Abstract
Background and Objective
The anti-epileptic drug levetiracetam is excreted renally. The objective of this trial was to evaluate the pharmacokinetics of levetiracetam in Japanese patients with renal impairment including end-stage renal disease (ESRD) to confirm that existing dosing instructions—based on data from European patients—are appropriate in a Japanese population.
Methods
This was a nonrandomised, open-label trial. Six participants were allocated to each of five groups (normal renal function, mild, moderate and severe renal impairment and ESRD); 30 participants in total. Participants received a single dose of levetiracetam 500 mg (normal or mild), 250 mg (moderate or severe), or 500 mg followed by 250 mg post-haemodialysis (ESRD). Blood and urine samples were obtained serially for levetiracetam and metabolite determinations. Noncompartmental pharmacokinetic parameters were calculated and steady-state profiles were simulated using the superposition method.
Results
In this trial, levetiracetam total clearance decreased proportionally with creatinine clearance: 52, 31, 25, 20 and 11 mL/min/1.73 m2 in healthy controls and in patients with mild, moderate, severe renal impairment, and ESRD, respectively. Simulated levetiracetam plasma profiles using the recommended dose adjustments were within the range for normal renal function. Overall, results from this trial were consistent with historical European data.
Conclusion
These findings confirm that the dosing instructions are appropriate for Japanese patients with renal impairment including ESRD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since levetiracetam is excreted via the kidneys, exposure increases with decreasing renal function and dose adjustment is required for patients with renal impairment. | |
Global dosing instructions for levetiracetam have been issued based on data from non-Japanese patients with renal impairment or end-stage renal disease. | |
Data from the current trial conducted among Japanese patients with varying degrees of renal impairment were consistent with historical European data, indicating that dosing instructions in the Japanese package insert are appropriate for Japanese patients. |
1 Introduction
Levetiracetam [E Keppra®; (−)-(S)-a-ethyl-2-oxo-1-pyrrolidine acetamide] is a second-generation antiepileptic drug (AED). It has been available as adjunctive therapy in Japan since 2010 for adults and since 2013 for children with partial onset seizures.
The pharmacokinetics of levetiracetam have been studied in American, European, Chinese and Japanese subjects [1, 2]. The studies conducted to date demonstrate consistently that there are no ethnic differences in pharmacokinetics [2, 3]. Absorption of levetiracetam is rapid and complete (>95 %), with maximum plasma concentrations being achieved within 0.5–2.3 h after dosing. The pharmacokinetics are dose-proportional and time-independent and the drug has a low potential for clinically relevant drug-drug interactions [3–8]. Levetiracetam is excreted via the kidneys, with two-thirds excreted as unchanged drug and one-third excreted as a pharmacologically inactive metabolite (ucb L057) formed by esterase hydrolysis of the acetamide group [9].
Since levetiracetam is renally excreted, exposure increases with decreasing renal function. Dosing instructions for patients with various degrees of renal impairment have been developed based on data from European patients [10–12]. These instructions are applied internationally, including in Japan (Table 1). This study was conducted to evaluate the pharmacokinetics of levetiracetam in Japanese patients with renal impairment or end-stage renal disease (ESRD) to confirm that dosing instructions included in the Japanese package insert are appropriate in the Japanese population [13]. A further objective was to assess the safety and tolerability of levetiracetam in Japanese patients with various degrees of renal impairment.
2 Methods
This nonrandomised, stratified, open-label trial (NCT01491113) was conducted between November 2011 and November 2012 at two centres in Japan. Subjects with normal renal function and mild, moderate or severe renal impairment (Groups A–D, respectively) were enrolled in the Kurume Clinical Pharmacology Clinic, Kurume-city, Fukuoka, while those with ESRD (Group E) were enrolled in the Department of Urology of Moriya Keiyu Hospital, Moriya-city, Ibaraki. The ethical principles for medical research in the 1964 Declaration of Helsinki and its subsequent revisions were followed and an Independent Review Board approved the trial. All trial participants gave written informed consent.
2.1 Trial Population
Thirty Japanese subjects (male or female), aged 20–80 years, were recruited to the trial (six in each of five groups). Subjects were excluded if they had clinically significant electrocardiogram (ECG) or laboratory findings at entry (other than those expected in subjects with renal impairment), or if they had a history of chronic alcohol or drug abuse, or any medical or psychiatric condition that could affect their ability to participate in the study. Healthy subjects (Group A) were similar in age and bodyweight to subjects with renal impairment (Groups B–D). Subjects with ESRD who were receiving haemodialysis three times a week were enrolled in Group E.
2.2 Trial Protocol
Subjects were allocated to treatment according to their baseline creatinine clearance (Table 2). On Day 1, subjects with normal or mild renal impairment (Groups A and B) received a single 500 mg levetiracetam dose and those with moderate or severe renal impairment (Groups C and D) received a single 250 mg dose (Table 2). Blood and urine samples were taken up to Day 4 (Group A), Day 5 (Group B), Day 6 (Group C) or Day 7 (Group D), with an additional blood sample at the safety follow up visit on Day 9 ± 1 day.
Patients with ESRD (Group E) received two doses of levetiracetam: 500 mg on Day 1 and 250 mg on Day 3. The second dose was administered 49 h after the first dose and 1 h after a scheduled haemodialysis procedure. Blood and urine samples were taken up to Day 7, with an additional blood sample at the safety follow-up visit on Day 11 ± 1 day. The haemodialysis procedure for subjects with ESRD was standardised. All subjects underwent a 4-h haemodialysis session on Day 3 (44 h after the first dose of levetiracetam) and on Day 7 (140 h after the first dose of levetiracetam). The same equipment was used for all subjects, with a blood flow rate of between 180 and 220 mL/min, a constant dialysate flow of 500 mL/min (pH 7.2–7.4) and a high flux membrane, polysulfone (PSF), with a surface area of 1.2–1.8 m2.
Safety was evaluated by monitoring adverse events, vital signs, 12-lead ECG, physical examination and standard clinical laboratory tests.
2.3 Bioanalytical Determinations
Validated bioanalytical methods were used to measure concentrations of levetiracetam in plasma, urine and dialysate fluid. Given that levetiracetam has a major metabolite, ucb L057 (24 % of the dose), all analyses were also conducted for this metabolite. Solid phase extraction was used to extract analyte and internal standard from the biological matrix. After extraction, levetiracetam and ucb L057 were separated by reverse-phase liquid chromatography using gradient elution and detected by electrospray ionization tandem mass spectrometry using multiple reaction monitoring in positive-ion mode. This method was described previously [14] for levetiracetam in plasma and was readily extended to metabolite quantification and to determination in urine and dialysis fluids.
In plasma and dialysate, the lower limit of quantification (LLOQ) was 0.100 μg/mL and the calibration range was 0.1–100 μg/mL. The intra- and inter-run precisions were ≤6.8 % and ≤ 6.2 % for levetiracetam, and ≤8.9 % and ≤5.4 % for ucb L057, respectively; and the intra- and inter-run accuracies were ≤14.1 % and ≤10.0 % for levetiracetam, and ≤8.0 % and ≤2.7 % for ucb L057, respectively. In urine, LLOQ was 1 μg/mL and the calibration range was 1–1,000 μg/mL for levetiracetam and ucb L057. The intra- and inter-run precisions were ≤5.9 % and ≤8.0 % for levetiracetam, and ≤6.3 % and ≤6.7 % for L057, respectively and the intra- and inter-run accuracy were ≤4.0 % and ≤3.0 % for levetiracetam, and ≤6.0 % and ≤5.4 % for L057, respectively.
2.4 Pharmacokinetic and Statistical Calculations
The pharmacokinetic calculations were performed using Phoenix WinNonLin 6.2 software (Pharsight Corporation, Sunnyvale CA, USA). Pharmacokinetic parameters were derived by noncompartmental methods, using actual sampling times. Given the linearity of levetiracetam pharmacokinetics, individual steady-state predictions of plasma concentration versus time profiles were generated from actual single-dose data by the superposition method. Descriptive statistics—geometric mean and 95 % confidence intervals—were calculated for each of the groups based on the six predicted concentrations-versus-time profiles.
For Groups A to D, the following parameters were calculated for levetiracetam and ucb L057: maximum measured concentration (C max), time to maximum concentration (t max), area under the concentration time curve from time 0 to infinity (AUC0–∞), area under the concentration time curve from time 0 to time t with last quantifiable concentration (AUC0–t), elimination half-life (t ½), renal clearance (CLR) and fraction of dose excreted in urine (fe). In addition, the following parameters were calculated for levetiracetam: apparent volume of distribution (V d/F), apparent total body clearance (CL/F) and non-renal clearance (CLNR). For Group E, the following parameters were calculated for levetiracetam and ucb L057: C max, t max, AUC0–44 h and AUC49–92 h. Additionally, t ½, V d/F and CL/F were calculated for levetiracetam. Calculation of the AUC parameters was performed using the linear trapezoidal method. The haemodialysis pharmacokinetic parameters calculated for levetiracetam and ucb L057 using the arterio-venous (A–V) difference method [15, 16] corrected by the physiological plasma flow were: t ½, fractional removal, reduction, extraction efficiency (E) of haemodialysis, dialysis clearance (CLD), ultrafiltration clearance (CLUF), and haemodialysis clearance (CLHD).
Statistical analysis was performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA). Mean, standard deviation (SD), geometric mean and geometric coefficient of variation (CV) were calculated, if at least two-thirds of the individual data at a specific sampling point were measured and were above or equal to the LLOQ. Plasma concentrations below the limit of quantification were substituted by LLOQ/2.
Linear/nonlinear regression of pharmacokinetic parameters on creatinine clearance was calculated for CLR, CLNR, CL/F and C max of levetiracetam and CLR and C max of the metabolite ucb L057 using SAS procedure regressions.
3 Results
3.1 Study Population
Thirty subjects with a mean age of 64.6 years (range 22–79 years) participated in the trial. Most (29/30) were male, with a mean bodyweight of 63.8 kg (range 47.9–85.0 kg) and mean height of 163.6 cm (range 148.5–175.6 cm). Baseline demographics were similar between the 5 groups. At study entry, creatinine clearance (median [range] mL/min/1.73 m2) was 118 (100.2–132.5) in Group A (normal renal function), 62 (58.7–82.8) in Group B (mild impairment), 49 (36.1–54.7) in Group C (moderate impairment) and 21 (12.7–26.0) in Group D (severe impairment) (Table 3). All subjects were included in the plasma pharmacokinetic analysis, but two in Group A were excluded from the urinary analysis due to incomplete sampling.
3.2 Pharmacokinetics in Renal Impairment
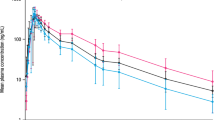
Levetiracetam plasma concentration versus time plots for Groups B–D were distributed within the range of plasma concentrations in group A (Fig. 1).
Levetiracetam plasma concentration versus time profiles after administration of single 250 mg (dashed line) or 500 mg (solid line) dose (geometric mean ± 95 % confidence interval). Normal renal function (A filled circle) and mild (B filled square), moderate (C filled triangle) and severe renal impairment (D filled diamond)
Absorption of levetiracetam was unaffected by renal impairment, as illustrated by a t max between 0.5 and 1 h in all groups (Table 3). The maximum plasma concentration (C max) of levetiracetam was approximately 50 % lower in subjects with moderate or severe renal impairment (given a 250 mg single dose) than in those with normal renal function or mild renal impairment (given a 500 mg dose). AUC was similar across all four groups, with the slope of the elimination phase becoming shallower as renal function worsened. Renal clearance of levetiracetam decreased with decreasing renal function (creatinine clearance), the relationship between CLR and creatinine clearance being linear (Fig. 2). Apparent total body clearance also decreased with decreasing renal function, from 51.5 mL/min/1.73 m2 in normal renal function to 20.3 mL/min/1.73 m2 in severe renal impairment and 10.9 mL/min/1.73 m2 ESRD; the latter value representing the non-renal clearance. Overall, the non-renal clearance was generally consistent among subjects with various degrees of renal impairment (Table 2).
Relationship between renal clearance (CLR) and total clearance (CL/F) of levetiracetam and creatinine clearance. Linear regression line (solid) for CL/F, Linear regression (dashed) line for CLR. Normal renal function (A filled circle/open circle), mild (B filled square/open square), moderate (C filled triangle/open triangle) and severe renal impairment (D filled diamond/open diamond) and end-stage renal disease (E filled inverted triangle)
For the metabolite ucb L057, both C max and AUC were higher in subjects with renal impairment compared with subjects with normal renal function (Table 2); however, with the compensation of the lower dose in subjects with moderate or severe renal impairment, the size of the difference was less than three-fold for C max. The AUC increased 10-fold in subjects with severe renal impairment compared with those with normal renal function.
The proportions of the levetiracetam dose excreted as parent drug and metabolite also changed with decreasing renal function. In subjects with normal renal function, approximately 60 % of the levetiracetam dose was excreted as parent compound; this decreased to 30 % in those with severe renal impairment (Group D). Conversely, approximately 17 % was excreted as metabolite in subjects with normal renal function, but this increased to 40 % in those with severe renal impairment. Overall, the urinary excretion of levetiracetam and metabolite taken together was similar for all groups (between 70 and 83 % in each group).
The steady-state prediction of concentrations versus time profiles for the subjects with normal renal function compared to those with renal impairment, based on single-dose data, shows the expected plasma concentrations at the minimum and maximum doses stated in the Japanese package insert (Fig. 3). The geometric mean plasma concentrations at steady state in subjects with normal renal function can range from 7 μg/mL (C min of lowest dose, 500 mg twice daily) to 75 μg/mL (C max of highest dose 1,500 mg twice daily) as shown by the shaded area, materialized on each predicted plasma concentration profile. The geometric mean plasma concentrations in subjects with various degrees of renal impairment, when following the dosing instructions contained in the package insert, fall within these extremes.
Simulated levetiracetam plasma concentration versus time profiles at steady state in subjects with various degrees of renal function (geometric mean and 95 % confidence intervals at recommended high and low doses in package insert). Comparison between subjects with normal renal function and those with impaired renal function. Solid lines and dotted lines show geometric mean and ±95 % confidential interval of simulated levetiracetam plasma concentrations at steady state at recommended high and low doses. Shaded areas in each figure show the range of simulated geometric mean levetiracetam plasma concentrations at recommended high and low doses in subject with normal renal function
3.3 Pharmacokinetics in End-Stage Renal Disease
The plasma concentration versus time profiles in ESRD (Fig. 4) show a rapid increase in levetiracetam plasma concentration after the first dose (500 mg), followed by a slow decrease in the period before haemodialysis (due to metabolism), a fast decrease during haemodialysis (44–48 h; due to elimination), and then an increase following the second dose at 49 h (250 mg). Outside the haemodialysis period, the geometric mean plasma concentration of levetiracetam ranged between 18 and 6 μg/mL; these plasma concentrations were similar to the expected concentrations in subjects with normal renal function receiving the minimum dose stated in the Japanese package insert.
In ESRD, t max for levetiracetam occurred approximately 0.7 h after dosing, similar to the t max seen in subjects with normal renal function (Table 3). Levetiracetam C max was 19 μg/mL after the 500 mg dose and 12 μg/mL after the 250 mg dose, both values being similar to C max in subjects with normal renal function (22 μg/mL). The half-life of levetiracetam (outside of haemodialysis) was approximately five times longer in ESRD than in subjects with normal renal function (approximately 40 and 8 h, respectively). The metabolite concentration increased continuously after dosing until the time of haemodialysis, with a C max of 8.8 μg/mL after the 500 mg dose and 9.1 μg/mL after the 250 mg dose.
Levetiracetam and ucb L057 plasma concentrations decreased rapidly during haemodialysis in ESRD (Table 4); t ½ was around 2 h for both compounds. The extraction efficiencies (E) of haemodialysis were around 0.8 and 0.9 for levetiracetam and ucb L057, respectively. The ultrafiltration clearance (CLUF) of both levetiracetam and ucb L057 was minimal (approximately 1 mL/min), with dialysis clearance (CLD) accounting for 99 % of the elimination. The haemodialysis clearance (CLHD), which is the sum of CLUF and CLD, was around 120 mL/min for both levetiracetam and ucb L057. At the end of the 4-h haemodialysis period, plasma concentrations were reduced by approximately 70 %, with low intersubject variability (less than 4 %).
The steady-state prediction generated from the single-dose data in ESRD in this study showed that the geometric mean plasma concentration range for patients with ESRD will be within the range for individuals with normal renal function (Fig. 5) when following the dosing instructions for ESRD contained in the package insert.
Simulated levetiracetam plasma concentration versus time profiles. Comparison between end stage renal disease and normal renal function at steady state over the recommended dose range. Solid lines and dotted lines show geometric mean and ±95 % confidential interval of simulated levetiracetam plasma concentrations at steady state at recommended high and low doses. Shaded areas in each figure show the range of simulated geometric mean levetiracetam plasma concentrations at recommended high and low doses in subjects with normal renal function
3.4 Safety Results
All 30 participants who received levetiracetam were included in the safety analysis. Five subjects reported 14 treatment-emergent adverse events (TEAEs); each TEAE occurred only once and most were observed in Group E (10 TEAEs in 2 subjects). No subjects in Groups A and C reported TEAEs. Non-serious TEAEs (white blood cell count increased and dizziness), reported by one subject in Group E, were considered to be related to the study drug by the investigator. Three TEAEs (pneumonia, acute respiratory distress syndrome and shunt malfunction) were considered to be serious, but were not related to levetiracetam. No clinically relevant changes in laboratory analyses, vital sign measurements, ECG, or physical examination findings were observed during this trial.
4 Discussion
The impact of renal impairment on the pharmacokinetic profile of renally-excreted drugs, such as levetiracetam, has to be evaluated in order to prescribe appropriate dosing regimens for patients with renal impairment [17–19]. This becomes increasingly important in older patients, not only because renal function deteriorates with age, but also because the incidence of new onset epilepsy is higher among older adults [20]. The impact of haemodialysis on elimination has to be evaluated as well, as this will affect the pharmacokinetic profile of levetiracetam in patients with ESRD [15].
The pharmacokinetic profile of levetiracetam in renal impairment and ESRD was previously established in non-Japanese patients and global dosing instructions were issued based on these data. Following levetiracetam registration in Japan in 2010, the Pharmaceuticals and Medical Devices Agency (PMDA) requested confirmation that the pharmacokinetic characteristics of levetiracetam were similar in Japanese patients and that the existing dosing instructions for patients with renal impairment were also relevant in Japan. While no clinically relevant differences in the Japanese population due to ethnicity were anticipated, to ensure appropriate pharmacokinetics and safety of Japanese patients, the current trial with Japanese patients with renal impairment, and healthy Japanese volunteers as controls was conducted.
For levetiracetam, steady state is reached after approximately 48 h using twice-daily administration when renal function is normal, but it is delayed with severe renal impairment (up to 5 days) or ESRD (up to 9 days). Taking into consideration the length of time taken to reach steady state in patients with renal impairment, a single-dose study design was chosen, with simulations to predict steady-state pharmacokinetics after multiple doses. This approach was deemed appropriate for a drug with linear pharmacokinetics, where simulations are known to give accurate predictions of steady-state pharmacokinetics.
Results from this trial were in agreement with those of the previous one that included European patients with renal impairment. As predicted, appropriate plasma concentrations of levetiracetam were achieved in Japanese patients following the recommended single dose. The dose reduction for subjects with moderate or severe renal impairment (from 500 to 250 mg) was appropriate to maintain similar plasma concentrations to those achieved in subjects with normal renal function with a 500 mg dose. Steady-state simulations showed that the dosing instructions in the Japanese package insert would produce similar exposure and plasma concentrations of levetiracetam over the whole range of renal impairment, including ESRD. As in previous studies, there was a linear relationship between creatinine clearance and renal clearance of both levetiracetam and its metabolite.
For patients with ESRD, there were some differences in the pharmacokinetic parameters observed in this trial and those in the previous clinical study of five European patients with ESRD [10, 11]. The main difference was in the dialysis clearance of levetiracetam and its metabolite during the 4-h dialysis period. In Japanese patients, levetiracetam clearance was 114 mL/min, whereas it was lower in European patients at 88 mL/min. The increase in clearance during dialysis in this study led to a greater percentage of levetiracetam removal during the 4-h dialysis session, with 69 % being removed compared with only 51 % in the European study. One possible explanation for this may be that the European study was conducted 14 years ago, and dialysis membranes now are more efficient, removing a greater percentage of levetiracetam during the 4-h dialysis period. The ultrafiltration clearances in both studies were in the same range (1.3 mL/min in the Japanese study and 3.6 mL/min in the European study) and accounted only for a small amount of the overall haemodialysis clearance.
Metabolite accumulation was observed in ESRD, with C max of approximately 9 µg/mL, 25 times higher than C max in normal renal function (0.36 µg/mL); however, the metabolite was efficiently eliminated during haemodialysis with a 74 % reduction in plasma concentration. Since the metabolite is not pharmacologically active, no safety concerns are anticipated with the levels of metabolite observed in patients with ESRD.
Levetiracetam was well tolerated in Japanese subjects with normal renal function, as well as in those with varying degrees of renal impairment. The majority of TEAEs (10/14) were reported by two patients with ESRD and only two of the TEAEs (white blood cell count increased and dizziness), reported by one patient, were considered to be related to the study drug.
The majority of participants in this study were male; however, levetiracetam pharmacokinetics show no significant gender differences. Indeed, gender was not found to be a significant covariate in a recent integrative population pharmacokinetic model for Japanese subjects [2]. Results of the model indicated that the slight difference observed in the concentrations could be explained mainly by differences in body weight between males and females. Therefore, the conclusions from this study are fully applicable to female Japanese patients.
5 Conclusion
Dose adjustments are required for levetiracetam in patients with renal impairment. The dosing instructions provided in the Japanese package insert are appropriate for Japanese patients with renal impairment, including ESRD, based on their creatinine clearance values, and no region-specific dose recommendations are required for Japan.
References
Stockis A, Lu S, Tonner F, Otoul C. Clinical pharmacology of levetiracetam for the treatment of epilepsy. Expert Rev Clin Pharmacol. 2009;2(4):339–50.
Toublanc N, Lacroix BD, Yamamoto J. Development of an integrated population pharmacokinetic model for oral levetiracetam in populations of various ages and ethnicities. Drug Metab Pharmacokinet. 2014;29(1):61–8.
Pigeolet E, Jacqmin P, Sargentini-Maier ML, Stockis A. Population pharmacokinetics of levetiracetam in Japanese and Western adults. Clin Pharmacokinet. 2007;46(6):503–12.
Patsalos PN. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther. 2000;85(2):77–85.
Perucca E, Gidal BE, Baltès E. Effects of antiepileptic comedication on levetiracetam pharmacokinetics: a pooled analysis of data from randomized adjunctive therapy trials. Epilepsy Res. 2003;53(1–2):47–56.
Gidal BE, Baltès E, Otoul C, Perucca E. Effect of levetiracetam on the pharmacokinetics of adjunctive antiepileptic drugs: a pooled analysis of data from randomized clinical trials. Epilepsy Res. 2005;64(1–2):1–11.
Ramael S, De Smedt F, Toublanc N, Otoul C, Boulanger P, Riethuisen JM, Stockis A. Single-dose bioavailability of levetiracetam intravenous infusion relative to oral tablets and multiple-dose pharmacokinetics and tolerability of levetiracetam intravenous infusion compared with placebo in healthy subjects. Clin Ther. 2006;28(5):734–44.
Toublanc N, Sargentini-Maier ML, Lacroix B, Jacqmin P, Stockis A. Retrospective population pharmacokinetic analysis of levetiracetam in children and adolescents with epilepsy: dosing recommendations. Clin Pharmacokinet. 2008;47(5):333–41.
Strolin-Benedetti M, Whomsley R, Nicolas JM, Young C, Baltès E. Pharmacokinetics and metabolism of 14C-levetiracetam, a new antiepileptic agent, in healthy volunteers. Eur J Clin Pharmacol. 2003;59(8–9):621–30.
Baltès E, Coupez R. Levetiracetam dose adjustment for patients on hemodialysis [abstract]. Epilepsia. 2000;41:254.
French J. Use of levetiracetam in special populations. Epilepsia. 2001;42(Suppl 4):40–3.
Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43(11):707–24.
E-Keppra, Japanese package insert. Available at http://www.info.pmda.go.jp/downfiles/ph/PDF/820110_1139010F1024_1_10.pdf. Accessed 19 Sept 2014.
Rouits E, Burton I, Guénolé E, Troenaru MM, Stockis A, Sargentini-Maier ML. Pharmacokinetics of levetiracetam XR 500 mg tablets. Epilepsy Res. 2009;84(2–3):224–31.
Lee CS, Marbury TC. Drug therapy in patients undergoing haemodialysis, clinical pharmacokinetic considerations. Clin Pharmacokinet. 1984;9:42–66.
Massry SG, Glassock RJ. Dialysis therapy. In: Massry and Glassock’s Textbook of Nephrology. vol. 84, 4th edn. Lippincott Williams & Wilkins; 2000, p. 1474–90.
Lalonde RL, Wagner JA. Drug development perspective on pharmacokinetic studies of new drugs in patients with renal impairment. Clin Pharmacol Ther. 2009;86(5):557–61.
Olyaei AJ, Steffl JL. A quantitative approach to drug dosing in chronic kidney disease. Blood Purif. 2011;31(1–3):138–45.
Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65(8):757–73.
Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol. 2009;8(11):1019–30.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17(6–2):863–71.
Acknowledgments
UCB Pharma sponsored this study and was involved in the design and conduct of the study, and collection, management, and analysis of the data. Nathalie Toublanc, Armel Stockis and Junichi Yamamoto are employees of UCB Pharma. Yuji Kumagai was the medical advisor and is a Professor of the Kitasato Clinical Research Center, Kitasato University, Japan. The authors thank Katsumi Yoshida, RPh for statistical assistance, Ludovicus Staelens (both UCB Pharma) who was responsible for the bioanalysis, and the principal investigators Teruo Fukumoto, MD, Tadayuki Hiroki, MD (both from Kurume Clinical Pharmacology Clinic), and Tokuro Kobayashi, MD (Moriya Keiyu Hospital). Fiona Swain of Mediwrite Ltd, United Kingdom provided medical writing support funded by UCB Pharma, and Azita Tofighy, UCB Pharma, provided editorial support and coordinated the manuscript development process.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yamamoto, J., Toublanc, N., Kumagai, Y. et al. Levetiracetam Pharmacokinetics in Japanese Subjects with Renal Impairment. Clin Drug Investig 34, 819–828 (2014). https://doi.org/10.1007/s40261-014-0237-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0237-7