Abstract
Management of metastatic renal cell carcinoma has drastically changed in the last few years, witnessing the advent of more and more target therapies and, recently, of immune-checkpoint inhibitors. On the other hand, the adjuvant setting still lacks a clear beneficial treatment. Medical treatment still remains a compelling challenge. A large number of clinical trials is ongoing with the aim to identify new therapeutic approaches to expand the options in our repertoire. Several strategies are under investigation in renal cell carcinoma (RCC). These include new targeted agents and combinations of target therapy and immunotherapy. Programmed death receptor-1 (PD-1), programmed death receptor ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA4) are just part of the intricate network that regulates our immune response to cancer cells. Co-stimulators, such as glucocorticoid-induced TNFR-related protein (GITR) and tumor necrosis factor receptor superfamily, member 4 (OX40), and co-repressors, example.g. T cell immunoglobulin and mucin domain 3 (TIM-3) and lymphocyte-activation gene 3 (LAG-3), also take part. As knowledge of the functioning of the immune system grows, so do these pathways to target with new drugs. This review is an overview of the current state of the clinical research, providing a report of ongoing Phase I, II and III clinical trials for localized and metastatic RCC, including novel target therapies, novel immunotherapy agents and new combinations strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We reported an overview of ongoing Phase I, II, III clinical trials for localized and metastatic RCC. |
Novel target therapies, including MET inhibitors, glutaminase inhibitors, histone deacetylates inhibitors, are under evaluation. |
New immunotherapeutic compounds are under investigation targeting co-modulatory pathways of the immune response, such as IDO, LAG-3, TIM-3, adenosine receptors. |
Immunotherapies and target therapies are being combined in multiple ways in order to achieve better outcomes. |
1 Introduction
Renal cell carcinoma (RCC) represents 5% of all cancers in men and 3% in women, with 65,340 estimated new cases and 14,970 estimated death in 2018 in the USA [1]. The biology of RCC, mainly depending on upregulation of angiogenic pathways, renders it very sensitive to anti-angiogenic therapies. Tyrosine kinase inhibitors (TKI)-targeting angiogenic pathways such as vascular endothelial growth factor (VEGF) such as sunitinib, axitinib, sorafenib, pazopanib, cabozantinib, and mammalian target of rapamycin (mTOR) inhibitors, like everolimus, are the cornerstone of the treatment of metastatic RCC [2]. Even tivozanib, a target of VEGFR, has recently been approved despite the lack of clear survival benefit on the basis of a prospective Phase III trial comparing tivozanib to sorafenib in previously untreated patients with metastatic RCC [3]. In addition to these target therapies, the immune-checkpoint inhibitor nivolumab, an anti-programmed death receptor-1 (PD-1), in monotherapy [4] or in combination with ipilimumab [5], an anti-cytotoxic T lymphocyte-associated protein 4 (CTLA4), entered in the treatment scenario of the metastatic disease. In patients with metastatic disease, cytoreductive nephrectomy could be an option even if recent evidence seems to resize the effective role of this approach [6, 7]. For the adjuvant setting, there is not yet a consensus since no TKI has been beneficial in this scenario—the only exception being sunitinib, that, in a recently published trial investigating its role in patients with clear cell (cc) RCC at high risk of relapse, showed a benefit in terms of disease-free survival [8, 9]. However, for several reasons, including lack of improvement in overall survival, these agents may not be suggested as preferred options in adjuvant setting. Of note, further evidence seems to suggest that better patient selection may be a key issue in evaluating a new adjuvant treatment [10, 11].
Although these treatments have improved the outcomes of patients with RCC, relapse or progression to therapies eventually occurs and, in some cases, it could be an early event. Clinical research is continually evolving, always experimenting with novel drugs or combinations in order to improve clinical outcomes of our patients. As the knowledge of resistance mechanisms and biological characteristics of the tumor and its interaction with the immune system grows, so do the researchers’ efforts to design more and more clinical trials to expand treatment options.
Here we present a review of the current state of ongoing active and recruiting Phase I, II and III clinical trials for patients with metastatic, locally advanced and resected RCC.
2 Combination of Immunotherapy and Target Therapy
Combining new immune-checkpoint inhibitors to ‘standard’ target therapy is a promising and emerging approach, which could potentially lead to a significant improvement of patient’s clinical outcomes. As known, most target drugs currently adopted in the management of RCC have angiogenesis as a primary target, which is mainly represented by the VEGFR/VEGF pathway. When we look at pathways regulating immune response and angiogenesis, we need to imagine a very complex and strongly braided net in which external factors acting in one, are inexorably involved with the other. As a consequence, factors acting to inhibit angiogenesis seem to enhance immunity against a tumor and, conversely, factors acting on the immune system can promote or repress angiogenesis. This happens mainly because of an indirect action of angiogenesis on immune-suppressive cytokines and cells other than a direct activation of immune-checkpoint on the surface of cancer cells promoted by VEGF [12,13,14,15,16].
Not surprisingly, the adoption of combination treatment with new immune-checkpoint inhibitors and target therapy represents a very attractive approach that has already been investigated in some clinical trials.
The PD-1 inhibitor, pembrolizumab, has been tested in combination with axitinib or lenvatinib in Phase Ib and Ib/II trials, respectively, showing a good safety profile and a promising response rate (73% and 63% in overall population in combination with axitinib and lenvatinib, respectively) [17, 18]. Furthermore, when added to the selective inhibitor of indoleamine 2,3-dioxygenase-1, pembrolizumab showed an objective response rate (ORR) of 47% [19].
Nivolumab and cabozantinib, with or without ipilimumab, showed an ORR of 54% even if treatment was associated with a significant rate of grade 3–4 toxicities in both arms [20]. Similar response rate has also been observed with the combination of nivolumab and tivozanib (44%) and avelumab—an anti-programmed death-ligand-1 (PD-L1), and axitinib (58%), even if grade 3–4 toxicities were frequent in both these studies (32% and 52%, respectively) [21, 22].
Furthermore, the combination of atezolizumab, an anti PD-L1, and bevacizumab has been tested in Phase I and II trials and the positive results obtained have led to the design of a Phase III trial (IMmotion151) that is currently ongoing [23, 24]. Although promising preliminary results have been observed in this last Phase III trial, final results are still awaited.
Due to the promising results achieved, the combination of immune-checkpoint inhibitors and target therapy is one of the most promising approaches under investigation.
2.1 Phase III Trials Exploring the Combination of Immunotherapy and Target Therapy
Several Phase III trials are currently ongoing (Table 1). The randomized Phase III clinical trial NCT02811861 (CLEAR) is currently exploring if the combination of pembrolizumab and lenvatinib or the combination of lenvatinib and everolimus could result in a significant improvement of outcomes over sunitinib in patients with untreated metastatic RCC (mRCC). This study has a planned enrollment of 1050 patients with an estimated primary completion date in April 2020.
Results of two Phase III randomized clinical trials: JAVELIN RENAL 101 and KEYNOTE 426 have been recently reported [25, 26]. In JAVELIN RENAL 101 trial, the combination of axitinib and avelumab has been compared to sunitinib in patients with metastatic RCC. Patients treated with the combination showed a longer progression free survival (PFS) [hazard ratio (HR) for PFS, 0.69; 95% CI 0.56–0.84] and higher objective response rate (55.2% vs 25.5%) [25]. In KEYNOTE 426, patients with metastatic RCC were randomized to receive sunitinib alone or the combination of pembrolizumab and axitinib. Again, patients in the combination group showed a significantly longer estimated 12-month overall survival (OS), HR 0.53, 95% CI 0.38–0.74), PFS (HR 0.69, 95% CI 0.57–0.84) and objective response rate (59.3% vs 35.7%) [26].
Nivolumab and cabozantinib with or without ipilimumab are under investigation versus sunitinib in previously untreated mRCC. With a planned recruitment of 630 patients, Checkmate 9ER (NCT03141177) is a randomized three-arm Phase III trial with PFS as primary endpoint in patients with intermediate/poor risk according to the International Metastatic RCC Database Consortium (IMDC) criteria.
The IMmotion 151 trial has investigated the combination of atezolizumab and bevacizumab over sunitinib in patients with metastatic RCC. Co-primary endpoints of the study were PFS in overall population and in patients expressing PD-L1. Despite that OS was immature at the first interim analysis, a PFS benefit was observed in all subgroups (HR 0.74 95% CI 0.57–0.96 in PD-L1-positive patients and HR 0.83 95% CI 0.7–0.97 in intention-to-treat population). ORR was 43% in the combination arm and 35% in the sunitinib arm. Despite these promising results, final survival analyses are still awaited [24].
2.2 Phase I and I/II Trials Exploring the Combination of Immunotherapy and Target Therapy
Other than combination with axitinib, bevacizumab and cabozantinib (NCT03341845, NCT02724878, NCT03141177, NCT03635892, NCT03172754, NCT03149822, NCT03595124, NCT03170960, NCT03200587, NCT03086174, NCT02496208), immune-checkpoint inhibitors are under investigation in combination with other target agents. As known, phosphorylation, acetylation, deacetylation, ADP ribosylation, sumoylation, citrullination, ubiquitination and deamination modulate histone functions and gene expression [27]. In particular, acetylation results in increased DNA accessibility as affinity between histones and DNA is reduced by addiction of acetyl groups. On the contrary, deacetylation results in lower DNA accessibility and gene silencing. Different agents targeting these functions are currently under investigation. Although a number of toxicities have been shown in some trials investigating molecules able to interact with histones physiology, the evaluation of deacetylase and acetylase histone inhibitors still remains an interesting approach mainly due to a strong biological rationale [28]. Thus, the class 1 histone deacetylases inhibitor entinostat is currently under investigation in combination with IL-2, bevacizumab and atezolizumab, and with the combination nivolumab and ipilimumab (NCT03501381, NCT03552380, NCT03024437). Panobinostat is another deacetylase inhibitor that is currently under investigation with the PD-1 inhibitor PDR001 and with LCL161 (a specific inhibitor of apoptosis protein IAP) (NCT02890069). Chidamide is a histone deacetylase inhibitor, which is able to inhibit several classes of histone deacetylases such as: HDAC1, HDAC2, HDAC3 and HDAC10. This drug is currently under investigation in combination with nivolumab (NCT02718066).
Sitravatinib (MGCD516) is a small multi-tyrosine kinase inhibitor, which is able to interact with several pathways including: TYRO3, AXL, MerTK, VEGFR, PDGFR, KIT, RET and MET, and has recently been demonstrated to enhance immune checkpoint blockade in refractory cancer models [29]. The combination of sitravatinib and nivolumab is currently under investigation in two Phase I/II clinical trials (NCT03015740, NCT03680521).
Savolitinib (AZD6094) is a selective MET tyrosine kinase inhibitor that has already been tested in a population of 109 patients with papillary RCC. In patients with recognized MET altered status, the administration of savolitinib resulted in an ORR of 18% with a PFS of 6.2 months [30]. A Phase I/II trial is currently testing the combination of savolitinib and durvalumab versus both the two treatments alone and versus the combination of durvalumab and tremelimumab (NCT02819596). Furthermore, the MET inhibitor bosutinib (CBT-101) in association with nivolumab is under investigation in previously treated metastatic hepatocellular carcinoma and RCC (NCT03655613). Bruton tyrosine kinase (BTK) is a key tyrosine kinase that drives lymphocyte B maturation and mast cell activation. As known, mutations in BTK gene lead to primary immunodeficiency (X-linked agammaglobulinemia). Ibrutinib (PCI-32765) is a selective BTK inhibitor, which has been shown to significantly improve clinical outcomes of patients with hematological malignancies [31, 32]. As BTK seems to be an interesting targetable pathway in solid tumors, this agent is currently being tested in solid malignancies [33]. In particular, the combination of nivolumab and ibrutinib is under investigation in a Phase I/II clinical trial (NCT02899078).
Other approaches under investigation involve the combination of the inhibitor of VEGF-A/B aflibercept and pembrolizumab while another Phase I trial is evaluating the combination using this latter PD-1 inhibitor and the angiopoietin 1–2 neutralizing peptibody (AMG386) (NCT02298959, NCT03239145).
A Phase II trial is investigating the combination of axitinib- and pembrolizumab-activated autologous D-CIK (cytokine-induced killer cells stimulated using mature dendritic cells). This approach consists of the acquisition of peripheral blood mononuclear cells by peripheral blood of patients, then these same cells are incubated with cytokines and pembrolizumab and finally re-infused in patients (NCT03736330).
As known, RCC, tumor cells often present an alteration of their metabolism, which mainly involves a switch, and thus increased production of pyruvate and lactate with a reduction of oxidative and mitochondrial activity. This switch is known as Warburg effect and several molecules that are able to interfere with these metabolic alterations have been tested in RCC [34]. In particular, metabolism of glutamine seems to be a key pathway for the production of lipids, amino acids, adenosine triphosphate (ATP) and nucleotides [35].
A Phase I/II clinical trial is currently evaluating if the combination of the glutaminase inhibitor CB-839 and nivolumab results in a safety profile and clinical activity in patients with melanoma, non-small cell lung cancer and clear cell RCC (ccRCC) (NCT02771626).
Curiously, another enzymatic inhibitor known as trigriluzole (BHV-4157) is under evaluation as combination treatment with nivolumab (NCT03170960). This agent has antidepressant and anxiolytic activity as it interferes with glutamate release and sodium channel activation and, due to its ability to interfere with glutamate transmission, it has also been evaluated in Alzheimer’s disease [36]. However, maybe due to its ability to interact with some metabolic pathways still unknown, it also presented an interesting anti-neoplastic activity.
Liver X receptor is a member of a nuclear receptor family of transcription factors, which modulate important functions such as cholesterol, fatty acid, and glucose homeostasis. Very recently, its role appears to be of particular interest as it seems to be altered in cancer cells where it drives key functions leading to cancer progression and development [37, 38]. The liver X receptor inhibitor RGX-104 and the anti-PD-1 nivolumab are under investigation in a Phase I trial (NCT02922764) [37, 38].
Of interest, synthetic protein able to bind specific proteins on the surface of cancer cells and stimulate immune response against these same cells, is under development. RO6874281 is a fusion protein under investigation in a Phase I trial in combination with atezolizumab alone or with atezolizumab and bevacizumab. This protein consists of a human anti-fibroblast activation protein-alpha (FAP) antibody and an engineered interleukin-2 (NCT03063762). By targeting FAP-positive tumor cells, this compound could enhance local immune response and promote tumor regression.
3 Combination of Immunotherapies
Targeting different pathways of the immune response system, which is made up of an intricate web of stimulatory and inhibitory signals, is a strategy used to enhance response to therapies. Different combinations of immune checkpoint inhibitors are being studied in Phase I, II and III trials in the adjuvant and metastatic settings. The results of the combination of nivolumab plus ipilimumab investigated in the Phase III trial Checkmate 214 [5] have already been published and show an advantage in term of OS and ORR for the combination compared to sunitinib among intermediate- and poor-risk patients with previously untreated advanced ccRCC.
Currently, the combination of nivolumab plus ipilimumab is being investigated in seven Phase II trials in the metastatic setting in first or following lines in clear cell and/or non-clear cell (ncc) RCC (NCT03203473, NCT03075423, NCT03297593, NCT03117309, NCT02960906, NCT02917772, NCT03177239) and one Phase III randomized trial versus placebo in the adjuvant setting in patients with ccRCC at high risk of relapse after nephrectomy (NCT03138512) (Table 2).
Durvalumab, an anti-PLD-L1, in monotherapy or in combination with tremelimumab, an anti-CTLA4, is under investigation in a randomized Phase III trial (NCT03288532) in patients with RCC (Table 1), both cc and ncc, at high or intermediate risk of relapse compared with active monitoring. Durvalumab in monotherapy or plus tremelimumab is also being investigated in the neoadjuvant setting in a Phase Ib trial (NCT02762006) in patients with any histological subtype RCC T2b-4 and/or N1, M0 disease, followed by nephrectomy.
The combination of pembrolizumab with low-dose interleukin-2 is being evaluated in a Phase I/II trial in advanced RCC after failure of anti-PD-1/PD-L1 and TKI therapies (NCT03111901).
4 Novel Target Agents
Although the research of new combination strategies appears to be one of the most promising approaches under investigation in RCC, the identification of new targets still remains a key issue in management of RCC (Table 2). Other than new target inhibitors developed to inhibit specific altered pathways of the disease, several efforts directing on the inhibition or stimulation of immune receptors are under investigation.
As already described, mutations in tumor cells resulting in metabolic alterations are perhaps the more common event in all RCC subtypes. It is important to observe that several different mutations inevitably lead to metabolic shift, which often, but not always, results in a ‘Warburg effect’ [34]. The anaerobic degradation of glucose- more than mitochondrial-mediated oxidative phosphorylation is the main consequence of this shift, which also leads to augmented dependence on pentose phosphate shunt, higher fatty acid production, higher intracellular level of lactate, and reduction of Krebs cycle activity.
Several altered genes could explain the metabolic alteration observed in RCC. VHL is certainly the more frequently altered gene in ccRCC. Other genes of particular interest, which are frequently mutated in RCC, are MET and mTOR. Alterations occurring in these genes lead to important and metabolic alterations which can be targeted by specific drugs. [39,40,41,42,43,44,45,46,47]. Alteration of these genes results in one of the most important alterations observed in RCC, that of neo-angiogenesis. Thus, agents able to inhibit pathways related to metabolic alteration and angiogenesis promotion represent a successful strategy for the management of RCC. Thus, the investigation of new agents able to interfere with these two hallmarks of RCC are of particular interest.
The VEGFR and PDGFR inhibitor vorolanib was designed to maintain the same clinical efficacy and pharmacodynamic proprieties of sunitinib with a better safety and toxicity profile. This agent was recently evaluated in a Phase I trial in patients with solid tumors, and confirmed a safety profile with a standard dose of 400 mg/daily [48]. It is currently under evaluation as monotherapy or in combination with the mTOR inhibitor everolimus in a Phase I and II/III trial (NCT02577458, NCT03095040). Other inhibitors have been developed to specifically target the hypoxia-inducible factor 1 and 2. As known, VHL is the most frequently altered gene in ccRCC. This gene is one of the major regulators of the ubiquitin-dependent degradation of the hypoxia-inducible factor 1 and 2 alpha (HIF1-2α). Accumulation of HIF1-2α occurring during hypoxia (physiological condition) or VHL loss (pathological condition) leads to up-regulation of hypoxia-response elements such as VEGF, PDGF, EGF and GLUT1 (the glucose transporter). Moreover HIF1α enhances glycolytic enzyme expression and reduces mitochondrial pyruvate consumption [39,40,41,42]. PT2977 is a HIF-2α inhibitor currently under investigation in a Phase I trial (NCT02974738) and in a Phase II trial in combination with cabozantinib (NCT03634540).
One attractive target in RCC is the MET tyrosine kinase receptor. This is mainly due to its key role in regulation of several functions of tumor cells such as: angiogenesis, resistance and progression to other TKIs targeting VEGFR, acquisition of aggressive behaviors by tumor, including metastatic proprieties and bone invasion [49]. The importance of this pathway could be further highlighted by the results obtained with MET inhibitors in papillary RCC [30], and by the results obtained by the multi-target inhibitor cabozantinib in RCC [50, 51]. Thus, other inhibitors targeting MET are under investigation. A randomized Phase III clinical trial is evaluating if administration of savolitinib could result in a PFS benefit over sunitinib in patients with metastatic or locally advanced MET-driven papillary RCC (NCT03091192). Capmatinib is a selective c-MET inhibitor currently under evaluation in a Phase II trial in patients with RCC (NCT02019693), while a Phase I study is currently evaluating the MET and multi-tyrosine kinase inhibitor sitravatinib in patients with advanced cancers (NCT02219711). Furthermore, volitinib is a MET inhibitor currently under investigation in a Phase I/II trial comparing sunitinib, cabozantinib and crizotinib in metastatic papillary RCC (NCT02761057).
As already described, agents able to interfere with altered metabolism of RCC are under investigation. Among these, agents able to inhibit function and synthesis of glutamine appear to be of particular interest, as this molecule seems to play a key role in driving disease progression and development [36]. Thus, CB-839, a glutaminase inhibitor, is under investigation in combination with nivolumab in a Phase I/II clinical trial (NCT02771626), as already mentioned, and in combination with the mTOR inhibitor everolimus and the multi-target inhibitor cabozantinib in two different Phase II trials (NCT03163667, NCT03428217, respectively).
Modulation of DNA transcription by regulation of chromatin conformation as driven by histone status seems to be an attractive approach. Indeed, histone conformation makes DNA more or less accessible for transcription modifying tumor-cell transcription and protein synthesis. Agents able to interfere with acetylation status of histones are under investigation and, as already described, several deacetylase inhibitors are under evaluation with immune-checkpoint inhibitors. However, a Phase 3 trial is investigating if the addition of abexinostat (a deacetylase inhibitor) to standard first-line therapy with pazopanib could lead to a clinical benefit over pazopanib and placebo. The RENAVIV trial (NCT03592472) is currently ongoing and recruiting patients – the primary completion date is estimated in January 2022.
Of interest, a study carried out on murine model of RCC cell lines showed that RCC expresses arginase II regulating l-arginine metabolism, resulting in a stimulation of cell growth and immune inhibition (in particular T-cell inhibition) [52]. Arginase inhibitors are also under investigation in RCC. Indeed, the arginase inhibitor CB-1158 is currently under investigation in a Phase I/II trial (NCT02903914).
5 Novel Immunotherapy Approaches
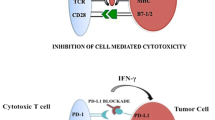
The immune response to cancer cells is modulated by many checkpoints, any one of which could be a potential target for the development of new drugs. As PD-1/PD-L1 and CTLA-4 inhibitors continue to be a cornerstone of immunotherapy changing the natural history of many cancer patients, other immunomodulatory pathways, such as co-inhibitory receptors such as TIM-3 and LAG-3 or co-stimulatory receptors such as GITR and OX40, need to be considered in order to enhance the response to other biological or immunological compounds.
Chimeric antigen receptor (CAR) T cells are a novel immunotherapy approach that is entering in the treatment of hematological diseases and is being tested in solid tumors including RCC in Phase I/II trials (NCT03393936, NCT03638206, NCT02830724). Second-generation CARs are engineered receptors consisting of an extracellular domain that binds to tumor antigen, a transmembrane domain, and an intracellular domain that is made of costimulatory domains (CD28 and 4-1BB and the CD3ζ chain) that direct the expansion of functional T cells. CAR genes are then transferred into the patient’s T cells and reinfused into the patients. CAR T cells can function independently from peptide-MHC presentation and possess the same cytotoxic effector function as endogenous CD8+ T cells [53, 54].
Lymphocyte activation gene-3 (LAG-3, CD223) is an immune checkpoint protein, and its upregulation prevents the onset of autoimmunity, but in the tumor setting can also lead to immunosuppression. The tumor microenvironment with its persistent antigen exposure leads to LAG3 overexpression. This can result in a state of immune exhaustion characterized by the negative regulation of T-cell function. LAG3 is also expressed on activated regulatory T cells (Tregs) at higher levels than on effector T cells (Teffs) [55, 56]. Relatlimab (BMS-986016) is an anti-LAG-3 antibody being tested in association with nivolumab in a Phase II trial on advanced RCC (NCT02996110). Other anti-LAG3 antibodies (LAG525, INCAGN02385) are being tested in Phase I and I/II trials alone (NCT02460224, NCT03538028) or in combination with an anti-PD1 (NCT02460224) in advanced malignancies including RCC.
Another interesting pathway that could mediate an important link between immune response and metabolic alterations in tumors is represented by adenosine interaction with adenosine receptors. Purinergic signaling is an important pathway that regulates the immune response and can lead to cancer immune evasion. Inflammation or cancer lead to cellular damage and a state of hypoxia that increases ATP levels, which is then dephosphorylated by ectonucleotidases (CD39, that dephosphorylates ATP to AMP, and CD73, that transforms AMP to adenosine) leading to accumulation of adenosine. Extracellular adenosine has a marked immunosuppressive effect, acting on effector cells by dampening their action and immunosuppressive regulatory cells by stabilizing them. Studies on these pathways are ongoing targeting both adenosine receptors and ectonucleotidases, which are overexpressed in the tumor microenvironment [57].
Adenosine activates cellular signaling pathways through G-protein-coupled adenosine receptors: in particular, adenosine receptors A2a (A2aR) and A2b (A2bR) are upregulated in response to immune cell activation. A2aR is expressed in T cells, natural killer, monocytes, macrophages, dendritic cells, while A2bR is expressed by macrophages and dendritic cells. The upregulation of these receptors leads to an immunosuppressive state in various ways: (1) suppressing the secretion of neutrophil chemoattractants, (2) impeding the maturation of dendritic cells, (3) altering dendritic cells to render them more suppressive by secreting IL-10, TGFβ, arginase and IDO (indoleamine 2,3-dioxygenase 1), (4) reducing IL-2 secretion by CD4 T cells thus reducing the expression of costimulatory receptor CD28, (5) inhibiting cell proliferation and cytotoxicity of CD8 T cells, (6) stabilizing Tregs, (7) increasing expression of checkpoint pathways such as PD-1, CTLA-4, and LAG-3 [57]. Therefore, adenosine signaling is an important checkpoint pathway that leads to suppression of immune response. Inhibitors of adenosine receptors are potential new drugs under development alone or in combination with anti-PD1/PDL1 or anti-CD73. Phase I and II trials on advanced malignancies are ongoing, testing NIR178 (NCT03207867, NCT03549000), CPI-444 (NCT03454451, NCT02655822) and AB928 (NCT03629756), which are immune checkpoint inhibitors that target the first two molecules A2aR and the third A2aR and A2bR. By inhibiting the interaction of adenosine with its receptors, these molecules reinstate the proliferation and activation of T lymphocytes and stimulate T-cell response against tumor cells. NIR178 is being tested in a Phase II trial in combination with PDR001, a new anti-PD1 (NCT03207867), and in a Phase I trial in combination with NZV930, a CD73 inhibitor (NCT03549000). Anti-CD73 inhibits the ectonucleotidase CD73 crucial for the production and accumulation of extracellular adenosine, thus reducing its formation and increasing activity of immune cells. CPI-444 is being tested alone or in association with atezolizumab in a Phase I/Ib trial (NCT02655822) or in combination with an anti-CD73 (CPI-006) in a Phase I trial (NCT03454451). AB928 is being evaluated in combination with a novel anti-PD1, AB122, in a Phase I study aimed to assess safety and toxicity profile (NCT03629756).
Glucocorticoid induced TNF receptor (GITR) is a costimulatory receptor of the TNF super family and is constitutively expressed at high levels on Tregs and at low levels on naïve and memory T cells. Its expression of Tregs and Teffs is increased after activation of T cells. Its ligand, GITRL, is expressed by activated antigen-presenting cells, including dendritic cells, macrophage and activated B cells. GITR expands CD8 T effector memory cell population and promotes the loss or inhibition of Tregs. GITR agonist antibodies could bind the activating Fcγ receptor in the Teff cells, thus shifting the balance of CD8 Teff/Treg in favor of effector cells. Therefore, the reduced immunosuppression derived by Treg depletion and the enhanced costimulatory function of CD8 T cells increase the antitumor immunity [58]. INCAGN01876 is an agonistic anti-GITR antibody that binds and activates GITRs on T cells, thus promoting the proliferation of Teff cells and suppressing the function of Tregs, leading to an improved immune response. This compound is being studied in Phase I/II trials alone (NCT02697591) and in combination with nivolumab and ipilimumab (NCT03126110) in metastatic malignancies.
T-cell immunoglobulin and mucin-domain containing-3 (Tim-3) is a type I transmembrane protein that acts as a checkpoint inhibitor of immune response against cancer. When overexpressed, it has been implicated in the suppression of T-cell responses and T-cell dysfunction, a state referred to as T-cell exhaustion [59]. Tim-3 has been found expressed in tumor cells, Teffs, Tregs, endothelial cells, dendritic cells. Tim-3 expressed on effector CD8 T cells in the tumor microenvironment bind to galectin-9 produced by myeloid-derived suppressor cells leading to apoptosis of effector T cells [59]. Tim-3 expression on CD8 TILs has been associated with PD-1 expression resulting in a subpopulation of T cells more exhausted than the Tim-3 negative PD-1+ CD8 T cells [60]. Tumor cells express PD-L1 and galectin-9 that bind PD-1 and Tim-3, respectively, resulting in downregulation of T-cell function that dampens anti-tumor immunity. In this immunosuppressive mechanism lies the rationale of combining PD-1 and Tim-3 blockade to restore Teff function. Tim-3 is also expressed on FoxP3+ Tregs within the tumor, resulting in higher expression of Treg effector molecules like IL-10 and inhibition of Teffs [59]. Furthermore, Tim-3 is expressed on tumor-infiltrating dendritic cells and its role is to bind high-mobility group box 1 (HMGB1) and block the transport of nucleic acids into endosomes, thus suppressing pattern-recognition receptor-mediated innate immune responses to tumor-derived nucleic acids [59]. High levels of Tim-3 expression have been associated with poor prognosis of patients with prostate cancer [61], ccRCC [62], colon cancer [63], bladder urothelial carcinoma [64], cervical cancer [65], and gastric cancer [66]. Currently, the TIM-3 inhibitors MBG453 and INCAGN02390 are being tested in a Phase I/II and I trial alone (MBG453 NCT02608268, INCAGN02390 NCT03652077) or in combination with PDR001 (MBG453 NCT02608268).
Indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase 2 (TDO2) are the enzymes that catalyze the first and rate-limiting step of the catabolic conversion of tryptophan into kynurenine, that is further converted in nicotinamide adenine dinucleotide (NAD) and ATP that fuel cellular metabolic functions.
IDO1 overexpression can impair the immune response in two ways. On one hand, tryptophan’s depletion has been associated with apoptosis and dysfunction of Teff cells. On the other hand, kynurenine accumulates and binds to the ligand-activated transcription factor aryl hydrocarbon receptor (AhR) leading to the generation of immune-tolerant dendritic cells and Tregs that create a tumor microenvironment defective in recognizing and eradicating cancer cells [67, 68]. Thus, IDO1 seems to be an important immune checkpoint and target for novel immunotherapy agents. A Phase II trial analyzing the combination of nivolumab and BMS-986205, an IDO1 inhibitor, in patients with advanced RCC (NCT02996110) is recruiting at present.
OX40 (CD134) is a member of the tumor necrosis factor receptor superfamily with co-stimulatory functions expressed by activated T cells. Its co-stimulation of T cells activates T-cell signaling, which includes NF-kB and nuclear factor of activated T cells that enhance the expression of cytokines, survivin and Bcl-2 anti-apoptotic molecules. Thus, the main role of OX40 is to enhance proliferation and survival of CD4 and CD8 T cells [69]. PF-04518600 is an agonistic antibody of OX40 under evaluation in a Phase II trial in combination with axitinib (NCT03092856) and in a Phase I alone, or in association with PF-05082566 (NCT02315066), an agonist of the receptor 4-1BB (CD-137) expressed on CD4 and CD8 T cells and natural killer cells.
Vaccines against tumor-specific antigens, called neoantigens, are being developed in the treatment of many solid tumors, including RCC. Phase I and I/II trials are ongoing, exploring vaccine therapy alone or in combination with anti-checkpoint inhibitors in the metastatic setting (NCT03548467, NCT03633110, NCT00722228, NCT03294083, NCT03311334, NCT03289962). Furthermore, an oncolytic virus comprising a thymidine kinase-deactivated vaccinia poxvirus plus granulocyte-macrophage colony-stimulating factor (JX-594) is being studied in combination with a novel anti-PD1 (REGN2810) in a Phase I trial in patients with metastatic RCC (NCT03294083).
Oncolytic viruses find their rationale in the compelling task of cancer therapy of targeting selected cancer cells and are designed to stimulate the immune system by infecting and replicating in tumor cells. Oncolytic viruses have been engineered in order to infect, replicate and induce transgene expression in cancer cells, thus causing lysis of tumoral cells and contributing to enhance antitumoral immunity [70].
6 Conclusion
In a few short years, the therapeutic scenario of RCC has been revolutionized by the advent of effective TKI, which have drastically changed the prognosis and clinical outcomes of patients. A second wave of progress has been represented by immune-checkpoint inhibitors, which have further increased the benefit and survival of patients with metastatic and advanced RCC. The next challenges will be directed towards investigating better approaches and treatment strategies in RCC. In particular, a combination of immune-checkpoint inhibitors as well as a combination of immune-checkpoint inhibitors and tyrosine kinases inhibitors seem to be reliable and effective strategies, and their role will be clearer in the near future. On the other hand, new targets are under investigation and so it is probable that other immune-checkpoint inhibitors or agonists and other targeted treatments will show promising activity in a few years. As the availability of active treatments is increasing, selection of patients who present specific clinical and genetic features are of particular interest in order to better select patients who are more likely to benefit from a specific treatment or treatment strategy.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. https://doi.org/10.3322/caac.21442.
Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–66. https://doi.org/10.1056/NEJMra1601333.
Santoni M, Massari F, Piva F, Carrozza F, Di Nunno V, Cimadamore A, et al. Tivozanib for the treatment of renal cell carcinoma. Expert Opin Pharmacother. 2018;19(9):1021–5. https://doi.org/10.1080/14656566.2018.1480722.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. https://doi.org/10.1056/NEJMoa1510665.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90. https://doi.org/10.1056/NEJMoa1712126.
Bhindi B, Abel EJ, Albiges L, Bensalah K, Boorjian SA, Daneshmand S, et al. Systematic review of the role of cytoreductive nephrectomy in the targeted therapy era and beyond: an individualized approach to metastatic renal cell carcinoma. Eur Urol. 2019;75(1):111–28. https://doi.org/10.1016/j.eururo.2018.09.016.
Massari F, Di Nunno V, Gatto L, Santoni M, Schiavina R, Cosmai L, et al. Should carmena really change our attitude towards cytoreductive nephrectomy in metastatic renal cell carcinoma? A systematic review and meta-analysis evaluating cytoreductive nephrectomy in the era of targeted therapy. Target Oncol. 2018;13(6):705–14. https://doi.org/10.1007/s11523-018-0601-2.
Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–54. https://doi.org/10.1056/NEJMoa1611406.
Massari F, Di Nunno V, Ciccarese C, Graham J, Porta C, Comito F, et al. Adjuvant therapy in renal cell carcinoma. Cancer Treat Rev. 2017;60:152–7. https://doi.org/10.1016/j.ctrv.2017.09.004.
Leonetti A, Zielli T, Buti S. Adjuvant tyrosine kinase inhibitors for renal cell carcinoma? No, thank you (at least for the present). Future Oncol. 2018;14(22):2223–4. https://doi.org/10.2217/fon-2018-0304.
Massari F, Di Nunno V, Mollica V, Graham J, Gatto L, Heng D. Adjuvant tyrosine kinase inhibitors in treatment of renal cell carcinoma: a meta-analysis of available clinical trials. Clin Genitourin Cancer. 2019. https://doi.org/10.1016/j.clgc.2018.12.011.
Tartour E, Pere H, Maillere B, Terme M, Merillon N, Taieb J, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30(1):83–95. https://doi.org/10.1007/s10555-011-9281-4.
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–51. https://doi.org/10.1038/nrc3237.
Guislain A, Gadiot J, Kaiser A, Jordanova ES, Broeks A, Sanders J, et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother. 2015;64(10):1241–50. https://doi.org/10.1007/s00262-015-1735-z (Epub 2015 Jun 24).
Liu XD, Hoang A, Zhou L, Kalra S, Yetil A, Sun M, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res. 2015;3(9):1017–29. https://doi.org/10.1158/2326-6066.CIR-14-0244 (Epub 2015 May 26).
Kuusk T, Albiges L, Escudier B, Grivas N, Haanen J, Powles T, Bex A. Antiangiogenic therapy combined with immune checkpoint blockade in renal cancer. Angiogenesis. 2017;20(2):205–15. https://doi.org/10.1007/s10456-017-9550-0 (Epub 2017 Apr 11).
Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion Phase 1b trial. Lancet Oncol. 2018;19:405–15. https://doi.org/10.1016/S1470-2045(18)30081-0 (Epub 2018 Feb 10).
Lee C-H, Makker V, Rasco DW, Taylor MH, Stepan DE, Shumaker RC, et al. Lenvatinib + pembrolizumab in patients with renal cell carcinoma: updated results. J Clin Oncol. 2018;36:4560. https://doi.org/10.1200/JCO.2018.36.15_suppl.4560.
Lara P, Bauer TM, Hamid O, Smith DC, Gajewski T, Gangadhar TC, et al. Epacadostat plus pembrolizumab in patients with advanced RCC: preliminary Phase I/II results from ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:4515. https://doi.org/10.1200/JCO.2017.35.15_suppl.4515.
Nadal RM, Mortazavi A, Stein M, Pal SK, Davarpanah NN, Parnes HL, et al. Results of Phase I plus expansion cohorts of cabozantinib (Cabo) plus nivolumab (Nivo) and CaboNivo plus ipilimumab (Ipi) in patients (pts) with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. J Clin Oncol. 2018;36:515. https://doi.org/10.1200/JCO.2018.36.6_suppl.515.
Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear- cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose- finding and dose-expansion, Phase 1b trial. Lancet Oncol. 2018;19:451–60. https://doi.org/10.1016/S1470-2045(18)30107-4 (Epub 2018 Mar 9).
Escudier B, Barthelemy P, Ravaud A, Negrier S, Needle MN, Albiges L. Tivozanib combined with nivolumab: phase Ib/II study in meta- static renal cell carcinoma (mRCC). J Clin Oncol. 2018;36:618. https://doi.org/10.1200/JCO.2018.36.6_suppl.618.
Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. https://doi.org/10.1038/ncomms12624.
Motzer R, Thomas Powles, Michael B. Atkins, Bernard Escudier, David F. McDermott, Cristina Suarez et al. IMmotion 151: randomized Phase III study of atezolizumab plus bevacizumab versus sunitinib in untreated metastatic renal cell carcinoma. J Clin Oncol. 2018. https://doi.org/10.1200/jco.2018.36.6_suppl.578.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019. https://doi.org/10.1056/nejmoa1816047.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019. https://doi.org/10.1056/nejmoa1816714.
Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14(11):1008–16. https://doi.org/10.1038/nsmb1337.
Qin HT, Li HQ, Liu F. Selective histone deacetylase small molecule inhibitors: recent progress and perspectives. Expert Opin Ther Pat. 2017;27(5):621–36. https://doi.org/10.1080/13543776.2017.1276565 (Epub 2016 Dec 29).
Du W, Huang H, Sorrelle N, Brekken RA. Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight. 2018. https://doi.org/10.1172/jci.insight.124184. (Epub ahead of print).
Choueiri TK, Plimack E, Arkenau HT, Jonasch E, Heng DYC, Powles T, et al. Biomarker-based Phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol. 2017;35(26):2993–3001. https://doi.org/10.1200/JCO.2017.72.2967 (Epub 2017 Jun 23).
Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018;378(25):2399–410. https://doi.org/10.1056/NEJMoa1802917.
Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378(13):1211–23. https://doi.org/10.1056/NEJMoa1715519.
Molina-Cerrillo J, Alonso-Gordoa T, Gajate P, Grande E. Bruton’s tyrosine kinase (BTK) as a promising target in solid tumors. Cancer Treat Rev. 2017;58:41–50. https://doi.org/10.1016/j.ctrv.2017.06.001 (Epub 2017 Jun 9).
Ramapriyan R, Caetano MS, Barsoumian HB, Mafra ACP, Zambalde EP, Menon H et al. Altered cancer metabolism in mechanisms of immunotherapy resistance. Pharmacol Ther. 2018. https://doi.org/10.1016/j.pharmthera.2018.11.004. (Epub ahead of print).
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. https://doi.org/10.1146/annurev-cellbio-092910-154237.
Liu J, Wang LN. The efficacy and safety of riluzole for neurodegenerative movement disorders: a systematic review with meta-analysis. Drug Deliv. 2018;25(1):43–8. https://doi.org/10.1080/10717544.2017.1413446.
Wang Q, Feng F, Wang J, Ren M, Shi Z, Mao X et al. Liver X receptor activation reduces gastric cancer cell proliferation by suppressing Wnt signalling via LXRβ relocalization. J Cell Mol Med. 2018. https://doi.org/10.1111/jcmm.13974. (Epub ahead of print).
Wu Y, Yu DD, Yan DL, Hu Y, Chen D, Liu Y, et al. Liver X receptor as a drug target for the treatment of breast cancer. Anticancer Drugs. 2016;27(5):373–82. https://doi.org/10.1097/CAD.0000000000000348.
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. https://doi.org/10.1038/20459.
Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG Jr. Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1(3):237–46. https://doi.org/10.1016/S1535-6108(02)00043-0.
Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65(8):3299–306. https://doi.org/10.1158/0008-5472.CAN-04-4130.
Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294(5544):1102–5. https://doi.org/10.1126/science.1063518.
Laplante M, Sabatini DM. MTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. https://doi.org/10.1016/j.cell.2012.03.017.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–84. https://doi.org/10.1016/j.cell.2006.01.016.
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39(2):171–83. https://doi.org/10.1016/j.molcel.2010.06.022.
Huffman TA, Mothe-Satney I, Lawrence JC Jr. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci USA. 2002;99(2):1047–52. https://doi.org/10.1073/pnas.022634399.
Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103. https://doi.org/10.1038/nrc3205.
Bendell JC, Patel MR, Moore KN, Chua CC, Arkenau HT, Dukart G et al. Phase I, First-in-human, dose-escalation study to evaluate the safety, tolerability, and pharmacokinetics of vorolanib in patients with advanced solid tumors. Oncologist. 2018. https://doi.org/10.1634/theoncologist.2018-0740. (Epub ahead of print).
Di Nunno V, Cubelli M, Massari F. The role of the MET/AXL pathway as a new target for multikinase inhibitors in renal cell carcinoma. Exp Rev Precis Med Drug Dev. 2017;2(3):169–75. https://doi.org/10.1080/23808993.2017.1347481.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23. https://doi.org/10.1056/NEJMoa1510016.
Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–7. https://doi.org/10.1200/JCO.2016.70.7398.
Tate DJ Jr, Vonderhaar DJ, Caldas YA, Metoyer T, Patterson JR 4th, Aviles DH, Zea AH. Effect of arginase II on l-arginine depletion and cell growth in murine cell lines of renal cell carcinoma. J Hematol Oncol. 2008;1:14. https://doi.org/10.1186/1756-8722-1-14.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. https://doi.org/10.1056/NEJMra1706169.
Baybutt TR, Flickinger JC Jr, Caparosa EM, Snook AE. Advances in chimeric antigen receptor (CAR)-T cell therapies for solid tumors. Clin Pharmacol Ther. 2018. https://doi.org/10.1002/cpt.1280. (Epub ahead of print).
Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96. https://doi.org/10.1111/imr.12519.
Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56. https://doi.org/10.1038/nri3790.
Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer. 2018;6(1):57. https://doi.org/10.1186/s40425-018-0360-8.
Knee DA, Hewes B, Brogdon JL. Rationale for anti-GITR cancer immunotherapy. Eur J Cancer. 2016;67:1–10. https://doi.org/10.1016/j.ejca.2016.06.028.
Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. https://doi.org/10.1111/imr.12520.
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targe ng Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. https://doi.org/10.1084/jem.20100643.
Piao YR, Piao LZ, Zhu LH, Jin ZH, Dong XZ. Prognostic value of T cell immunoglobulin mucin-3 in prostate cancer. Asian Pac J Cancer Prev. 2013;14:3897–901. https://doi.org/10.7314/APJCP.2013.14.6.3897.
Yuan J, Jiang B, Zhao H, Huang Q. Prognostic implication of TIM-3 in clear cell renal cell carcinoma. Neoplasma. 2014;61:35–40. https://doi.org/10.4149/neo_2014_006.
Zhou E, Huang Q, Wang J, Fang C, Yang L, Zhu M, et al. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol. 2015;8:8018–27.
Yang M, Yu Q, Liu J, Fu W, Cao Y, Yu L, et al. T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: clinicopathologic correlations and association with survival. J Surg Oncol. 2015;112:430–5. https://doi.org/10.1002/jso.24012.
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang L, et al. Tim-3 expression in cervical cancer promotes tumor metastasis. PLoS One. 2013;8:e53834. https://doi.org/10.1371/journal.pone.0053834.
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia Z, et al. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS One. 2013;8:e81799. https://doi.org/10.1371/journal.pone.0081799.
Cheong JE, Sun L. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy—challenges and opportunities. Trends Pharmacol Sci. 2018;39(3):307–25. https://doi.org/10.1016/j.tips.2017.11.007.
Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular pathways: targeting IDO1 and other tryptophan dioxygenases for cancer immunotherapy. Clin Cancer Res. 2015;21(24):5427–33. https://doi.org/10.1158/1078-0432.CCR-15-0420.
Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A, et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. https://doi.org/10.1016/j.ejca.2015.08.021.
Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. https://doi.org/10.1038/nature10358.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors declare that no source of funding was obtained for this research.
Conflict of interest
Mollica V, Di Nunno V, Gatto L, Santoni M, Cimadamore A, Cheng L, Lopez- Beltran A, Montironi R, Pisconti S, Battelli N and Massari F declare no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Rights and permissions
About this article
Cite this article
Mollica, V., Di Nunno, V., Gatto, L. et al. Novel Therapeutic Approaches and Targets Currently Under Evaluation for Renal Cell Carcinoma: Waiting for the Revolution. Clin Drug Investig 39, 503–519 (2019). https://doi.org/10.1007/s40261-019-00773-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00773-w