Abstract
Background and Objective
The optimal choice for first- and second-line antiseizure medications for pediatric patients with convulsive status epilepticus remains ambiguous. The present study aimed to estimate the comparative effect on the efficacy and safety of different antiseizure medications in pediatric patients with status epilepticus and provide evidence for clinical practice.
Methods
We searched PubMed, EMBASE, and the Cochrane Library for eligible randomized controlled trials. Inclusion criteria included: (1) pediatric patients; (2) diagnosis of status epilepticus; and (3) randomized controlled trials. Exclusion criteria were: (1) mixed population without a pediatric subgroup analysis; (2) not status epilepticus; (3) received the study drug prior to admission; (4) sample size fewer than 30; and (5) not randomized controlled trials. Primary outcome was seizure cessation. Secondary outcomes were seizure recurrence within 24 h, respiratory depression, and admission to an intensive care unit. The hierarchy of competing antiseizure medications was presented using the surface under the cumulative ranking curve.
Results
Eight first-line antiseizure medication studies involving 1686 participants and eight second-line antiseizure medication studies involving 1711 participants were eligible for analysis. Midazolam, diazepam, lorazepam, and paraldehyde were administered as first-line antiseizure medications. Valproate, phenobarbital, phenytoin, fosphenytoin, and levetiracetam were investigated as second-line antiseizure medications. No significant differences were observed across first- and second-line antiseizure medications. Midazolam ranked the best for primary and secondary outcomes among the first-line antiseizure medications. Phenobarbital ranked the best for seizure cessation and a lower risk of admission to the intensive care unit. Valproate had superiority in preventing recurrence within 24 h. Levetiracetam had the lowest probability of developing respiratory depression.
Conclusions
This study demonstrated the hierarchy of competing interventions. Midazolam could be a better option for first-line treatment. Phenobarbital, levetiracetam, and valproate had their respective superiority in the second-line intervention. This study may provide useful information for clinical decision making under different circumstances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This network meta-analysis revealed the hierarchy of preferential antiseizure medications in first- and second-line treatment and provided useful information for clinical decision making. |
We found midazolam could be a better option as a first-line antiseizure medication, while phenobarbital, valproate, and levetiracetam showed their respective advantages in efficacy and safety as second-line treatments. |
1 Introduction
Status epilepticus (SE) is one of the most common pediatric neurological emergencies. The incidence of pediatric SE is 3–42 episodes per 100,000 population per year [1]. Failure of SE cessation could cause irreversible neuronal injury and complications related to consequent changes in the neuronal network [2]. Therefore, it is vitally important to receive intervention as early as possible to terminate SE. Following the American Epilepsy Society (AES) guideline, benzodiazepines were commonly used as first-line antiseizure medications (ASMs), while paraldehyde was an alternative option [3]. In approximately one third of cases, SE cannot be terminated by a first-line intervention and required additional anticonvulsants. Valproate, phenobarbital, phenytoin, fosphenytoin, and levetiracetam are commonly used for second-line treatment. However, no evidence supported the most efficacious ASM as a second-line intervention for pediatric convulsive SE (CSE).
So far, the evidence of optimal ASMs for pediatric patients with CSE remains limited because of the small sample sizes and paucity of high-quality studies. To estimate the comparative effect on the efficacy and safety of different ASMs in eligible randomized controlled trials (RCTs) and provide hierarchical evidence on first- and second-line drug selection, we performed this network meta-analysis.
2 Methods
2.1 Search Strategy
We searched PubMed, EMBASE, and the Cochrane Library for any available study up to 20 May, 2020. Terms “status epilepticus”, “prolonged seizure”, “antiepileptic drug”, “anticonvulsant”, “randomized clinical trial”, and “random” were searched in “title/abstract” and “Mesh term” or “Emtree” if available. There was no limitation in language or publication date. Details of the search strategy are reported in Appendix S1 of the Electronic Supplementary Material (ESM). A prespecified protocol registration has been submitted to PROSPERO (https://www.crd.york.ac.uk/prospero/) for review prior to the initiation of this study (registration number: CRD42020184940).
2.2 Selection Criteria
Inclusion criteria included: (1) pediatric patients (age: 1 month to 18 years); (2) patients diagnosed with CSE with a single convulsive seizure that lasted for at least 5 min, two or more sequential seizures without recovery of consciousness, or three or more sequential seizures [4]; and (3) randomized controlled trials (RCTs). The following studies were excluded: (1) mixed population (pediatric and adult) without an age group analysis; (2) studies with participants who did not meet the definition of CSE; (3) participants who received the study drug prior to admission; (4) a sample size fewer than 30; and (5) study design that was not randomized and controlled. Any disagreement was discussed within the group and evaluated by a third reviewer to reach a final consensus.
2.3 Data Extraction
Two independent reviewers (YZ and YL) screened articles by reading titles and abstracts. Full texts were further assessed for the eligibility. The following information was extracted: authors, publication year, country, study design, patient characteristics, etiology of seizures, intervention, dosage, route, sample size, primary outcome, and secondary outcomes. Primary outcome was defined as seizure cessation after drug infusion. Secondary outcomes included seizure recurrence within 24 h, patient admission to an intensive care unit (ICU), and respiratory depression. Modified intention-to-treat population data are preferred for data analysis. The modified intention to treat population is a subset of the intention-to-treat population, which allows exclusion of participants under justified conditions. The intention-to-treat population may include participants who never receive an intervention or are ineligible after randomization, which could be problematic especially in non-inferiority trials [5]. Therefore, the modified intention-to-treat population is appropriate for data synthesis.
Study quality was evaluated by Cochrane’s Risk-of-Bias Tool 2 (randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result) [6]. Any disagreement was discussed within the group and evaluated by a third reviewer to reach a final consensus.
2.4 Data Analysis
We performed a network meta-analysis within a Bayesian framework to directly and indirectly compare the efficacy and safety of different first- and second-line ASMs. The number of chains in our model was four. Considering that seizure cessation and seizure recurrence were dichotomous data, we used binomial and logit values for likelihood and links function with a random-effect model and reported our data as odds ratios and 95% credible intervals for mixed comparisons or 95% confidence intervals (CIs) for direct comparisons. Convergence of the model was assessed after 10,000 iteration adaptations with a thinning factor of ten, and 50,000 iteration simulations to estimate the effect. Because event occurrence was relatively low for respiratory depression and admission to the ICU, the risk difference was calculated to estimate the effect size [7]. We assessed inconsistency between direct and indirect comparisons in the network using a nodesplit approach. A p value larger than 0.05 was considered as no inconsistency, hence a consistency model was used if eligible. Heterogeneity was evaluated using the I2 test and p value. A p value less than 0.05 was considered of significant heterogeneity. Cumulative probability of efficacy and safety was presented as a surface under the cumulative ranking curve or a stacked column graph.
For the above-mentioned data analysis and image synthesis, we used freely available R packages GeMTC (https://CRAN.R-project.org/package=gemtc), pcnetmeta (https://cran.r-project.org/web/packages/pcnetmeta/index.html), and ggplot2 (https://cloud.r-project.org/package=ggplot2).
3 Results
3.1 Study Characteristics
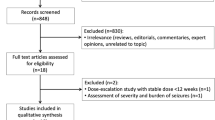
We identified 1286 articles from PubMed, EMBASE, and the Cochrane Library. An additional review of references identified another five articles. Sixteen RCTs involving 3397 pediatric participants were included in the data analysis [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23] (Fig. 1). The definition of CSE was homogenous between studies. Etiology of included studies varied remarkably across studies. The following drugs were included for comparison: midazolam, diazepam, lorazepam, paraldehyde, valproate, phenobarbital, phenytoin, fosphenytoin, and levetiracetam. Dosage and administration route of intervention, as well as study characteristics are summarized in Table 1. Eight studies were conducted in untreated participants [8,9,10,11, 13,14,15,16], while eight studies were conducted in participants who required second-line therapy [12, 17,18,19,20,21,22,23].
3.2 Risk of Bias
Selection bias was the most frequent bias observed in 13 studies (seven studies judged to be high risk), followed by bias due to deviations from intended interventions in seven studies, randomization bias in five studies, and measurement bias in six studies (Fig. S1 of the ESM). Despite interventions not being blinded to participants and caregivers in some studies, outcome measurement was unlikely to be affected. Missing outcome and report selection biases were low.
3.3 Efficacy in First-Line Antiseizure Medications
Figure 2 shows the network of first- and second-line ASM comparisons. In total, 1686 patients were randomized to receive four ASMs as the first-line intervention, including midazolam, diazepam, lorazepam, and paraldehyde. The most frequently studied drug was diazepam. Direct and mixed comparison for seizure cessation between these drugs did not show significant differences (Figs. 3a, 5a). The point estimates of seizure cessation indicated that midazolam had superiority, followed by diazepam, lorazepam, and paraldehyde (probmidazolam = 49.44% vs probdiazepam = 31.34% vs problorazepam = 13.96% vs probparaldehyde = 5.27%, Fig. 4a).
Differences for recurrence within 24 h among first-line ASMs did not show statistical significance (Figs. 3c, 5b). Midazolam was superior to lorazepam, paraldehyde, and diazepam with respect to seizure recurrence within 24 h (probmidazolam = 58.26% vs probdiazepam = 8.42% vs problorazepam = 17.43% vs probparaldehyde = 15.89%, Fig. 4b). Therefore, midazolam had the greatest probability of controlling seizure and preventing recurrence within 24 h. There was no inconsistency between direct and indirect comparisons (p > 0.05). No evidence of heterogeneity was found (I2 < 25%, p > 0.05).
Outcome comparison of antiseizure medications. Data were presented as odd ratios with 95% credible intervals for mixed comparisons or 95% confidence intervals for direct comparisons. AEDs antiepileptic drugs, DZP diazepam, fPHY fosphenytoin, LZP lorazepam, LEV levetiracetam, MDL midazolam, PAD paraldehyde, PHB phenobarbital, PHY phenytoin, VPA valproate
3.4 Efficacy in Second-Line Antiseizure Medications
In total, 1711 patients were randomized to receive five ASMs as the second-line intervention, including valproate, phenobarbital, phenytoin, fosphenytoin, and levetiracetam. These ASMs showed similar effects in seizure cessation (Figs. 3b, 5c). Phenobarbital and levetiracetam had superiority with respect to seizure cessation (probvalproate = 17.39% vs probphenobarbital = 39.10% vs probphenytoin = 6.04% vs probfosphenytoin = 5.61% vs problevetiracetam = 31.87%, Fig. 4c), whereas valproate was likely to have the best effect in preventing seizure recurrence (probvalproate = 68.49% vs probphenobarbital = 2.18% vs probphenytoin = 0.93% vs probfosphenytoin = 10.09% vs problevetiracetam = 18.32%, Figs. 4d, 5d). There was no inconsistency between direct and indirect comparisons (p > 0.05). No evidence of heterogeneity was found (I2 < 25%, p > 0.05).
3.5 Safety
The most common ASM-relevant adverse event was respiratory depression. Other adverse events such as arrhythmia, hypotension, and death were not eligible for quantitative analysis because of missing outcomes and null values. Hence, only respiratory depression was compared between studies. Seven first-line ASM studies [8,9,10,11, 13, 15, 16] and five second-line ASM studies [12, 17, 20, 21, 23] were eligible for quantitative synthesis. Compared with midazolam, diazepam and lorazepam had a slightly increased risk for respiratory depression (RDdiazepam-midazolam = 0.07, 95% CI -0.12 to 0.32; RDlorazepam-midazolam = 0.06, 95% CI − 0.14 to 0.29). Administration of midazolam had a lower probability of respiratory depression compared with diazepam and lorazepam (Figs. S2A and C of the ESM).
Among all these second-line ASMs, the risk of respiratory depression was slightly lower in levetiracetam compared with valproate (RDlevetiracetam-valproate = − 0.01, 95% CI − 0.30 to 0.48, Fig. S2B and D of the ESM). Phenobarbital, phenytoin, and fosphenytoin showed an increased risk compared with valproate (RDphenobarbital-valproate = 0.06, 95% CI − 0.17 to 0.53; RDphenytoin-valproate = 0.11, 95% CI − 0.25 to 0.53; RDfosphenytoin-valproate = 0.18, 95% CI − 0.11 to 0.89). Analysis on admission to the ICU was performed exclusively in second-line ASM studies because most first-line ASM studies did not have sufficient information for pooled data. Phenobarbital significantly lowered the risk of admission to the ICU compared with fosphenytoin (RDfosphenytoin-phenobarbital = 0.56, 95% CI 0.05–0.89). Despite phenobarbital not having significant differences compared to other second-line ASMs, it was more likely to reduce the probability of admission to the ICU (Fig. S2e, f of the ESM).
4 Discussion
The present study provided evidence from a systematic analysis of RCTs conducted in pediatric patients with CSE. Four first-line ASMs and five second-line ASMs were assessed for their efficacy and safety in the pediatric population. The absence of significant differences between these ASMs may reflect the flaws of the small sample size and heterogeneity in current RCTs. Otherwise, the differences were only of small magnitude yet to be demonstrated in a larger population.
This study provided a profile of the hierarchy of comparative effect in different ASMs. The best ranked first-line ASMs was midazolam, which had superiority to both primary and secondary outcomes (seizure cessation, recurrence, and respiratory depression). Diazepam was usually more frequently used in the emergency room. It required continuous intravenous infusion, which may cause acute respiratory depression, sedation, and hypotension. Our finding suggested that midazolam was probably a better option as a first-line treatment.
Phenobarbital ranked best among second-line ASMs for seizure cessation and admission to the ICU. Similar to the conclusion drawn by the present study, one high-quality study comparing phenobarbital with phenytoin found a significant difference in seizure cessation (p = 0.003) [17]. Phenobarbital as a traditional anticonvulsant may be more acceptable in resource-limited regions considering that it ranked best in the likelihood of achieving seizure cessation and reducing admission to the ICU. These advantages may help ease the financial burden and terminate seizure efficaciously. However, the side effects in cognition and behavior should also be taken into consideration.
Valproate had superiority with respect to preventing recurrence within 24 h and had an equally lower risk in respiratory depression compared with levetiracetam. The vast majority of valproate was metabolized by the cytochrome P450 enzyme in the liver [24]. A considerable adverse event of valproate was hepatotoxicity. However, few included studies reported gastrointestinal and hepatic adverse events.
Levetiracetam was a novel ASM safely used in children aged older than 4 years. The maximum dose of 60 mg/kg per day was acceptable [25]. According to a recent pairwise meta-analysis [26], the efficacy of levetiracetam was not significantly superior to phenytoin, which was consistent with this study. Despite no differences observed between levetiracetam and other second-line ASMs, it ranked the second-best option for cessation and preventing recurrence. Among six RCTs evaluating levetiracetam, only one low-quality study demonstrated levetiracetam was superior to phenytoin [20]. Two high-quality studies found that levetiracetam was not superior to phenytoin or other ASMs, e.g., fosphenytoin and valproate [18, 21]. Its unique metabolic kinetics independent of the cytochrome P450 enzyme exerted less effect on drug–drug interactions. Few adverse events were reported, e.g., sedative and behavioral effects [27]. Consistently, we found that levetiracetam had the lowest probability of developing respiratory depression. However, this drug was not available in many regions worldwide. Therefore, selection of the optimal second-line ASMs should be balanced between accessibility, tolerability, etiology, and patient characteristics.
Brivaracetam is structurally similar to levetiracetam. Despite these two drugs targeting the same molecule, SV2A, brivaracetam showed a more than 15-fold higher binding affinity and faster response [28]. Several randomized clinical studies evaluated the efficacy and safety of brivaracetam in adult focal seizures. A meta-analysis demonstrated that brivaracetam was not inferior to eslicarbazepine acetate, lacosamide, and perampanel [29]. To date, few clinical studies have evaluated brivaracetam in the treatment of SE. According to the latest systematic review, brivaracetam could be safely used but still lacks evidence to evaluate its efficacy in the treatment of SE [30].
Studies have highlighted the neuronal machinery of SE, involving loss of GABA-mediated inhibition and sustained glutamate-mediated excitation [4]. The AMPA receptor induced seizures by synchronizing glutamatergic excitatory signals, thus it may be a potential therapeutic target. Perampanel is an orally active, selective, non-competitive AMPA receptor agonist that inhibits AMPA-mediated Ca2+ influx and neuronal excitation [31]. Because of its pharmacodynamics, perampanel could be a promising treatment for seizures. It becomes a welcome adjunctive treatment for focal seizures and primary generalized tonic–clonic seizures, but few studies investigated its role in the treatment of SE [32, 33]. A systematic review summarized current evidence supporting the administration of perampanel in SE. Perampanel is well tolerated in patients with SE. However, the efficacy of perampanel in SE treatment remains elusive [32]. Therefore, these studies revealed the urgent need for more well-designed high-quality studies investigating the efficacy of novel drugs, e.g., perampanel and brivaracetam in the treatment of SE.
Paraldehyde was commonly used as first- and second-line ASMs in sub-Saharan Africa. Focal irritation, inconvenience for infusion, and high cost were major disadvantages. Moreover, patients treated with paraldehyde had a higher probability of receiving additional dose of ASMs (lorazepam, 10% vs paraldehyde, 26%, p = 0.007) [9]. In the present study, paraldehyde ranked worst for efficacy among other first-line ASMs. However, only one study investigating paraldehyde was eligible for analysis [9]. The evidence was insufficient to validate its efficacy and safety.
In second-line ASMs, phenytoin and fosphenytoin ranked worst for seizure cessation. This may reflect the flaws of restricted infusion speed and prolonged infusion duration to avoid respiratory and cardiac adverse events. Phenytoin and fosphenytoin did not show significant differences in seizure cessation or recurrence (Fig. 3b, d). The risk of respiratory depression was similar (Fig. S2b of the ESM).
Some clinical studies have evaluated ASMs in adult patients with SE. A recent network meta-analysis revealed that high-dose phenobarbital ranked best for SE control and preventing seizure recurrence, while phenytoin ranked worst for seizure cessation. Lacosamide and valproate represented a safer option. Phenobarbital ranked worst in safety outcomes because of the very high dosage used in patients with SE [34]. In adult patients with SE, the beneficial effect obtained in the phenobarbital arm was most likely due to the very high dosage. However, the dosage used in pediatric patients was regularly recommended (20 mg/kg), suggesting that the dosage did not largely affect the superiority of phenobarbital over other ASMs in pediatric patients with SE. Moreover, an updated network meta-analysis including the Established Status Epilepticus Treatment Trial (ESETT) was presented to compare those ASMs with appropriate dosages in clinical practice [35]. No significant difference was identified for seizure cessation, seizure freedom at 24 h, respiratory depression, and hypotension. Valproate ranked the best with respect to the efficacy outcome and had the lowest probability in respiratory depression. In the present study, similar results were observed. Valproate and levetiracetam were comparable in efficacy and ranked as the best two medications for safety outcomes, whereas phenytoin and fosphenytoin ranked as the worst medications for seizure cessation and safety outcome.
The sample size in half of the included studies was small, which may increase the risk for statistical type II error and cause a false-negative result in RCTs [36]. Clinical heterogeneity in age, etiology, and admission interval may also cause potential biases. The most common cause of SE was febrile SE, which limited to children aged less than 5 years, whereas cryptogenic and remote symptomatic SE were common in older children [1]. Acute symptomatic SE including metabolic disorders, cerebrovascular disease, central nervous system infection, anoxia, electrolyte abnormality, and traumatic brain injury was strongly associated with a poorer prognosis [37]. A selected population may produce a distinct outcome, interfering with the validity of pooled data. Moreover, SE may have persisted for a different time interval before receiving any intervention. A longer duration of SE predicted a worse outcome. Therefore, clinical heterogeneity could be another reason for the absence of clear-cut differences between ASMs. Most included studies were not designed to investigate clinical outcomes of ASMs in stratified subgroups. Consequently, it was impossible to identify interfering covariates by a subgroup analysis or a meta-regression in the present study. Because of these limitations, the interpretation of this study should be cautious.
The present study was the first to compare the efficacy and safety of different ASMs for CSE in pediatric patients. We provided a hierarchy of different ASMs for first- and second-line interventions in pediatric patients with CSE. Although a network meta-analysis could not be a substitute for head-to-head clinical trials, it may provide solid evidence and supplementary guidance for clinical decision making.
5 Conclusions
This study demonstrated the hierarchy of comparative effect on the efficacy and safety of different ASMs in pediatric patients with CSE. Midazolam ranked as the best first-line treatment. Levetiracetam and valproate are better tolerated options with no inferiority compared to other second-line treatments. Phenobarbital had superiority in seizure cessation and ICU admission, thus could be a better option in resource-limited regions. To date, few high-quality studies have demonstrated the superiority of one ASM over the other. This study highlighted current limitations and the need for high-quality studies. It may provide useful information for clinical practice under different circumstances.
References
Gurcharran K, Grinspan ZM. The burden of pediatric status epilepticus: epidemiology, morbidity, mortality, and costs. Seizure. 2019;68:3–8.
Trinka E, Kalviainen R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure. 2017;44:65–73.
Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61.
Trinka E, Brigo F, Shorvon S. Recent advances in status epilepticus. Curr Opin Neurol. 2016;29(2):189–98.
Beckett RD, Loeser KC, Bowman KR, Towne TG. Intention-to-treat and transparency of related practices in randomized, controlled trials of anti-infectives. BMC Med Res Methodol. 2016;16(1):106.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Lin L, Zhang J, Hodges JS, Chu H. Performing arm-based network meta-analysis in R with the pcnetmeta package. J Stat Softw. 2017;80:5.
Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–6.
Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet. 2006;367(9522):1591–7.
Mpimbaza A, Ndeezi G, Staedke S, Rosenthal PJ, Byarugaba J. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121(1):e58–64.
Sreenath TG, Gupta P, Sharma KK, Krishnamurthy S. Lorazepam versus diazepam-phenytoin combination in the treatment of convulsive status epilepticus in children: a randomized controlled trial. Eur J Paediatr Neurol. 2010;14(2):162–8.
Malamiri RA, Ghaempanah M, Khosroshahi N, Nikkhah A, Bavarian B, Ashrafi MR. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. 2012;16(5):536–41.
Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311(16):1652–60.
Malu CKK, Kahamba DM, Walker TD, Mukampunga C, Musalu EM, Kokolomani J, et al. Efficacy of sublingual lorazepam versus intrarectal diazepam for prolonged convulsions in sub-Saharan Africa. J Child Neurol. 2014;29(7):895–902.
Momen AA, Azizi Malamiri R, Nikkhah A, Jafari M, Fayezi A, Riahi K, et al. Efficacy and safety of intramuscular midazolam versus rectal diazepam in controlling status epilepticus in children. Eur J Paediatr Neurol. 2015;19(2):149–54.
Welch RD, Nicholas K, Durkalski-Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population. Epilepsia. 2015;56(2):254–62.
Burman RJ, Ackermann S, Shapson-Coe A, Ndondo A, Buys H, Wilmshurst JM. A comparison of parenteral phenobarbital vs. parenteral phenytoin as second-line management for pediatric convulsive status epilepticus in a resource-limited setting. Front Neurol. 2019;10:506.
Dalziel SR, Borland ML, Furyk J, Bonisch M, Neutze J, Donath S, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet. 2019;393(10186):2135–45.
Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet. 2019;393(10186):2125–34.
Noureen N, Khan S, Khursheed A, Iqbal I, Maryam M, Sharib SM, et al. Clinical efficacy and safety of injectable levetiracetam versus phenytoin as second-line therapy in the management of generalized convulsive status epilepticus in children: an open-label randomized controlled trial. J Clin Neurol. 2019;15(4):468–72.
Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020;395(10231):1217–24.
Nalisetty S, Kandasamy S, Sridharan B, Vijayakumar V, Sangaralingam T, Krishnamoorthi N. Clinical effectiveness of levetiracetam compared to fosphenytoin in the treatment of benzodiazepine refractory convulsive status epilepticus. Indian J Pediatr. 2020;87:512–9.
Vignesh V, Rameshkumar R, Mahadevan S. Comparison of phenytoin, valproate and levetiracetam in pediatric convulsive status epilepticus: a randomized double-blind controlled clinical trial. Indian Pediatr. 2020;57(3):222–7.
Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci. 2004;25(4):193–200.
Egunsola O, Choonara I, Sammons HM. Safety of levetiracetam in paediatrics: a systematic review. PLoS ONE. 2016;11(3):e0149686.
Li L, Zhang Y, Jia L, Jia D, Faramand A, Chong W, et al. Levetiracetam versus phenytoin for the treatment of established status epilepticus: a systematic review and meta-analysis of randomized controlled trials. Seizure. 2020;78:43–8.
Verrotti A, Prezioso G, Di Sabatino F, Franco V, Chiarelli F, Zaccara G. The adverse event profile of levetiracetam: a meta-analysis on children and adults. Seizure. 2015;31:49–55.
Stephen LJ, Brodie MJ. Brivaracetam: a novel antiepileptic drug for focal-onset seizures. Ther Adv Neurol Disord. 2018;11:1756285617742081.
Brigo F, Bragazzi NL, Nardone R, Trinka E. Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: results of an indirect comparison meta-analysis of RCTs. Seizure. 2016;42:29–37.
Brigo F, Lattanzi S, Nardone R, Trinka E. Intravenous brivaracetam in the treatment of status epilepticus: a systematic review. CNS Drugs. 2019;33(8):771–81.
Steinhoff BJ. The AMPA receptor antagonist perampanel in the adjunctive treatment of partial-onset seizures: clinical trial evidence and experience. Ther Adv Neurol Disord. 2015;8(3):137–47.
Brigo F, Lattanzi S, Rohracher A, Russo E, Meletti S, Grillo E, et al. Perampanel in the treatment of status epilepticus: a systematic review of the literature. Epilepsy Behav. 2018;86:179–86.
Kim HD, Chi CS, Desudchit T, Nikanorova M, Visudtibhan A, Nabangchang C, et al. Review of clinical studies of perampanel in adolescent patients. Brain Behav. 2016;6(9):e00505.
Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S. Intravenous antiepileptic drugs in adults with benzodiazepine-resistant convulsive status epilepticus: a systematic review and network meta-analysis. Epilepsy Behav. 2019;101(Pt B):106466.
Brigo F, Del Giovane C, Nardone R, Trinka E, Lattanzi S. Second-line treatments in benzodiazepine-resistant convulsive status epilepticus: an updated network meta-analysis including the ESET trial: what did change? Epilepsy Behav. 2020;106:107035.
Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial: survey of 71 "negative" trials. N Engl J Med. 1978;299(13):690–4.
Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol. 2010;67(8):931–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Yihao Zhang, Yingjie Liu, Qiao Liao, and Zhixiong Liu have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
The data used in this study were fully available in databases.
Code availability
Not applicable.
Author contributions
YZ and YL equally contributed to this manuscript in conception, data extraction, quality assessment, data analysis, and drafting. QL and ZL revised the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Liu, Y., Liao, Q. et al. Preferential Antiseizure Medications in Pediatric Patients with Convulsive Status Epilepticus: A Systematic Review and Network Meta-Analysis. Clin Drug Investig 41, 1–17 (2021). https://doi.org/10.1007/s40261-020-00975-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00975-7