Abstract
Appropriate use of antidepressant in patients with hepatic impairment requires careful consideration of how the hepatic illness may affect pharmacokinetics. This review aims to analyze pharmacokinetic profile, plasma level variations so as the metabolism of several antidepressants relating to their use in patients with an hepatic impairment. Due to the lack of data regarding hepatic impairment itself, the review is focused mainly on studies investigating pharmacokinetics in hepatic cirrhosis or alcohol-related conditions. More data on reduced hepatic metabolism can be extrapolated by drug studies conducted in elderly populations. Dose adjustment of antidepressants in these patients is important as most of these drugs are predominantly metabolized by the liver and many of them are associated with dose-dependent adverse reactions. As no surrogate parameter is available to predict hepatic metabolism of drugs, dose adjustment according to pharmacokinetic properties of the drugs is proposed. There is a need for a more balanced assessment of the benefits and risks associated with antidepressants use in patients with hepatic impairment, particularly considering pharmacokinetic profile of the drugs to ensure that patients, who would truly benefit from these agents, are not denied appropriate treatment. In conclusion, kinetic studies for centrally acting drugs including antidepressants with predominant hepatic metabolism should be carried out in patients with liver disease to allow precise dose recommendations for enhanced patient safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The different pharmacokinetic properties of the antidepressants are altered by hepatic impairment. |
Assessment of hepatic function is necessary so that appropriate dose adjustment of antidepressants can be made to allow an appropriate treatment for patients. |
1 Introduction
Pharmacokinetic parameters of many drugs including antidepressants depend on adequate hepatic function. Every drug, especially those with a narrow therapeutic range (i.e. little difference between toxic and therapeutic doses) run the risk of accumulating and causing toxicity in patients with hepatic disease.
The liver receives a dual blood supply with about 20 % of blood coming from the hepatic artery and 80 % from the portal circulation. The blood flow to the liver is around 20–25 % of the total cardiac output. Toxins, infectious agents, medications and serum inflammatory mediators may result in a various range of processes leading to a reduction of hepatic functioning and causing a loss of normal histological architecture of liver, reduction of cell mass and loss of blood flow. Consequently, functional liver capacity may be lost.
A reduction in hepatic blood flow can occur in some hepatic diseases, such as liver cirrhosis, causing a decrease in the pre-systemic elimination (i.e., first-pass effect). This lead to a significant increase in the extent of systemic absorption.
Liver dysfunction can not only reduce the blood/plasma clearance of drugs eliminated by hepatic metabolism or biliary excretion, but it can also affect plasma protein binding, which in turn could influence the processes of distribution and elimination.
Aging is associated with a reduction of ~40 % in hepatic blood flow and 30 % in liver mass and an impairment in hepatic drug metabolism in older people has been attributed to these changes: many pharmacokinetic studies have documented a decline in the clearance of drugs undergoing liver metabolism in aging individuals. The reduction in hepatic metabolism with age is also important in first pass metabolism [1, 2].
Chronic liver diseases are associated with variable and non-uniform reductions in drug-metabolizing activities. For example, the activity of the various cytochrome P450 enzyme system (CYP) enzymes seems to be differently affected in patients with cirrhosis. Glucuronidation is often considered to be affected to a lesser extent than CYP-mediated reactions in mild to moderate cirrhosis but can also be substantially impaired in patients with advanced cirrhosis [3].
Alterations of these metabolic and/ or excretory functions in patients with liver disease, most pronounced in patients with liver cirrhosis, can lead to drug accumulation or, less often, failure to form an active metabolite.
On the contrary, an initial, mild or moderate hepatic impairment can lead to an induced hepatic metabolism (e.g. alcoholism, drug abuse) that could reduce drug plasma levels [4]. Very recent data on the effects of mild or moderate hepatic impairment on the pharmacokinetics of a peripherally acting µ-opioid receptor antagonist (naloxegol) reported a shorter terminal half-life in patients with mild and moderate hepatic impairment versus healthy subjects [5].
Assessing hepatic function is necessary so that drug dose appropriate adjustment can be made. However, this is not always straightforward as there is no single test that reliably measures liver function. There is no simple endogenous marker to predict hepatic function with respect to the elimination capacity of specific drugs. Several quantitative liver tests that measure the elimination of marker substrates such as galactose, sorbitol, antipyrine, caffeine, erythromycin, and midazolam, have been developed and evaluated, but no single test has gained widespread clinical use to adjust dosage regimens for drugs in patients with hepatic dysfunction.
The semi-quantitative Child–Pugh score [6] is frequently used to assess the severity of liver function impairment, but it offers only the clinician rough guidance for dosage adjustment because it lacks the sensitivity to quantitate the specific ability of the liver to metabolize individual drugs.
The recommendations of the US Food and Drug Administration and the European Medicines Evaluation Agency to study the effect of liver disease on the pharmacokinetics of drugs under development is clearly aimed at generating, if possible, specific dosage recommendations for patients with hepatic dysfunction.
In any case, characterization of the status of hepatic function would benefit by being quantified on the basis of an independent measure of metabolism of a marker known to be influenced by liver disease in addition to clinical assessment by a semi-quantitative Child–Pugh score.
The Child–Turcotte score was designed to estimate the operative risk of patients with cirrhosis [7]. The parameters used include serum concentrations of bilirubin and albumin, prothrombin time, nutritional status and ascites. These parameters were modified to substitute degree of encephalopathy for nutritional status and then became known as the Child–Pugh classification (Table 1). The grades A, B and C may also be a useful indicator of an individual’s ability to effectively metabolise a drug. An alternative method for assessing liver dysfunction is the Model for End-Stage Liver Disease score [7]. This may be a more accurate method but is less accessible to most clinicians because it involves calculating the score.
Another of the most widely used liver function tests is galactose elimination capacity (GEC). It is relatively simple but still involves a number of capillary blood samples: simplified formulas have been proposed [8].
On the other hand, from a more strictly clinical point of view, it is possible to assess liver function by using blood tests such as serum albumin and bilirubin, as well as prothrombin time. Moreover, liver enzyme concentrations may be useful indicators of hepatocellular damage or enzyme induction.
Drugs acting on the central nervous system including antidepressants are often prescribed to cirrhotic patients because of a variety of psychiatric symptoms or illnesses associated with liver cirrhosis. In fact, chronic depressive symptoms are not uncommon in patients with cirrhosis.
Most of the psychotropic drugs including antidepressants are lipophilic and are extensively metabolized through the liver, involving also biotransformation by CYP iso-enzymes.
In patients with cirrhosis, the decrease in hepatic clearance and hepatic extraction results in an increased risk for dose-related adverse drug reactions. But not only pharmacokinetic changes should be considered when prescribing centrally acting drugs, pharmacodynamic changes have also been reported in patients with liver cirrhosis.
Prescribing to patients with liver cirrhosis requires careful drug selection and dose adjustment based on the pharmacokinetic profile may prevent adverse effects. Classification according to pharmacokinetic properties and results from clinical trials in patients with liver cirrhosis and/ or other liver diseases can therefore help to select and administer drugs more rationally in this group of patients [9].
In other words, drugs, including antidepressants, must be given with caution to patients with severe hepatic insufficiency such as is the case of cirrhosis. Before administering drugs that are largely eliminated by hepatic mechanisms, their potential therapeutic benefits must be carefully counterbalanced with their risk for toxic reactions. If these drugs are needed by the cirrhotic patient, they should be started at a low dose which may subsequently be titrated to obtain the desired therapeutic effect.
2 Pharmacokinetics of Antidepressants
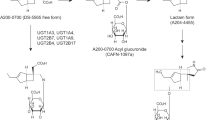
The pharmacokinetics of antidepressants are often described by a two-compartment model. These substances have to be lipophilic in order to pass the blood–brain barrier and thus are likely to distribute into peripheral compartments. This lipophilic property may be one of the reasons why they undergo extensive metabolism in the liver and show a first-pass effect, leading to variable bioavailability ranging from 30 to 80 %. The time until peak plasma concentration is reached varies between 1 and 12 h. These drugs are mainly metabolized by the liver via oxidation by the CYP and glucuronidation.
In particular, oxidative drug metabolism is catalysed by the hepatic CYP enzyme system. Five enzymes (CYP3A4, CYP1A2, CYP2C9, CYP2C19, CYP2D6) account for the metabolism of the majority of antidepressants. CYP3A4 is the most frequent enzyme in the liver followed by CYP1A2, CYP2C9, CYP2C19 and CYP2D6.
In general, the half-life of antidepressants ranges from 9 to 40 h. Most antidepressants are highly bound to plasma proteins. A linear relationship between dose and plasma concentrations exists for most antidepressants, except for paroxetine, fluvoxamine and clomipramine. On the other hand, although many attempts were made to date, convincing evidence of a relationship between plasma concentrations and clinical efficacy doesn’t exist. The pharmacokinetic parameters of the drugs that were analysed in this work are detailed in Table 2 [10–13, 15, 16].
The pharmacokinetic behaviour of a drug is altered by factors affecting the absorption, distribution or elimination process. In particular elimination is altered by liver or renal function, by the activity of metabolizing enzymes or transporters. Co-medication can influence every pharmacokinetic process. Nowadays, controlled clinical studies evaluate the influence of factors that are likely to be relevant in patients where the drug will be administered. However, these studies consist of a small number of carefully selected participants and except for one particular factor all others influencing the pharmacokinetics are excluded.
Studies evaluating the influence of several covariates in a naturalistic clinical setting are rare because of the lack of dense pharmacokinetic data, but over the years new pharmacokinetic methods were developed that are based on a population approach rather than modelling individual pharmacokinetics.
As the mechanisms underlying pharmacokinetic variability have been intensively studied over the last twenty years, this knowledge is now included in drug development. Nevertheless, old drugs still remain less well studied [17, 18].
We will now analyse the variation of single pharmacokinetic parameters of the classical and new generation of antidepressants due to the hepatic impairment (Table 3).
A literature search of the US National Library of Medicine’s PubMed database for the text words ‘antidepressants and liver disease’, ‘hepatic impairment and antidepressants’, ‘pharmacokinetics and hepatic impairment’, ‘liver disease and pharmacokinetics’, ‘hepatic dysfunctions and antidepressants’, ‘pharmacokinetics of—the name of antidepressant—’have been made.
Data sheets of antidepressants reported in the text have been consulted.
3 Tricyclic Antidepressants
The pharmacokinetics of tricyclic antidepressants (TCAs) are characterized by substantial presystemic first-pass metabolism, a large volume of distribution, extensive protein binding, and an elimination half-life averaging about 1 day.
Tricyclics undergo multiple biotransformation actions in the liver, producing progressively more polar metabolites which can be readily excreted by the kidneys [19]. Less than 5 % of a dose of a TCA is eliminated unchanged. Through the processes of demethylation, oxidation, and/or hydroxylation, metabolites, which are generally pharmacologically active, are formed. These reactions are catalyzed primarily by the hepatic mixed function P450 enzymes, a family of more than 30 isoenzymes in the hepatocyte endoplasmic reticulum, through two major pathways [20].
Tertiary amine TCAs are N-demethylated to secondary amine forms. Both tertiary and secondary amine tricyclics undergo aromatic hydroxylation [21, 22].
There are very limited data concerning the use of TCAs in patients with liver disease. Some clinical indications can be obtained from data on their use in elderly where there is a “physiological” impairment of hepatic drug clearance.
3.1 Amitriptyline and its Metabolite Nortriptyline
Amitriptyline undergoes extensive first-pass hepatic metabolism, the systemic bioavailability being of 45 %. There is wide individual variation in the pharmacokinetic profile of amitriptyline. It is metabolised in the liver, the primary routes of metabolism being demethylation, hydroxylation and conjugation. It is considered that the metabolic pathways are mediated by the enzymes CYP2D6 and CYP2C19, although other enzymes are probably also involved [23]. The major active metabolites formed are nortriptyline, 10-hydroxyamitriptyline, and 10-hydroxynortriptyline. Both nortriptyline and 10-hydroxynortriptyline contribute significantly to the antidepressant effect [24]. Amitriptyline is excreted mainly in the urine as conjugated and unconjugated metabolites. Less than 5 % is excreted as unchanged drug [25].
Hepatic impairment: reduced metabolic capacity in liver impairment results in accumulation of amitriptyline [26]. There is some evidence to suggest that higher plasma concentrations of amitriptyline occur in females over the age of 50 than in males of a similar age [23, 27]. Similar data on nortriptyline indicated a longer plasma half-life and slower clearance in elderly depressed patients [28].
From data extrapolated by aged patients and product informations is possible to indicate a range of amitriptyline oral dosage in patients with hepatic impairment of 30–40 mg/die with a possible increase to a maximum of 100 mg/die.
Regarding nortriptyline, the dosage should be of 25 mg/die not exceeding 150 mg/die. In hepatopathic patients is indicated the plasma level determination of the drug considering that the therapeutic range in the adults is of 50–140 ng/ml [29, 30]. However in case of liver disease the use of second generation antidepressants is advisable.
3.2 Imipramine and its Metabolite, Desipramine
Available data report that patients with an initial hepatic dysfunction (e.g. alcoholics) had a threefold greater intrinsic clearance of imipramine [31]. These patients had been found to have significantly greater total body clearance of imipramine (0.93 vs. 0.48 L/h/kg) and desipramine (1.00 vs. 0.62 L/h/kg) than did control subjects. The mean elimination half-life for imipramine was significantly decreased (8.7 vs. 19.9 h) after intravenous infusion and 10.9 vs. 19.6 h after oral administration. The mean elimination half-life for desipramine was decreased after intravenous infusion (16.5 vs. 22.4 h). These findings suggested that initial liver dysfunction (e.g. detoxified alcoholics) might require higher doses of imipramine. Desipramine clearance was affected to a lesser degree than imipramine, suggesting that from a pharmacokinetic standpoint it may be the preferred drug for the treatment of depressed patients with an initial liver dysfunction.
Considering the reduction in hepatic metabolism with age, the clearance of imipramine has been shown to be reduced by 10–50 % in elderly depressed patients. In particular, imipramine half-life has been shown to be markedly prolonged in elderly vs young males (28.6 vs. 16.5 h) and females (30.2 vs. 17.8 h) due to decreased clearance (males 567 vs. 945 ml/min, females 599 vs. 975 ml/min) with no change in volume of distribution. In contrast, after p.o. desipramine more limited age-related changes were noted. Desipramine half-life was slightly prolonged in elderly males (30.8 vs. 21.2 h) [32, 33].
There are no data on dose adjustments in patients with liver dysfunction. However caution is recommended especially in cases of severe liver dysfunction. From data extrapolated by elderly patients and product informations it is possible to indicate a range of imipramine oral dosage in patients with hepatic impairment of 30–50 mg/die. For desipramine the initial dose is 10–25 mg/day orally given as a single dose or in divided doses. The dose should be gradually increased according to tolerance and clinical response. The maximum dose is 150 mg/day. Monitoring plasma levels can facilitate treatment response by providing objective guidelines for dosage adjustment [31, 34].
However, in case of liver disease, the use of second generation antidepressants is advisable.
4 Second Generation Antidepressants
4.1 Fluoxetine
In most countries, fluoxetine was the first SSRI that became available for clinical use.
Due to hepatic first-pass metabolism, the oral bioavailability is below 90 % [12, 35]. It undergoes extensive metabolic conversion, leading to the active metabolite norfluoxetine and multiple other metabolites. For norfluoxetine, t½ ranges even between 7 and 15 days [36, 37]. Because of the long t½, 1–22 months are required to achieve steady-state conditions [35]. Fluoxetine exhibits nonlinear kinetics, indicated by a disproportionate increase in its blood concentrations after dose escalation. Under multiple dosing, longer t½ and reduced oral clearance result, compared with single doses [38].
Abnormalities in the elimination of fluoxetine have not been reported for patients with renal impairment, whereas the pharmacokinetics of fluoxetine were affected by hepatic dysfunction. The t½β (terminal half life) was significantly longer (7.6 vs. 2.8 days) and plasma clearance was lower (14.5 vs. 43.31 L/h) in patients with alcohol-related cirrhosis of the liver than in individual with normal hepatic function. The Vd was similar in patients with cirrhosis and healthy individuals (46.8 and 42.5 L/h, respectively) [11, 37, 39].
The kinetics of norfluoxetine are also altered: apparent oral clearance is decreased by 30 % and t½ prolonged (12 vs. 6.4 days in healthy individuals). Therefore, upon repeated administration, excessive accumulation of the drug can be expected, thus increasing the risk of toxicity and exaggerated pharmacological response. Based on the pharmacokinetic modifications observed in the patients with cirrhosis, a lower dosage (about a 50 % reduction) or prolonged interval should be used [40]. In other words the reduction of the maximum oral dosage to 40 mg/day is indicated. Plasma level determination of fluoxetine and norfluoxetine can be only orientative because they were not reported to be related to clinical outcome [41].
4.2 Fluvoxamine
The pharmacokinetic profile of fluvoxamine are well established. Despite complete absorption, oral bioavailability in man is ~50 % on account of first-pass hepatic metabolism. Fluvoxamine displays nonlinear steady-state pharmacokinetics over the therapeutic dose range, with disproportionally higher plasma concentrations with higher dosages. Plasma fluvoxamine concentrations show no clear relationship with antidepressant response or severity of adverse effects. Fluvoxamine undergoes extensive oxidative liver metabolism. Nine metabolites have been identified, none of which are known to be pharmacologically active. CYP2D6, which is crucially involved in the metabolism of paroxetine and fluoxetine, appears to play a clinically insignificant role in the metabolism of fluvoxamine. The drug is excreted in the urine, predominantly as metabolites, with only negligible amounts (<4 %) of the parent compound [42].
Blood concentrations of fluvoxamine in patients with severe renal impairment treated with 100 mg/day fluvoxamine maleate were similar to those observed in healthy volunteers, indicating that the pharmacokinetics of fluvoxamine do not primarily depend on the renal function [42]. In contrast, in patients with hepatic cirrhosis, the area under the concentration-time curve (AUC) and t½ were significantly increased compared with healthy controls [12] so as its elimination is prolonged in patients with hepatic cirrhosis.
In 13 patients with alcoholic liver cirrhosis, after a single oral dose of fluvoxamine 100 mg the AUC and the t½ were about 50 % higher than in healthy volunteers [43]. This increase is evidenced by a reduced metabolism (clearance 54.8 L/h).
Pharmacokinetics were found to be similar in elderly (mean age 73 years) and young subjects (mean age 28 years) [44] indicating no oral dosage adjustment in case of mild liver impairment.
On the other hand it is recommended that patients with moderate or severe liver dysfunction should be given a lower initial daily dosage with a longer interval between doses and this should be followed by careful monitoring. The oral dosage should be limited to a maximum of 100–150 mg/die. Plasma level determination of fluvoxamine can be orientative to adjust the oral dosage [45].
4.3 Paroxetine
In patients with hepatic impairment (e.g. cirrhosis) although no significant differences in pharmacokinetic parameters were observed after administration of a single dose of paroxetine (20 mg), repeated administration of paroxetine (20–30 mg daily) over 14 days resulted in a doubling of steady-state plasma concentration (CSS) and t½ compared with values for healthy controls. In particular paroxetine was administered orally for 14 days to 12 patients with hepatic cirrhosis and 6 healthy controls. Patients with a GEC of >30 but <70 % of normal received 20 mg/day of paroxetine, those with a GEC of >70 to 80 % received 30 mg/day and healthy volunteers received 30 mg/day. In the patients with hepatic impairment, the mean minimum steady-state plasma concentration and the mean maximum steady-state plasma concentration and AUC over 24 h were approximately twice, and the t½β (terminal half life) more than twice, those in the healthy volunteers. The rate of urinary excretion of unchanged drug was low and did not differ significantly between patients with hepatic impairment and healthy volunteers (mean 0.67 vs. 0.5 mg/day), indicating that most of the paroxetine dose was cleared by metabolism despite hepatic impairment [46].
Moreover plasma concentrations at steady-state and the elimination t½ are prolonged in elderly subjects where a reduced metabolic hepatic activity generally occur [47, 48].
In conclusion while renal impairment has almost no effect on the pharmacokinetics of paroxetine, hepatic dysfunction may reduce the clearance of paroxetine [46, 49].
Although considerable interindividual variation in pharmacokinetic values was observed in all of these studies, the results suggest that paroxetine dosages should be titrated carefully in patients who are elderly or have severe renal or hepatic impairment (starting dosage of 10 mg/die) and should be kept at the lower end of the range recommended for the general population (it should not increased beyond 40 mg/day) [50, 51]. Plasma level determination can be an useful tool to allow adjusting the dose in each individual patient [52].
4.4 Sertraline
As sertraline is metabolised in the liver, its clearance and that of its primary metabolite desmethylsertraline are reduced in patients with hepatic impairment. Therefore, either dose reduction or prolongation of the dosage interval is advised for this patient group [53].
Although the hepatic metabolism is the most important elimination pathway, with only 0.2 % of an oral dose being excreted unchanged in the urine [54], information on the metabolism of sertraline is rather limited. N-demethylation is the main metabolic step in the biotransformation of sertraline [55]. The N-demethylated metabolite is more slowly eliminated and has a three times longer t½ (60–100 h) [55] than its parent drug. Hence, the plasma concentration of N-desmethylsertraline is 1–3 times that of sertraline. Since N-desmethylsertraline has only 5–10 % of the serotonin reuptake inhibitor potency of sertraline [56], a contribution to clinical effects of sertraline can be neglected. The N-demethylation correlates with the activity of CYP3A4 [57].
The effects of hepatic impairment on the pharmacokinetics of sertraline were determined in ten patients with chronic stable hepatic insufficiency (due to cirrhosis) who received a single 100 mg dose of the drug. After 264 h, median sertraline maximum plasma concentration (Cmax) and AUC values were approximately 1.7 and 4 times higher in the patients with cirrhosis than in ten healthy volunteers who had also received a single 100 mg dose [58]. The presence of hepatic disease resulted in a 3.2-fold increase in the median t½β value of sertraline, compared with that in the healthy volunteer group. Sertraline time to reach Cmax (tmax) values were similar for both groups of patients. The median Cmax of desmethylsertraline was 1.5 times higher (p < 0.05) and tmax significantly longer in patients with liver cirrhosis than in the healthy volunteers [58].
In ten patients with stable hepatic impairment of varying severity, multiple-dose administration of sertraline 50 mg daily for 21 days resulted in AUC0-24 h and Cmax values that were three times greater than values in ten healthy volunteers with normal hepatic function who received the same dosage of the drug [59]. In addition, the mean sertraline t½β was prolonged to 44.1 h in patients with hepatic impairment compared with 26.5 h in the healthy volunteers.
In conclusion while the pharmacokinetics are not significantly different between healthy controls and patients suffering from renal impairment [59], in patients with liver cirrhosis, the clearance of sertraline is markedly reduced [60]. The oral dosage should be adjusted in patients with hepatic impairment at a dose regimen of 50–100 mg/day. Plasma level determination can be an useful tool to adjust the oral dosage [61, 62].
4.5 Citalopram
The pharmacokinetics of citalopram and its metabolites demethylcitalopram and didemethylcitalopram in subjects with moderate renal insufficiency and subjects with hepatic cirrhosis with that in healthy subjects was investigated in a study conducted by Joffe et al. [63]. Pharmacokinetic parameters from three individual, open-label, phase I trials were derived following single oral or intravenous citalopram dose (40 mg) to healthy subjects and a single oral dose (20 mg) to patients. The absolute bioavailability of citalopram tablets in healthy subjects was 80 %. The renal clearance was a minor component (<20 %) of the total elimination of citalopram. Serum Cmax and tmax for citalopram were essentially unaffected by the occurrence of renal or hepatic disease. In comparison with healthy subjects, renal impairment was associated with a significant reduction in the renal elimination of citalopram and its two metabolites and a slight prolongation of serum citalopram t ½ (49.5 vs. 36.8 h in healthy subjects).
Previous data supported similar results: as for the renal impairment, the Cmax in patients with hepatic impairment was unchanged compared with that of healthy volunteers [64]. The t½ was significantly increased to 50 h and the renal clearance of citalopram and desmethylcitalopram was significantly lower [64].
Similarly, product data reported that citalopram oral clearance was reduced by 37 % and t½ was doubled so as steady-state plasma concentrations increased approximately twofold in patients with reduced hepatic function compared to normal subjects [65]. Cirrhosis resulted in significant decrease in citalopram oral clearance (0.21 vs. 0.331) in healthy subjects) and increase in distribution volume with an approximately twofold increase in t½ (83.4 vs. 36.8 h in healthy subjects).
Indices of renal (creatinine or 51Cr-EDTA clearances) and hepatic (GEC or Child–Pugh score) function were reported poor predictors of the changes in the pharmacokinetics of citalopram and its metabolites in these populations.
Dose- and weight-corrected serum concentrations of citalopram and parent drug plus demethylcitalopram increased linearly with age in a group of 169 psychiatric patients (aged 10–89 years). The fraction of demethylcitalopram decreased significantly with increasing age. Mean clearance decreased and mean t½ increased in 11 elderly depressed patients when compared with values obtained for younger healthy volunteers and patients in other studies [65]. Therefore, lower doses are recommended for elderly patients than for young ones.
In conclusion no reduction of citalopram dosage is warranted in patients with moderately impaired renal function even if that may not apply for patients with severe renal failure. In patients with impaired hepatic function, prescription of a lower dosage of citalopram may be appropriate, 20 mg/day being the maximum recommended dose for hepatically impaired patients. Plasma level determination of the drug could be useful to evaluate possible toxicity [66].
4.6 Escitalopram
After a single oral dose of escitalopram 20 mg, mean AUC values were elevated in patients with mild (Child–Pugh score 5–6; 51 % increase but not significant) or moderate (Child–Pugh score 7–8; 71 % significant in-crease) hepatic impairment relative to individuals with normal hepatic function. Study participants with moderately impaired, rather than normal, hepatic function also had a significantly lower escitalopram clearance (16 vs. 25 L/h) with an half-life about two fold longer [67, 68].
In patients with hepatic impairment (A and B Child–Pugh criteria) escitalopram dosage adjustments are advocated. The recommended initial dose for the first two weeks is 5 mg/day. The dosages could be adjusted at the maximum dose of 10 mg/day. A further major attention should be kept in case of severe hepatic impairment.
4.7 Mirtazapine
Mirtazapine is extensively metabolized and eliminated via the urine and faeces within a few days. Major pathways of biotransformation are demethylation and oxidation, followed by conjugation. In vitro data from human liver microsomes indicate that cytochrome P450 enzymes CYP2D6 and CYP1A2 are involved in the formation of the 8-hydroxy metabolite of mirtazapine, whereas CYP3A4 is considered to be responsible for the formation of the N-demethyl and N-oxide metabolite. The desmethyl metabolite is pharmacologically active and appears to have the same pharmacokinetic profile as the parent compound.
The clearance of mirtazapine may be decreased as a result of renal or hepatic insufficiency.
The effects of mild to moderate hepatic impairment on the pharmacokinetics of mirtazapine were investigated in a single dose (15 mg) study in two age matched parallel groups consisting of eight elderly males (control group: mean age 68 ± 5 years, mean bodyweight 71.3 ± 10.3 kg; patients with hepatic impairment: mean age 67 ± 5 years, mean bodyweight 73.0 ± 12.0 kg). The individuals were categorised by their hepatic function as measured by antipyrine clearance. The oral clearance of mirtazapine decreased by 33 % in the patient group (0.32 ± 0.14 vs. 0.49 ± 0.18 L/h/kg) and was associated with a corresponding decrease of 30 % in antipyrine clearance; the t½β of mirtazapine increased by 39 % (44.0 ± 4.8 vs. 31.6 ± 7.5 h) [16].
In summary, following a single 15 mg oral dose of mirtazapine, the clearance of mirtazapine was ~35 % decreased in patients with mild or moderate hepatic impairment compared to subjects with normal hepatic function. The average plasma concentration of mirtazapine was about 55 % increased.
Dosage adjustments of mirtazapine may be necessary in patients with hepatic impairment maintaining a dose range of 15–30 mg/day. Plasma level determination of the drug could be useful to evaluate possible toxicity [66].
4.8 Venlafaxine/Desvenlafaxine
Venlafaxine and its major metabolite, O-desmethylvenlafaxine (desvenlafaxine), appear to be equipotent with respect to their overall action on neurotransmitter re-uptake and receptor binding. They are potent inhibitors of serotonin and noradrenaline reuptake, and also weakly inhibit dopamine reuptake.
Desvenlafaxine, a new serotonin-norepinephrine reuptake inhibitor, is the major active metabolite of venlafaxine and exists as a racemic (RS) mixture.
Clinical studies indicate that 45 % of desvenlafaxine is eliminated unchanged in urine. The minor hepatic metabolic pathway for desvenlafaxine involves CYP3A4-mediated metabolism to N,O-didesmethylvenlafaxine [69]. This elimination profile indicates that desvenlafaxine has a comparatively uncomplicated metabolism, primarily through high-capacity systems (eg, glucuronidation). In contrast, the parent compound, venlafaxine, is extensively metabolized by the liver. A 50 % reduction in total daily dose is recommended in patients with mild to moderate hepatic impairment. Increases in exposure for Child–Pugh class B and C hepatically impaired subjects were seen (31 and 35 % increase in AUC values for moderate and severe hepatic impairment, respectively). Although the increases were not statistically significant (changes <50 %) relative to matched healthy subjects, the clinical significance of mean increases in desvenlafaxine exposure of ≥30 % in hepatically impaired patients is not known. CYP2D6 is not involved in the metabolism of desvenlafaxine, which indicates that hepatic metabolism plays a modest role in the elimination of desvenlafaxine. The current findings suggest that among with mild hepatic impairment, most desvenlafaxine pharmacokinetic and elimination parameters, including AUC and Cl/F, were similar to those seen in healthy subjects. Such data suggest that hepatic metabolism plays a definite but limited role in desvenlafaxine removal [70–72].
In summary also in the patients with compensated hepatic cirrhosis, the pharmacokinetic disposition of both venlafaxine and desvenlafaxine was significantly altered. The reduction in both the metabolism of venlafaxine and elimination of desvenlafaxine resulted in higher plasma concentrations of both the drugs. The drug dosages for venlafaxine should be limited to a maximum of 150 mg/day while the recommended dose of desvenlafaxine in patients with moderate to severe hepatic impairment is 50 mg/day. Dose escalation above 100 mg/day is not recommended. Plasma level determination can be of some utility to adjust the oral dosage [73].
4.9 Duloxetine
Patients with clinically evident hepatic insufficiency exhibit a substantial decrease in the ability to metabolize and eliminate duloxetine. Following a single, 20 mg oral dose of duloxetine, subjects with moderate hepatic impairment (Child–Pugh Class B) had a mean plasma duloxetine exposure that was fivefold higher [74] and elimination took approximately three times longer compared to age- and sex-matched healthy subjects, but Cmax was unaffected [75].
On the other hand alcohol abuse and pre-existing chronic liver disease have been cited as potential risk factors for duloxetine reported hepatotoxicity [76–78].
In patients with underlying liver disease, slow and deliberate dosage titration with careful monitoring for adverse hepatic effects in the first few months of duloxetine therapy is suggested. Daily dosage should be limited to 30–60 mg. Moreover use of duloxetine in patients with hepatic insufficiency is not ordinarily recommended.
4.10 Bupropion
Bupropion is extensively metabolized in humans. The parent drug is transformed to three active metabolites: hydroxybupropion, which is the major metabolite, threohydrobupropion and erythrohydrobupropion. CYP 2B6 is responsible for the conversion of bupropion to hydroxybupropion.
The effects of hepatic impairment on the pharmacokinetic properties of bupropion have been assessed in 8 patients with alcoholic hepatic disease of unspecified severity [79] and in 17 patients with mild to severe hepatic cirrhosis [80]. In an open-label study, De Vane et al. [79] showed that the mean tl/2 of hydroxybupropion was significantly longer in eight patients with alcoholic/hepatic disease compared with that in eight healthy volunteers (32.2 vs. 21.1 h). The AUCs of bupropion and its metabolite were more variable in the patients with alcoholic hepatic disease but were statistically similar compared with those in the healthy subjects.
A separate, open-label study [80] found no statistically significant differences in the pharmacokinetic properties of bupropion or its active metabolites between nine patients with mild to moderate hepatic cirrhosis and eight healthy volunteers. However, in eight patients with severe hepatic cirrhosis, the bupropion Cmax and AUC values were significantly increased (mean differences, −70 % and threefold, respectively) and showed more interpatient variability compared with values in healthy subjects. The mean bupropion t½ was statistically similar in patients with severe hepatic cirrhosis compared with that in healthy subjects (29 vs. 19 h). The hydroxybupropion Cmax however, was significantly lower (−69 %) in these cirrhotic patients.
Based on these data, it is recommended in the product information that bupropion be used with caution and at a reduced frequency of administration and/or dose reduction be considered in patients with mild to moderate hepatic impairment, and that bupropion be used with extreme caution in patients with severe hepatic cirrhosis, and that the dose not exceed 150 mg every other day in patients with severe cirrhosis [81].
4.11 Agomelatine
The presence of hepatic impairment causes a substantial increase in bioavailability and the drug is contraindicated in patients with cirrhosis or active liver disease and caution should be exercised when agomelatine is administered to patients who consume substantial quantities of alcohol or who are treated with medicinal products associated with risk of hepatic injury.
Following a single oral dose of 25 mg agomelatine in patients with hepatic impairment, Cmax increased by a factor of ~60 and ~110, while AUC increased by ~70- and ~140-times, in mild (Child–Pugh score of 5 or 6) and moderate (Child–Pugh score of 7–9) hepatic impairment, respectively compared to healthy subjects. Both mild and moderate liver impairment increased the half-life of agomelatine by a factor of ~3. The unbound fraction of agomelatine was also increased in subjects with hepatic insufficiency. The inter-individual variability decreased with mild hepatic impairment, with a further decrease in moderate hepatic impairment, suggesting a progressive saturation of the hepatic first-pass effect. Agomelatine is therefore contraindicated in patients with hepatic impairment [82].
4.12 Trazodone
Trazodone is extensively metabolized in the liver, but the effects of trazodone in patients with renal or hepatic impairment have not been well studied. An early study conducted in the late 1970s assessed the effects of 12 days’ treatment with trazodone (75 mg) in patients with mixed neuroses and normal or impaired renal function [83]. The authors concluded that renal impairment is not a contraindication of treatment with low-dose trazodone [83].
On the other hand a recent case of severe liver toxicity resulting in fulminant hepatic failure has been reported following treatment with venlafaxine and trazodone for 4 months [84].
Given the available data on the use of trazodone in patients with renal or hepatic impairment, trazodone product labelling advises careful dosing and regular monitoring in patients with hepatic impairment, particularly in cases of severe hepatic impairment, and severe renal impairment (usually, no dosage adjustment is necessary for mild to moderate renal impairment [85]). The maximum dose for geriatric outpatients and similarly in patients with hepatic impairment should not exceed 400 mg/day in divided doses.
5 Conclusions
As the liver is responsible for the metabolism of many compounds, knowledge of a patient’s hepatic function is required for the safe prescribing of many drugs. Assessing liver function by way of a patient history, examination and blood tests such as serum albumin and bilirubin, as well as prothrombin time, is recommended before prescribing several medications including antidepressants. Liver enzyme concentrations may be useful indicators of hepatocellular damage or enzyme induction. For drugs dependent on hepatic elimination, careful choice of compounds and their dose is prudent if liver function is significantly compromised.
The liver plays a central role in the absorption, distribution, and elimination kinetics of most drugs and many active or inactive drug metabolites. It is not only the most important biotransformation site, but parameters such as liver blood flow, binding to plasma proteins, and biliary excretion, which can all potentially influence drug pharmacokinetics, depend on the normal functioning of the liver. In addition, patients with hepatic dysfunction may also be more sensitive to the effects, both desired and adverse, of several drags. Dosage adjustment in patients with liver dysfunction is therefore essential for many drugs to avoid excessive accumulation of the drug, and possibly of active drug metabolite(s), which may lead to serious adverse reactions. Therapeutic drug monitoring can be an useful tool to evaluate and eventually adjust the oral dosages at least for antidepressants where a plasma level range has been recognized.
References
Woodhouse KW, Wynne HA. Age related changes in liver size and hepatic blood flow. The influence on drug metabolism in the elderly. Clin Pharmacokinet. 1988;15:287–94.
Hilmer SN, Shenfield GM, Le Couter DG. Clinical implications of changes in hepatic drug metabolism in older patients. Ther Clin Risk Manag. 2005;1(2):151–6.
Verbeeck RV. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–61.
Palatini P, De Martin S, Pegoraro P, Orlando R. Enzyme inhibition and induction in liver disease. Curr Clin Pharmacol. 2008;3:56–69.
Bui K, She F, Sostek M. The effects of mild or moderate hepatic impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J Clin Pharmacol. 2014. doi:10.1002/jcph.348.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9.
Brown RS, Kumar KS, Russo MW, Kinkhabwala M, Rudow DL, Harren P, Lobritto S, Emond JC. Model for end-stage liver disease and Child–Turcotte–Pugh score predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in united network for organ sharing status 2A patients. Liver Transpl. 2002;8(3):278–84.
Bergström M, Söderman C, Eriksson LS. A simplified method to determine galactose elimination capacity in patients with liver disease. Scand J Clin Lab Invest. 1993;53(7):667–70.
Schlatter C, Egger SS, Tchambaz L, Krähenbühl S. Pharmacokinetic changes of psychotropic drugs in patients with liver disease. Drug Saf. 2009;32(7):561–78.
Gram LF. Pharmacokinetics and clinical response to tricyclic antidepressants. Acta Psychiatr Scand 1980;280(S):169–180.
Altamura AC, Moro AR, Percudani M. Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet. 1994;26(3):201–14.
van Harten J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1993;24(3):203–20.
Fleishaker JC. Clinical Pharmacokinetics of reboxetine, a selective norepinephrine reuptake inhibitor for the treatment of patients with depression. Clin Pharmacokinet. 2000;39(6):413–27.
Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281–90.
Knadler MP, Lobo E, Chappell L, Bergstrom R. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281–94.
Timmer CJ, Ad Sitsen JM, Delbressine LP. Clinical pharmacokinetics of mirtazapine. Clin Pharmacokinet 2000;38(6):461–474
Federal Drug Administration. Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. 2003. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072123.pdf. Accessed 22 Sep 2014.
Goodnick PJ. Pharmacokinetic optimisation of therapy with newer antidepressants. Clin Pharmacokinet. 1994;27(4):307–30.
Rudorfer MV, Potter WZ. Metabolism of tricyclic antidepressants. Cell Mol Neurobiol. 1999;19(3):373–409.
Watkins PB. Role of cytochromes P450 in drug metabolism and hepatotoxicity. Semin Liver Dis. 1990;10:235–50.
Balant-Gorgia AE, Gex-Fabry M, Balant LP. Clinical pharmacokinetics of clomipramine. Clin Pharmacokinet. 1991;20(6):447–62.
Nordin C, Bertilsson L. Active hydroxymetabolites of antidepressants. Emphasis on E-10-hydroxy-nortriptyline. Clin Pharmacokinet. 1995;28(1):26–40.
Schmider J, Greenblatt DJ, von Moltke LL, Harmatz JS, Shader RI. N-demethylation of amitriptyline in vitro: role of cytochrome P-450 3A (CYP3A) isoforms and effect of metabolic inhibitors. J Pharmacol Exp Ther. 1995;275(2):592–7.
Bertilsson L, Mellström B, Sjöqvist F. Pronounced inhibition of noradrenaline uptake by 10-hydroxymetabolites of nortriptyline. Life Sci. 1979;25(15):1285–92.
Schulz P, Dick P, Blaschke TF, Hollister L. Discrepancies between pharmacokinetic studies of amitriptyline. Clin Pharmacokinet. 1985;10(3):257–68.
Hrdina PD, Lapierre YD, Koranyi EK. Altered amitriptyline kinetics in a depressed patient with porto-caval anastomosis. Can J Psychiatry. 1985;30(2):111–3.
Preskorn SH, Mac DS. Plasma levels of amitriptyline: effect of age and sex. J Clin Psychiatry. 1985;46(7):276–7.
Dawling S, Crome P, Braithwaite R. Pharmacokinetics of single oral doses of nortriptyline in depressed elderly hospital patients and young healthy volunteers. Clin Pharmacokinet. 1980;5(4):394–401.
Kragh-Sørensen P, Asberg M, Eggert-Hansen C. Plasma-nortriptyline levels in endogenous depression. Lancet. 1973;20(1):113–5.
Altamura AC, Mauri MC. Plasma concentrations, information and therapy adherence during long-term treatment with antidepressants. Br J Clin Pharmacol. 1985;20:714–6.
Ciraulo DA, Bamhill JM, Jaffe JH. Clinical pharmacokinetics of imipramine and desipramine in alcoholics and normal volunteers. Clin Pharmacol Ther. 1988;43:509–18.
Abernethy DR, Greenblatt DJ, Shader RI. Imipramine and desipramine disposition in the elderly. J Pharmacol Exp Ther. 1985;232:183–8.
Sallee FR, Pollock BG. Clinical pharmacokinetics of imipramine and desipramine. Clin Pharmacokinet. 1990;18(5):346–64.
Preskorn SH. Tricyclic antidepressant plasma level monitoring: an improvement over the dose-response approach. J Clin Psychiatry. 1986;47(S1):24–30.
Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203–8.
Gram L. Fluoxetine. N Engl J Med. 1994;17;331(20):1354–1361.
Benfield P, Heel RC, Lewis SP. Fluoxetine: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in depressive illness. Drugs. 1986;32:481–508.
Hiemke C, Härtter S. Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther. 2000;85:11–28.
Schenker S, Bergstrom RF, Wolen RL, Lemberger L. Fluoxetine disposition and elimination in cirrhosis. Clin Pharmacol Ther. 1988;44:353–9.
Rodighiero V. Effects of liver disease on pharmacokinetics. An update. Clin Pharmacokinet. 1999;37(5):399–431.
Amsterdam JD, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Michelson D, Hornig-Rohan M, Beasley CM. Fluoxetine and norfluoxetine plasma concentrations in major depression: a multicenter study. Am J Psychiatry. 1997;154:963–9.
van Harten J. Overview of the pharmacokinetics of fluvoxamine. J Clin Pharmacokinet 1995;29(S1):1–9.
van Harten J, Duchier J, Devissaguet JP, van Bemmel P, de Vries MH, Raghoebar M. Pharmacokinetics of fluvoxamine maleate in patients with liver cirrhosis after single-dose oral administration. Clin Pharmacokinet. 1993;24(2):177–82.
de Vries MH, Raghoebar M, Mathlener IS, van Harten J. Single and multiple oral dose fluvoxamine kinetics in young and elderly subjects. Ther Drug Monit. 1992;14(6):493–8.
DeVane CL, Gill HS. Clinical pharmacokinetics of fluvoxamine: application to dosage regimen design. J Clin Psychiatry. 1997;58(5):7–14.
Dalhoff K, Almdal TP, Bjerrum K, Keiding S, Mengel H, Lund J. Pharmacokinetics of paroxetine in patients with cirrhosis. Eur J Clin Pharmacol. 1991;41(4):351–4.
Lundmark J, Scheel Thomsen I, Fjord-Larsen T, Manniche PM, Mengel H, Møller-Nielsen EM, Pauser H, Wålinder J. Paroxetine: pharmacokinetic and antidepressant effect in the elderly. Acta Psychiatr Scand Suppl. 1989;350:76–80
Bayer AJ, Roberts NA, Allen EA, Horan M, Routledge PA, Swift CG, Byrne MM, Clarkson A, Zussman BD. The pharmacokinetics of paroxetine in the elderly. Acta Psychiatr Scand Suppl. 1989;350:85–6.
Doyle GD, Laher M, Kelly JG, Byrne MM, Clarkson A, Zussman BD. The pharmacokinetics of paroxetine in renal impairment. Acta Psychiatr Scand Suppl. 1989;350:89–90.
Foster RH, Goa KL. Paroxetine. A review of its pharmacology and therapeutic potential in the management of panic disorder. CNS Drugs. 1997;8(2):163–88.
Gunasekara NS, Noble S, Benfield P. Paroxetine. An update of its pharmacology and therapeutic use in depression and a review of its use in other disorders. Drugs. 1998;55(1):85–112.
Mauri MC, Laini V, Bitetto A, Boscati L, Scalvini M, Mapelli L, Rudelli R. Long term efficacy of paroxetine in major depression: a study with plasma levels. Int J Clin Psychiatr Clin Pract. 1999;3(2):115–9.
Perry CM, Benfield P. Sertraline. An overview of its pharmacological properties and a review of its therapeutic efficacy in obsessive-compulsive disorder. CNS Drugs. 1997;7(6):480–500.
Murdoch D, McTavish D. Sertraline A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive-compulsive disorder. Drugs. 1992;44(4):604–24.
Rudorfer MV, Potter WZ. The role of metabolites of antidepressants in the treatment of depression. CNS Drugs. 1997;7:273–312.
Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–22.
Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1997;32(Suppl. 1):1–21.
Démolis JL, Angebaud P, Grangé JD, Coates P, Funck-Brentano C, Jaillon P. Influence of liver cirrhosis on sertraline pharmacokinetics. Br J Clin Pharmacol. 1996;42:394–7.
Wilner KD, Baris BA, Foulds GH, Anziano RJ, Berl T, Ziegler MG. Multiple dose pharmacokinetics of sertraline in subjects with varying degrees of renal impairment. Eur Neuropsychopharmacol. 1996;6(Suppl. 3):41.
Wilner KD, Everson G, Foulds GH, Hansen RA, Shrestra R, McKinley C, McClain CJ. Multiple dose pharmacokinetics of sertraline in subjects with varying degrees of hepatic impairment. Eur Neuropsychopharmacol. 1996;6(suppl. 3):40.
Mauri MC, Laini V, Cerveri G, Scalvini ME, Volonteri LS, Regispani F, Malvini L, Manfrè S, Boscati L, Panza G. Clinical outcome and tolerability of sertraline in major depression: a study with plasma levels. Prog Neuro-Psychopharmacol Biol Psychiatr. 2002;26:597–601.
Mauri MC, Fiorentini A, Cerveri G, Volonteri LS, Regispani F, Malvini L, Boscati L, Lo Baido R, Invernizzi G. Long-term efficacy and therapeutic drug monitoring of sertraline in major depression. Hum Psychopharmacol Clin Exp. 2003;18:385–388.
Joffe P, Larsen FS, Pedersen V, Ring-Larsen H, Aaes-Jørgensen T, Sidhu J. Single-dose pharmacokinetics of citalopram in patients with moderate renal insufficiency or hepatic cirrhosis compared with healthy subjects. Eur J Clin Pharmacol. 1998;54(3):237–42.
Baumann P, Larsen F. The pharmacokinetics of citalopram. Rev Contemp Pharmacother. 1995;6:287–95.
Noble S, Benfield P. Citalopram. A review of its pharmacology, clinical efficacy and tolerability in the treatment of depression. CNS Drugs. 1997;8(5):410–31.
Duverneuil C, de la Grandmaison GL, de Mazancourt P, Alvarez JC. A high performance liquid chromatography method with photodiode array UV detection for therapeutic drug monitoring of the nontricyclic antidepressant. Drugs Ther Drug Monit. 2003;25(5):565–573.
Areberg J, Christophersen JS, Poulsen MN, Larsen F, Molz KH. The pharmacokinetics of escitalopram in patients with hepatic impairment. AAPS J. 2006;8(1):14–9.
Murdoch D, Keam SJ. Escitalopram. A review of its use in the management of major depressive disorder. Drugs. 2005;65(16):2379–404.
Nichols AI, Richards LS, Behrle JA, Posener JA, McGrory SB, Paul J. The pharmacokinetics and safety of desvenlafaxine in subjects with chronic renal impairment. Int J Clin Pharmacol Ther. 2011;49:3–13.
Wyeth Pharmaceuticals. Pristiq [prescribing information]. Philadelphia, Pa: Wyeth Pharmaceuticals, Inc, a subsidiary of Pfizer Inc.; 2013.
Muth EA, Moyer JA, Haskins JT, Andree TH, Husbands GEM. Biochemical, neurophysiological, and behavioral effects of Wy-45,233 and other identified metabolites of the antidepressant venlafaxine. Drug Dev Res. 1991;23:191–9.
Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M, Andree TH. Desvenlafaxine succinate: a new serotonin and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2006;318:657–65.
Charlier C, Pinto E, Ansseau M, Plomteux G. Venlafaxine: the relationship between dose, plasma concentration and clinical response in depressive patients. J Psychopharmacol. 2002;16(4):369–72.
Eli Lilly Pharmaceuticals. Cymbalta (duloxetine hydrochloride) capsules [package insert]. Indianapolis: Eli Lilly Pharmaceuticals; 2008.
Suri A, Reddy S, Gonzales C, Knadler MP, Branch RA, Skinner MH. Duloxetine pharmacokinetics in cirrhotics compared with healthy subjects. Int J Clin Pharmacol Ther. 2005;43(2):78–84.
Gahimer J, Wernicke J, Yalcin I, Ossanna MJ, Wulster-Radcliffe M, Viktrup L. A retrospective pooled analysis of duloxetine safety in 23,983 subjects. Curr Med Res Opin. 2007;23:175–84.
Wernicke J, Acharya N, Strombom I, et al. Hepatic effects of duloxetine-II: spontaneous reports and epidemiology of hepatic events. Curr Drug Saf. 2008;3:143–53.
Hanje AJ, Pell LJ, Votolato NA, Frankel WL, Kirkpatrick RB. Case report: fulminant hepatic failure involving duloxetine hydrochloride. Clin Gastroenterol Hepatol. 2006;4:912–7.
DeVane CL, Laizure SC, Stewart JT, Kolts BE, Ryerson EG, Miller RL, Lai AA. Disposition of bupropion in healthy volunteers and subjects with alcoholic liver disease. J Clin Psychopharmacol. 1990;10(5):322–8.
GlaxoSmithKline. Study AK1A1001. Research Triangle Park, NC; 1996.
Thomson PDR. Wellbutrin (bupropion) tablets. In: Physicians’ desk reference. Montvale, 2005, p. 1655–1659.
European Medicines Agency (EMA). European Public Assessment Report (EPAR) for Valdoxan EMEA/C/000915:66 pages; 2008.
Catanese B, Dionisio A, Bacillari G, De Martino C. A comparative study of trazodone serum concentrations in patients with normal or impaired renal function. Bull Chim Farm. 1978;117(7):424–7.
Detry O, Delwaide J, De Roover A, et al. Fulminant hepatic failure induced by venlafaxine and trazodone therapy: a case report. Transplant Proc. 2009;41(8):3435–6.
Fagiolini A, Comandini A, Dell’Osso MC, Kasper S. Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs. 2012;26:1033–49.
Rickels K, Schweizer E. Clinical overview of serotonin reuptake inhibitors. J Clin Psychiatry. 1990;51(Suppl B):9–12.
DeVane CL. Pharmacokinetics of the selective serotonin reuptake inhibitors. J Clin Psychiatry. 1992;53(Suppl):13–20.
Grimsley SR, Jann MW. Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors. Clin Pharm. 1992;11(11):930–57.
Ereshefsky L, Riesenman C, Lam YW. Antidepressant drug interactions and the cytochrome P450 system. The role of cytochrome P450 2D6. Clin Pharmacokinet. 1995;29(Suppl 1):10–18. (Discussion 18–19).
Ereshefsky L, Riesenman C, Lam YW. Serotonin selective reuptake inhibitor drug interactions and the cytochrome P450 system. J Clin Psychiatry. 1996;57(Suppl 8):17–24. (Discussion 25).
Wille SM, Cooreman SG, Neels HM, Lambert WE. Relevant issues in the monitoring and the toxicology of antidepressants. Crit Rev Clin Lab Sci. 2008;45(1):25–89.
Karhu D, Gossen ER, Mostert A, Cronjé T, Fradette C. Safety, tolerability, and pharmacokinetics of once-daily trazodone extended-release caplets in healthy subjects. Int J Clin Pharmacol Ther. 2011;49(12):730–43.
Nilsen OG, Dale O, Husebø B. Pharmacokinetics of trazodone during multiple dosing to psychiatric patients. Pharmacol Toxicol. 1993;72(4–5):286–9.
Conflict of interest
No sources of funding were used in the preparation of this review. All authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mauri, M.C., Fiorentini, A., Paletta, S. et al. Pharmacokinetics of Antidepressants in Patients with Hepatic Impairment. Clin Pharmacokinet 53, 1069–1081 (2014). https://doi.org/10.1007/s40262-014-0187-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0187-5