenditure. Ann Paediatr Rheum. 2012;1:120–5.
Kovarik JM, Offner G, Broyer M, Niaudet P, Loirat C, Mentser M, et al. A rational dosing algorithm for basiliximab (Simulect) in pediatric renal transplantation based on pharmacokinetic-dynamic evaluations. Transplantation. 2002;74:966–71.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry and FDA staff. Qualification process for drug development tools. Jan 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM230597.pdf. Accessed 14 Feb 2014.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–32.
Weisman LE, Thackray HM, Garcia-Prats JA, Nesin M, Schneider JH, Fretz J, et al. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrob Agents Chemother. 2009;53:2879–86.
Kokai-Kun JF, Mould D, Weisman L, Bloom B, Eyal F, Polack M, et al. Predicted and measured pagibaximab serum levels in high-risk neonates [poster no. 1380]. Pediatr Res. 2010;68:683.
Höcker B, Kovarik JM, Daniel V, Opelz G, Fehrenbach H, Holder M, et al. Pharmacokinetics and immunodynamics of basiliximab in pediatric renal transplant recipients on mycophenolate mofetil comedication. Transplantation. 2008;86:1234–40.
Nagai T, Gotoh Y, Watarai Y, Tajima T, Arai K, Uchida K. Pharmacokinetics and pharmacodynamics of basiliximab in Japanese pediatric renal transplant patients. Int J Clin Pharmacol Ther. 2010;48:214–23.
De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–90.
Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20:vi3–9.
Niebecker R, Kloft C. Safety of therapeutic monoclonal antibodies. Curr Drug Saf. 2010;5:275–86.
Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14:296–302.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry: immunogenicity assessment for therapeutic protein products. 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338856.pdf. Accessed 22 Oct 2014.
Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond R, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53.
Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases. Ann Rheum Dis. 2013;72:165–78.
Wang S-L, Ohrmund L, Hauenstein S, Salbato J, Reddy R, Monk P, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012;382:177–88.
Gómez-Mantilla JD, Trocóniz IF, Parra-Guillén Z, Garrido MJ. Review on modeling anti-antibody responses to monoclonal antibodies. J Pharmacokinet Pharmacodyn. Epub 2014 Jul 16.
Siegrist C-A, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94.
Angiolillo AL, Yu AL, Reaman G, Ingle AM, Secola R, Adamson PC, et al. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a children’s oncology group report. Pediatr Blood Cancer. 2009;53:978–83.
Kuemmerle-Deschner JB, Hachulla E, Cartwright R, Haweekins PN, Tran TA, Bader-Meunier B, et al. Two-year results from an open-label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin-associated periodic syndrome across different severity phenotypes. Ann Rheum Dis. 2011;70:2095–102.
Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360:2416–25.
Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2396–406.
Ruperto N, Quartier P, Wulffraat N, Woo P, Ravelli A, Mouy R, et al. A phase II, multicenter, open-label study evaluating dosing and preliminary safety and efficacy of canakinumab in systemic juvenile idiopathic arthritis with active systemic features. Arthritis Rheum. 2012;64:557–67.
Baldassano R, Braegger CP, Escher JC, DeWoody K, Hendricks DF, Keenan GF, et al. Infliximab (Remicade) therapy in the treatment of pediatric Crohn’s disease. Am J Gastroenterol. 2003;98:833–8.
Hadigan C, Baldassano R, Braegger CP, Vasiliauskis E, Escher JC, Sinaasappel M, et al. Pharmacokinetics of infliximab (Anti-TNF) in children with Crohn’s disease: a multicenter trial [abstract]. J Pediatr Gastroenterol Nutr. 1999;29:525.
Abarca K, Jung E, Fernández P, Zhao L, Harris B, Connor EM, et al. Safety, tolerability, pharmacokinetics, and immunogenicity of motavizumab, a humanized, enhanced-potency monoclonal antibody for the prevention of respiratory syncytial virus infection in at-risk children. Pediatr Infect Dis J. 2009;28:267–72.
Weisman LE, Thackray HM, Steinhorn RH, Walsh WF, Lassiter HA, Dhanireddy R, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128:271–9.
Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184:350–4.
Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359:810–20.
Imagawa T, Takei S, Umebayashi H, Yamaguchi K, Itoh Y, Kawai T, et al. Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clin Rheumatol. 2012;31:1713–21.
Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn’s disease in children. Gastroenterology. 2012;143:365–74.
Joy MS, Gipson DS, Powell L, MacHardy J, Jennette JC, Vento S, et al. Phase 1 trial of adalimumab in focal segmental glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis. 2010;55:50–60.
Kingsbury DJ, Bader-Meunier B, Patel G, Arora V, Kalabic J, Kupper H. Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2–4 years. Clin. Rheumatol. 2014;33:4133–41.
Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a children’s oncology group study. J Clin Oncol. 2008;26:399–405.
Trippett TM, Herzog C, Whitlock JA, Wolff J, Kuttesch J, Bagatell R, et al. Phase I and pharmacokinetic study of cetuximab and irinotecan in children with refractory solid tumors: a study of the pediatric oncology experimental therapeutic investigators’ consortium. J Clin Oncol. 2009;27:5102–8.
Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:256–62.
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81.
Buckwalter M, Dowell JA, Korth-Bradley J, Gorovits B, Mayer PR. Pharmacokinetics of gemtuzumab ozogamicin as a single-agent treatment of pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Pharmacol. 2004;44:873–80.
Candon S, Mosca A, Ruemmele F, Goulet O, Chatenoud L, Cézard J-P. Clinical and biological consequences of immunization to infliximab in pediatric Crohn’s disease. Clin Immunol. 2006;118:11–9.
Adedokun OJ, Xu Z, Padgett L, Blank M, Johanns J, Griffiths A, et al. Pharmacokinetics of infliximab in children with moderate-to-severe ulcerative colitis: results from a randomized, multicenter, open-label, phase 3 study. Inflamm Bowel Dis. 2013;19:2753–62.
Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–73 (quiz 1165–6).
Hyams J, Walters TD, Crandall W, Kugathasan S, Griffiths A, Blank M, et al. Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn’s disease in children: REACH open-label extension. Curr Med Res Opin. 2011;27:651–62.
Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–106.
Fernández P, Trenholme A, Abarca K, Griffin MP, Hultquist M, Harris B, et al. A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season. BMC Pediatr. 2010;10:38.
Grundy J, Pan W, Kugathasan S, Nagpala F, Hilton D, Taylor J, et al. Pharmacokinetics and pharmacodynamics of natalizumab in a phase 2 study of adolescent patients with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2005;41:493–563.
Hyams JS, Wilson DC, Thomas A, Heuschkel R, Mitton S, Mitchell B, et al. Natalizumab therapy for moderate to severe Crohn disease in adolescents. J Pediatr Gastroenterol Nutr. 2007;44:185–91.
Sáez-Llorens X, Moreno MT, Ramilo O, Sánchez PJ, Top FH, Connor EM. Safety and pharmacokinetics of palivizumab therapy in children hospitalized with respiratory syncytial virus infection. Pediatr Infect Dis J. 2004;23:707–12.
Sáez-Llorens X, Castaño E, Null D, Steichen J, Sánchez PJ, Ramilo O, et al. Safety and pharmacokinetics of an intramuscular humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. The MEDI-493 Study Group. Pediatr Infect Dis J. 1998;17:787–91.
Subramanian KN, Weisman LE, Rhodes T, Ariagno R, Sánchez PJ, Steichen J, et al. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatr Infect Dis J. 1998;17:110–5.
Barth MJ, Goldman S, Smith L, Perkins S, Shiramizu B, Gross TG, et al. Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia: a children’s oncology group report. Br J Haematol. 2013;162:678–83.
Pranzatelli MR, Tate ED, Verhulst SJ, Bertolone SJ, Bhatla D, Granger M, et al. Pediatric dosing of rituximab revisited: serum concentrations in opsoclonus-myoclonus syndrome. J Pediatr Hematol Oncol. 2010;32:e167–72.
Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol. 2009;24:1321–8.
Brunner H, Ruperto N, Zuber Z, Keane C, Harari O, Kenwright A, et al. Efficacy and safety of tocilizumab in patients with polyarticular juvenile idiopathic arthritis: data from a phase 3 trial [abstract no. 1597]. Arthritis Rheum. 2012;64:s682.
Woo P, Wilkinson N, Prieur A-M, Southwood T, Leone V, Livermore P, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–8.
Zhang X, Morcos PN, Saito T, Terao K. Clinical pharmacology of tocilizumab for the treatment of systemic juvenile idiopathic arthritis. Expert Rev Clin Pharmacol. 2013;6(2):123–37.
Yokota S, Imagawa T, Mori M, Miyamae T, Aihara Y, Takei S, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006.
Zhang X, Morcos PN, De Benedetti F, Brunner H, Ruperto N, Schaefer F, et al. Pharmacokinetics and pharmacodynamics of tocilizumab in systemic juvenile idiopathic arthritis [abstract]. Pharmacotherapy. 2011;31(10):381e.
Kakkar T, Sung C, Gibiansky L, Vu T, Narayanan A, Lin S-L, et al. Population PK and IgE pharmacodynamic analysis of a fully human monoclonal antibody against IL4 receptor. Pharm Res. 2011;28:2530–42.
Turner DC, Navid F, Daw NC, Mao S, Wu J, Santana VM, et al. Population pharmacokinetics of bevacizumab in children with osteosarcoma: implications for dosing. Clin Cancer Res. 2014;20:2783–92.
Pescovitz MD, Knechtle S, Alexander SR, Colombani P, Nevins T, Nieforth K, et al. Safety and pharmacokinetics of daclizumab in pediatric renal transplant recipients. Pediatr Transplant. 2008;12:447–55.
Xu Z, Mould D, Hu C, Ford J, Keen M, Davis HM, et al. Population pharmacokinetic analysis of infliximab in pediatrics using integrated data from six clinical trials. Clin Pharmacol Drug Dev. 2012;4:203.
Zhao L, Roskos L, Griffin P, Losonsky G, Jallal GB, Robbie G. 891 Population pharmacokinetics analysis of motavizumab in children at risk for respiratory syncytial virus infection. Pediatr. Res. 2010;68:447.
Marian M, Sun Y-N, Sinclair D, Kenkare S, Sallas W, Mortensen D, et al. PI-23 Clincal pharmacokinetics (PK) and IgE pharmacodynamics (PD) of omalizumab, a recombinant humanized monoclonal antibody to IgE. Clin Pharmacol Ther. 2001;69:P7.
Bennett CM, Rogers ZR, Kinnamon DD, Bussel JB, Mahoney DH, Abshire TC, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107:2639–42.
Hagopian W, Ferry RJ, Sherry N, Carlin D, Bonvini E, Johnson S, et al. Teplizumab preserves C-Peptide in recent-onset type 1 diabetes. Two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901–8.
Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487–97.
Acknowledgments
The authors wish to thank Corina Becker (Bayer Pharma) for fruitful discussion, Tamara Richter and Caroline Fongern (both Freie Universitaet Berlin) for their support during the literature search, and the reviewers of this manuscript for their valuable input into the manuscript. No sources of funding were used for the preparation of this review. The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edlund, H., Melin, J., Parra-Guillen, Z.P. et al. Pharmacokinetics and Pharmacokinetic–Pharmacodynamic Relationships of Monoclonal Antibodies in Children. Clin Pharmacokinet 54, 35–80 (2015). https://doi.org/10.1007/s40262-014-0208-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0208-4