Abstract
Objectives
The objective of the study was to determine the relative bioavailability of an extended-release multilayer bead formulation of methylphenidate hydrochloride (MPH-MLR) 80 mg vs. methylphenidate immediate-release (IR; Ritalin®) tablets as single and multiple doses in the fed state.
Methods
A single-center, multiple-dose, randomized, open-label, two-period crossover study conducted in 26 healthy adults assigned to 4 days of once-daily MPH-MLR 80 mg or IR methylphenidate 25 mg three times daily.
Results
MPH-MLR 80 mg produced reproducible biphasic profiles of plasma methylphenidate concentrations characterized by a rapid initial peak, followed by a moderate decline reaching a plateau ~5 h post dose, then a gradual increase culminating in an attenuated second peak ~7 h post dose. Maximum concentration was lower for MPH-MLR 80 mg than IR methylphenidate 25 mg three times daily on day 1 (23.70 vs. 31.47 ng/mL); exposure was similar. The geometric mean ratios (MPH-MLR/IR methylphenidate [90 % CI]) of log-transformed area under the plasma drug concentration-time curve to the last measurable observation (day 1: 0.88 [84.75–91.80]; day 4: 0.84 [81.16–86.94]), and area under the plasma drug concentration extrapolated to infinity (day 1: 0.93 [88.57–97.28]; day 4: 0.88 [84.48–91.17]) were within the 80–125 % bioequivalence range. The mean ± SD MPH-MLR 80-mg capsule day 4 area under the plasma drug concentration vs. time curve from 0 to 4 h (74.5 ± 15.2 ng·h/mL) was greater than IR methylphenidate 25 mg three times daily (66.0 ± 17.4 ng·h/mL), confirming steady-state levels during the study period. All treatment regimens were safe and well tolerated.
Conclusion
MPH-MLR 80-mg capsule once daily or IR methylphenidate 25 mg three times daily provides comparable maximum methylphenidate concentrations and systemic exposure in the fed state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common neurobehavioral disorder with a parent-reported prevalence of 3.5–9.5 % in children [1–4] and a prevalence of 2.5–4.4 % in adults [5–7]. In both age groups, the central nervous system stimulant methylphenidate treats the core ADHD symptoms of inattention, distractibility, hyperactivity and impulsivity, and difficulty in psychosocial functioning [8–10]. Over the previous 14 years, the development of methylphenidate extended-release (ER) preparations has enabled convenient once-a-day dosing, potentially minimizing tolerance and abuse, and prolonging the duration of therapeutic effect beyond that of methylphenidate immediate-release (IR) preparations [11].
Current ER formulations variably modulate methylphenidate absorption kinetics by providing a rapid pulse of methylphenidate release soon after administration followed by a protracted period of drug delivery throughout the day [12, 13]. The proportion of the total methylphenidate dose intended for IR varies widely [i.e., 20 % for Quillivant XR® (Pfizer Inc., New York, NY, USA), 22 % for Concerta® (Janssen Pharmaceuticals, Inc., Titusville, NJ, USA), 30 % for Metadate CD® (UCB, Inc., Smyrna, GA, USA), 50 % for Ritalin LA® (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA), and 50% (dexmethylphenidate) for Focalin XR® (Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA)], as does the proportion intended for ER preparations [12, 14–16]. While each of these formulations was designed to replace or mimic the pharmacokinetic profile of IR methylphenidate administered either two or three times daily at a comparable dose level, none is bioequivalent to the IR formulation or to each other based on reproducible peak plasma drug concentration-time course profiles and aberrant peak plasma drug concentration (C max) [12–14, 17, 18]. These between-formulation differences in methylphenidate pharmacokinetics offer clinicians options for individualizing therapy by prescribing an ER formulation with a plasma methylphenidate pharmacokinetic profile best suited to their patients’ needs in terms of duration of action and timing of effect [13, 14].
The initial drug absorption phase has been associated with behavioral improvements in children with ADHD receiving IR methylphenidate and optimization of this phase has minimized tolerance [19–21]. The US Food and Drug Administration (FDA) mandates partial area under the plasma concentration-time curve (pAUC) to be a primary metric in bioequivalence studies of methylphenidate ER products [21] as a reflection of the importance of this absorption phase, especially when compared with IR forms of the drug. To account for the effect of prandial status on absorption kinetics, the methylphenidate ER pAUC bioequivalence metrics that are most appropriate in the fasting and fed states are AUC calculated from 0 to 3 h (AUC0–3) and from 0 to 4 h (AUC0–4), respectively [21].
A novel ER multilayer bead capsule formulation of methylphenidate hydrochloride (MPH-MLR; Aptensio XR™Footnote 1) formulation currently approved in Canada for the treatment of ADHD in children, adolescents, and adults [22]. Each MPH-MLR 80-mg hard gelatin capsule contains controlled-release beads that facilitate a rapid initial delivery of ~37 % of the total methylphenidate dose with an onset of action similar to IR methylphenidate formulations [23, 24]. The remaining 63 % of methylphenidate, following a morning administration of MPH-MLR, was designed to sustain a plateau in plasma drug concentration ~5 h post dose, followed by a second more moderate ascent in plasma drug concentration in the afternoon. Once-daily MPH-MLR was reported to be associated with improvements in behavior and cognition as evaluated using a variety of measures (e.g., Clinical Global Impressions scale, Inattention/Overactivity With Aggression Conners scale, Child’s Behavior in Problem Situations scale, Communicative Pragmatics scale, and Conners’ Adult ADHD rating scale) when given to children [23, 25, 26] and adults [27] with ADHD across home, simulated school, and work settings.
The objectives of the present study were to assess the relative bioavailability of the MPH-MLR 80-mg capsule and IR methylphenidate tablets (75 mg administered as three equally divided doses) as a single dose and at steady state under fed conditions in healthy adults.
2 Methods
2.1 Study Design
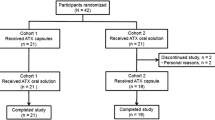
This was a single-center, multiple-dose, randomized, open-label, two-period crossover study (Fig. 1) approved by the IntegReview Ethics Review Board (protocol number, RP-BP-PK002; approved May 13, 2011). Each subject was required to provide written informed consent before enrollment. The study was conducted by Frontage Clinical Services at Frontage Laboratories, Inc., Hackensack, NJ, USA, and undertaken in compliance with the Good Clinical Practice guidelines of the International Conference on Harmonization and the principles of the Declaration of Helsinki.
The test product was MPH-MLR. The comparator product Ritalin® (IR methylphenidate 20-mg and 5-mg tablets) is commercially available from Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. The 5-mg tablets used in this study were from lot number F0103, expiration date March 2014. The 20-mg tablets used in this study were from lot number F0125, expiration date December 2013.
Subjects were screened ≤3 weeks of initial dosing and returned to the research facility the night before the first period of study drug administration (day −1). In the morning after an overnight fast (~08:00 hours on day 1), subjects consumed a high-fat breakfast (~1,000 calories, ~50 % fat). The high-fat breakfast consisted of two eggs, bacon, toast, hash browns, and whole milk. The standard breakfast consisted of toast, jam, cereal with 2 % milk, and orange juice. Approximately 5 min later, they received either a single MPH-MLR 80-mg capsule or IR methylphenidate (20 and 5 mg) tablets, both of which were taken with 240 mL water. Subjects randomized to the IR methylphenidate arm received two further assigned doses 4 and 8 h after treatment initiation and ~5 min after completing a meal or snack. The same treatment regimens were administered on days 2, 3, and 4, except that subjects received a standard rather than a high-fat breakfast. The standard breakfast subjects remained sitting upright and ambulation was limited during the immediate 1-h post-dose period. On the days of dosing, standard meals were consumed at ~4 and 10 h after study drug administration. An evening snack was offered at ~9 p.m. on the evenings of admission and the days of dosing.
Serial blood samples for the determination of methylphenidate plasma concentration and pharmacokinetic analysis were obtained on: day 1 and day 4 at time 0 (≤15 min pre dose) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 4.5, 5, 5.5, 6, 6.5, 7, 8, 8.5, 9, 9.5, 10, 10.5, 12, 15, 19, and 24 h post dose; and on day 2 and day 3 at 4, 8, 12, 16, and 24 h post dose. Subjects were discharged from the research facility on day 5, ~24 h after receiving their day 4 dose of study drug. Vital signs were measured ≤60 min of dosing and before study discharge.
To enable an 8-day washout period, subjects returned to the research facility on day 11 at ~8 p.m. On the morning of day 12 after an overnight fast, they were crossed over to the alternate treatment and the same procedures were performed as before. Blood and urine were collected for clinical laboratory tests (chemistry, hematology, and urinalysis) ~24 h after the administration of the last doses of study drug during this second treatment period, an abbreviated physical examination was performed, vital signs were collected, and subjects were discharged from the study on day 16.
2.2 Subjects
The study enrolled 26 methylphenidate treatment-naïve male and female subjects aged 18–45 years inclusive who were ≤15 % of ideal weight based on height and body frame (based on the Metropolitan Life Insurance Company height and weight tables [28]). Subjects were in good health as evidenced by results of physical examinations, vital signs evaluation, routine clinical laboratory tests, and 12-lead electrocardiograms (ECGs) performed ≤21 days of study drug administration. Female subjects of child-bearing potential were required to use appropriate contraceptive measures throughout the duration of the study and have a negative urine pregnancy test result at screening and before all dosing periods. In addition, all subjects must have had a negative drug/alcohol test result at screening and at each admission to the research facility. Exclusion criteria included: (1) a true allergy to methylphenidate; (2) infection with hepatitis B, hepatitis C, or human immunodeficiency virus; (3) administration of any prescription drug therapy ≤14 days or any over-the-counter drugs or supplements ≤48 h of receiving study drug; (4) current smoker or use of any tobacco-containing products; (5) consumption of grapefruit or grapefruit-containing juices ≤72 h; (6) and consumption of caffeine-containing foods or beverages ≤24 h of receiving study drug.
2.3 Assays
Blood samples (6 mL) were collected from indwelling catheters into chilled blood collection tubes containing ethylenediaminetetraacetic acid dipotassium, immediately chilled on crushed ice and centrifuged for 10 min in a refrigerated centrifuge (4–8 °C) at 2,000 × g ≤ 30 min after collection. Duplicate plasma samples (~1.5 mL per tube) were transferred into two polypropylene tubes. One plasma sample was the primary assay sample and the second served as the backup sample. Plasma samples were stored at −70 °C or lower until ready for analysis by Frontage Laboratories, Inc. Absence of a reaction between collection/storage vessels and methylphenidate was determined as part of the bioanalytical method validation. Harvested plasma samples were extracted into ethylenediaminetetraacetic acid dipotassium and analyzed for total plasma MPH concentration determination using a fully validated liquid chromatography tandem mass spectrometry analysis method using methylphenidate-d3 hydrochloride as the internal standard [23]. Calibrations were performed similarly to the Quinn et al. study [23], with a quadratic regression (weighted 1/x) on the calibration standards for curve determination. Curve parameters for the assay method were stable throughout the runs, specifically, coefficient of determination met acceptance criteria (R 2 ≥ 0.99), lower limit of quantitation was 50 pg/mL, and >2/3 of the analyzed incurred sample reanalysis samples had no more than ±20 % difference when compared with the original analysis results.
2.4 Pharmacokinetic Analysis
Individual plasma concentration-time data were used to calculate methylphenidate pharmacokinetic parameters using standard noncompartmental methods (WinNonlin version 5.3®, Pharsight Corporation, Mountain View, CA, USA). The primary pharmacokinetic endpoints were C max and AUC calculated to 4 h (AUC0–4), to the last measurable observation (AUC0–t ), and extrapolated to infinity (AUC0–∞). AUC values were calculated using the linear trapezoidal rule. The terminal phase rate constant (k el) was calculated as the negative of the slope of the log-linear terminal portion of the plasma concentration-time curve using linear regression. Secondary methylphenidate pharmacokinetic variables were the respective times to C max (t max) and the elimination half-life (t ½). The t ½ of the terminal elimination phase was estimated by use of the following ratio: 0.693/k el. The fluctuation index was estimated from the average plasma concentration (C avg) and trough concentration (C trough) for each formulation using the calculation: (C max − C trough)/C avg.
2.5 Safety Assessments
Safety was evaluated by performing vital sign measurements, 12-lead ECGs, clinical laboratory testing (hematology, chemistry, and urinalysis), and physical examinations. The type, incidence, severity, and relationship of adverse events (AEs) to study drugs were assessed throughout the study by nursing and medical observations of the staff. A treatment-emergent AE (TEAE) was defined as an AE that followed exposure to study treatment. The study investigator assessed AEs for severity and relationship to study drug. AEs were coded and summarized using the Medical Dictionary for Regulatory Activities, version 13.1 (MeDRA MSSO, McLean, VA, USA).
2.6 Analysis Populations
All enrolled subjects who received one or more doses of study drug were included in the safety analysis set. The subset of subjects from the safety analysis set who completed both treatment periods without any major protocol violations and provided plasma methylphenidate concentration data were included in the pharmacokinetic analysis set.
2.7 Statistical Analysis
All pharmacokinetic and safety data analyses were performed using descriptive statistics compiled by SAS® version 9.2 (SAS® Institute Inc., Cary, NC, USA). All plasma concentrations below the lower limit of quantitation were treated as missing in the pharmacokinetic analyses, except those that occurred before the first quantifiable concentration on the day of dosing or after the last quantifiable concentration, which were set to zero. At each time point, summary statistics (mean, standard deviation, minimum, maximum, and n) were calculated for methylphenidate concentrations in plasma. Ninety percent confidence intervals (CIs) were constructed around the geometric mean ratio of the MPH-MLR 80-mg capsule to IR methylphenidate 25 mg administered three times daily for the primary pharmacokinetic parameters and C max, AUC0–4, AUC0–t , and AUC0–∞. Relative bioavailability was based on log-transformation of these parameters, which were analyzed using an analysis of variance model. The model included terms for sequence, study treatment, and period as fixed effects and subject nested within sequence as a random effect. Sequence was tested using subject nested within sequence as the error term. Bioequivalence was concluded if the 90 % CIs for the ratio of the geometric means for the MPH-MLR 80-mg capsule vs. IR methylphenidate 75 mg were within the 80–125 % range for all primary pharmacokinetic parameters.
3 Results
3.1 Subject Disposition and Baseline Data
All of the 26 subjects enrolled were evaluable for safety analyses. Five subjects did not complete the study. Four subjects requested to be withdrawn prematurely from the study for reasons other than AEs, while one subject receiving IR methylphenidate had an AE of high blood pressure. Thus, 21 subjects completed the study and were included in the pharmacokinetic analysis set.
The safety analysis set comprised 21 men and 5 women, with a mean age of 32 years and a mean body mass index of 24.7 kg/m2 (Table 1). The majority (16 of 26) of subjects enrolled in the study were white.
3.2 Pharmacokinetic Assessments
Based on visual inspection, IR methylphenidate 25 mg administered at time 0, 4, and 8 h resulted in a triphasic profile following 1 day of dosing and after 4 days of dosing at steady state (Fig. 2a, b). On days 1 and 4 (when serial blood sampling was most frequent), the C max values associated with each successive IR methylphenidate 25-mg dose became progressively higher and fell away sharply ~8 h post dose. The plasma concentration-time profile of the once-daily MPH-MLR 80-mg capsule was different from that of IR methylphenidate 25 mg administered three times daily on days 1 and 4 (Fig. 2a, b). Administration of the MPH-MLR 80-mg capsule resulted in more sustained concentrations of methylphenidate over an ~8-h period and the biphasic profile had fewer fluctuations in plasma drug concentrations (Fig. 2c). Eight hours after the administration of the MPH-MLR 80-mg capsule on days 1 and 4, there was a gradual decline in plasma methylphenidate concentrations.
Mean plasma methylphenidate concentration-time profiles following single-dose administration of the MPH-MLR 80-mg capsule and IR MPH 25 mg administered three times daily after a high-fat breakfast (day 1; a), after a standard breakfast (day 4; b), and after multiple daily doses (days 1–4; c). IR MPH immediate-release methylphenidate, MPH-MLR extended-release multilayer bead formulation of methylphenidate
On day 1 after a high-fat breakfast and day 4 after a standard breakfast, pharmacokinetic analysis revealed that that total systemic exposure (i.e., AUC0–t and AUC0–∞) to methylphenidate was similar following the administration of either the MPH-MLR 80-mg capsule or IR methylphenidate 25 mg three times daily (Table 2). However, there were differences between acute exposure of the MPH-MLR and IR methylphenidate formulations regarding C max on days 1 and 4 and regarding AUC0–4 on day 4. On day 1, the C max values associated with each successive dose of IR methylphenidate 25 mg (C max1, C max2, C max3) were progressively higher (C max1, 21.9 ng/mL at 2 h; C max2, 26.7 ng/mL at 6 h; C max3, 28.8 ng/mL at 10 h) such that the average of the three IR methylphenidate C max values was higher than that associated with a single dose of the MPH-MLR 80-mg capsule (31.5 vs. 23.7 ng/mL). Comparability between C max and t max of the first dose of IR methylphenidate 25 mg (C max1 and t max1) and C max and t max of the MPH-MLR 80-mg capsule translated into similar AUC0–4 values for each treatment (55.9 vs. 55.5 ng·h/mL). On day 4 at steady state, the average C max associated with IR methylphenidate 25 mg administered three times daily remained higher (32.9 ng/mL) than the C max for the MPH-MLR 80-mg capsule (28.1 ng/mL at 2 h). The estimated fluctuation index suggested that the peak to trough variability of steady-state plasma concentrations after MPH-MLR administration was less than with IR methylphenidate resulting in sustainable plasma concentrations with MPH-MLR. Again, there was a trend towards increasing peak plasma methylphenidate concentrations after each successive 25-mg dose of IR methylphenidate (C max1, 25.6 ng/mL at 2 h; C max2, 29.6 ng/mL at 6 h; C max3, 30.1 ng/mL at 10 h). C max in the first 4 h after ingestion for the MPH-MLR 80-mg capsule was higher than the C max for the first dose of IR methylphenidate 25 mg and the similarity in corresponding t max values meant that the MPH-MLR 80-mg capsule was associated with a greater AUC0–4 than IR methylphenidate 25 mg (74.5 vs 66.0 ng·h/mL) on day 4 (Table 2). The MPH-MLR 80-mg capsule also had a longer mean t ½ than IR methylphenidate 25 mg administered three times daily after a single dose (on day 1, 6.0 vs. 3.4 h) and at steady state (day 4, 5.4 vs. 3.5 h).
Estimates from the bioequivalence results met design criteria for MPH-MLR, and showed that neither the MPH-MLR 80-mg capsule administered as a single dose after a high-fat breakfast nor the MPH-MLR 80-mg capsule administered as multiple doses to achieve steady state in the fed state were bioequivalent to IR methylphenidate 25 mg administered three times daily (Table 3) based on C max. On day 1, the geometric mean ratio of log-transformed C max (0.71) and the lower limit of the 90 % CI (66.81) were below the 80 % threshold. On day 4, the lower 90 % CI limits for the geometric mean ratios of log-transformed C max (0.80) and AUC0–∞ (84.48) met the 80 % threshold. Total systemic exposure to methylphenidate after the administration of the MPH-MLR 80-mg capsule and IR methylphenidate 25 mg administered three times daily was equivalent, as evidenced by comparable AUC0–t and AUC0–∞ values.
Bioequivalence results were also assessed using pAUC0–4 data. On day 1, the geometric mean ratio of log-transformed AUC0–4 (0.98) and 90 % CIs (85.14–113.46) were contained within the 80–125 % threshold for bioequivalence. On day 4, the geometric mean ratio of log-transformed AUC0–4 (1.14) and lower 90 % CI (100.38) were contained within the bioequivalence interval; however, the upper 90 % CI (129.95) was outside of the 125 % threshold.
3.3 Intra-Subject Variability
Intra-subject variability was assessed for MPH-MLR compared with IR methylphenidate. As expected, on day 1 intra-subject coefficients of variance (CVs) were slightly higher (44–136 %) for MPH-MLR during the first 2 h post dose in the absorption phase compared with the IR product (20–112 %), but were quite similar after reaching C max (Fig. 3). Intra-subject CVs were tighter through day 4 for MPH-MLR (28–56 %) compared with the reference comparator (26–108 %).
Intra-subject variability in plasma methylphenidate concentrations during the 4-day pharmacokinetic study of extended-release MPH-MLR 80 mg once daily and IR methylphenidate administered three times daily in healthy subjects on day 1 (a) and at steady state (day 4; b). CV coefficient of variation, IR MPH immediate-release methylphenidate, MPH-MLR extended-release multilayer bead formulation of methylphenidate hydrochloride
3.4 Safety Assessments
Single and multiple doses of the MPH-MLR 80-mg capsule administered once daily and IR methylphenidate 25 mg administered three times daily were safe and well tolerated in this study. The proportion of subjects who received the MPH-MLR 80-mg capsule who had TEAEs was similar when the same subjects were crossed over to receive IR methylphenidate 25 mg administered three times daily (38 vs. 35 %). The most common TEAEs recorded were headache, nausea, and decreased appetite (Table 4). All TEAEs were mild in intensity, except for one episode of elevated blood pressure that was moderate in intensity and resulted in discontinuation from study treatment. The elevation in blood pressure was identified in a 44-year-old white man following the second IR methylphenidate dose. Blood pressure rose from a pre-dose level of 123/73 to 159/93 mmHg ~3 h after the second dose. The AE was considered to be probably related to study drug. The subject recovered without residual effects and blood pressure had returned to normal ~2.5 h after the high value had been recorded. No serious AEs were reported and no clinically significant findings from clinical laboratory test results, or following physical examinations or ECG evaluations, were observed. No trends or changes in vital sign assessments were noted.
4 Discussion
This multiple-dose, randomized, crossover study identified two relevant pharmacokinetic findings following the administration of the MPH-MLR 80-mg capsule administered once daily and IR methylphenidate 25 mg administered three times daily. First, MPH-MLR produced expected differences in C max; the lower 90 % CI limits for the geometric mean ratios of log-transformed C max on day 1 and day 4 breached the bioequivalence thresholds mandated by the FDA. While the average of the three IR methylphenidate C max values was higher than that associated with the MPH-MLR 80-mg capsule on days 1 and 4, IR methylphenidate C max1 was lower and t max1 was greater than those of MPH-MLR. The initial drug absorption observed with the MPH-MLR 80-mg capsule was greater than that for IR methylphenidate 25 mg on day 4 as evidenced by the upper 90 % CI limit for the geometric mean ratio of log-transformed AUC0–4 being above the 125 % bioequivalence threshold. Second, the total extent of methylphenidate systemic exposure delivered by the two treatment regimens was similar after administration as a single dose with a high-fat meal (day 1) and after multiple daily dosing with a standard meal at steady state (day 4). A high-fat meal is known to slow gastric emptying and delay methylphenidate C max [12]. In our study, MPH-MLR coadministration with a high-fat meal delayed C max by 1 h relative to coadministration with a standard meal, but the methylphenidate pharmacokinetic profiles observed on days 1 and 4 were qualitatively and quantitatively similar.
As expected, intra-subject variability, evidenced by the % intra-subject CV, was greater during the absorption phase with the ER formulation MPH-MLR than was observed following administration of IR methylphenidate. However, once the initial C max was reached, intra-subject variability was more pronounced with the IR formulation than was observed with MPH-MLR. This finding may have contributed to a much lower fluctuation index for the ER compared with the IR product.
Similar pharmacokinetic findings were observed in a single-dose study of healthy adult subjects who received the MPH-MLR 80-mg capsule, MPH-MLR 80 mg sprinkled on applesauce, and IR methylphenidate 25 mg administered three times daily in the fasted state [29]. That is, total systemic exposure to both MPH-MLR 80-mg formulations was equivalent to that of the IR methylphenidate treatment regimen, but between-treatment differences in the initial rate and extent of methylphenidate absorption manifested as greater AUC0–4 values in favor of MPH-MLR 80 mg administered as an intact capsule or sprinkled on applesauce.
The finding that the once-daily MPH-MLR 80-mg capsule produces a higher AUC0–4 than the first dose of IR methylphenidate 25 mg administered three times daily at steady state fulfills the design objective for the MPH-MLR bead formulation. It has been postulated that the greatest changes in ADHD symptoms in children occur during the drug absorption phase, which coincides with morning activities [19, 20, 30, 31]. The release profile of the MPH-MLR 80-mg capsule provides its highest levels of plasma methylphenidate concentrations in the morning after dose administration.
IR methylphenidate 75 mg/day was the closest total daily dose achievable to the once-daily MPH-MLR 80-mg capsule and thus represents a confounding issue in our data as absolute bioavailability calculations were based on dose normalization. Dose normalization was accomplished by dividing the pharmacokinetic parameters by the administered dose for each subject and treatment group in the study. Comparisons of the dose-normalized parameters were used to assess bioequivalence. These calculations were hypothetical. Nevertheless, the pharmacokinetic profiles for the MPH-MLR 80-mg capsule and IR methylphenidate 25 mg administered three times daily on day 1 and day 4 were markedly different. In contrast to the initial peak and relatively constant methylphenidate concentration that persisted throughout the day with the MPH-MLR 80-mg capsule, marked peaks and troughs in plasma methylphenidate concentration were associated with IR methylphenidate 25 mg administered three times daily. Lower plasma methylphenidate concentrations were observed throughout the morning with IR methylphenidate than that with the MPH-MLR regimen, and vice versa, far higher drug concentrations were observed with the methylphenidate regimen through the afternoon and in the early evening. Fluctuating plasma methylphenidate concentrations, as produced by IR methylphenidate tablets twice and three times daily, are reported to be less desirable than gradually rising methylphenidate plasma concentrations throughout the day [12, 32, 33].
Overall, single and multiple doses of the MPH-MLR 80-mg capsule were safe and well tolerated by healthy male and female subjects, with no major safety concerns. All AEs were consistent with the known safety profile of methylphenidate and no new safety signals were observed.
5 Conclusion
In conclusion, MPH-MLR produced a biphasic profile of plasma methylphenidate concentrations characterized by rapid initial drug release throughout the morning, slowly falling levels until the ~5 h post dose, and delayed secondary release over the afternoon and early evening. While total systemic exposure to methylphenidate following the administration of the once-daily MPH-MLR 80-mg capsule was similar to that of IR methylphenidate 25 mg administered three times daily in the fed steady state, the treatment regimens were not bioequivalent because of lower C max and higher AUC0–4 values in the morning associated with MPH-MLR.
Notes
Rhodes Pharmaceuticals L.P. has received conditional acceptance from the US Food and Drug Administration to use the name Aptensio XR™ for this extended-release methylphenidate product.
References
Lecendreux M, Konofal E, Faraone SV. Prevalence of attention deficit hyperactivity disorder and associated features among children in France. J Atten Disord. 2011;15:516–24.
Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children–United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59:1439–43.
Döpfner M, Breuer D, Wille N, Erhart M, Ravens-Sieberer U. How often do children meet ICD-10/DSM-IV criteria of attention deficit-/hyperactivity disorder and hyperkinetic disorder? Parent-based prevalence rates in a national sample: results of the BELLA study. Eur Child Adolesc Psychiatry. 2008;17(suppl 1):59–70.
Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161:857–64.
Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–9.
Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–23.
Simon V, Czobor P, Balint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–11.
Greenhill LL, Pliszka S, Dulcan MK, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2001;40:1352–5.
Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–22.
National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. 2008. http://publications.nice.org.uk/attention-deficit-hyperactivity-disorder-cg72. Accessed 12 Feb 2014.
Rothenberger A, Döpfner M. Editorial: Observational studies in ADHD: the effects of switching to modified-release methylphenidate preparations on clinical outcomes and adherence. Eur Child Adolesc Psychiatry. 2011;20(suppl 2):S235–42.
Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23:1281–99.
Maldonado R. Comparison of the pharmacokinetics and clinical efficacy of new extended-release formulations of methylphenidate. Expert Opin Drug Metab Toxicol. 2013;9:1001–14.
Quillivant XR (methylphenidate hydrochloride) [prescribing information]. New York: NextWave Pharmaceuticals; 2013.
Brams M, Turnbow J, Pestreich L, et al. A randomized, double-blind study of 30 versus 20 mg dexmethylphenidate extended-release in children with attention-deficit/hyperactivity disorder: late-day symptom control. J Clin Psychopharmacol. 2012;32:637–44.
Fischer R, Schutz H, Grossmann M, Leis HJ, Ammer R. Bioequivalence of a methylphenidate hydrochloride extended-release preparation: comparison of an intact capsule and an opened capsule sprinkled on applesauce. Int J Clin Pharmacol Ther. 2006;44:135–41.
González MA, Pentikis HS, Anderl N, et al. Methylphenidate bioavailability from two extended-release formulations. Int J Clin Pharmacol Ther. 2002;40:175–84.
Schutz H, Fischer R, Grossmann M, Mazur D, Leis HJ, Ammer R. Lack of bioequivalence between two methylphenidate extended modified release formulations in healthy volunteers. Int J Clin Pharmacol Ther. 2009;47:761–9.
Swanson J, Gupta S, Guinta D, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305.
Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–8.
Polli JE, Cook JA, Davit BM, et al. Summary workshop report: facilitating oral product development and reducing regulatory burden through novel approaches to assess bioavailability/bioequivalence. AAPS J. 2012;14:627–38.
Swanson JM, Wigal SB, Wigal T, et al. COMACS Study Group: a comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics. 2004;113:e206–16.
Quinn D, Bode T, Reiz JL, Donnelly GA, Darke AC. Single-dose pharmacokinetics of multilayer-release methylphenidate and immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Pharmacol. 2007;47:760–6.
Reiz JL, Donnelly GA, Michalko K. Comparative bioavailability of single-dose methylphenidate from a multilayer-release bead formulation and an osmotic system: a two-way crossover study in healthy young adults. Clin Ther. 2008;30:59–69.
Weiss M, Hechtman L, Turgay A, et al. Once-daily multilayer-release methylphenidate in a double-blind, crossover comparison to immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:675–88.
Schachar R, Ickowicz A, Crosbie J, et al. Cognitive and behavioral effects of multilayer-release methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18:11–24.
Jain U, Hechtman L, Weiss M, et al. Efficacy of a novel biphasic controlled-release methylphenidate formula in adults with attention-deficit/hyperactivity disorder: results of a double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2007;68:268–77.
Metropolitan Life Insurance Company. Metropolitan height and weight tables. Stat Bull Metrop Life Insur Co. 1999.
Adjei A, Teuscher NS, Kupper RJ, et al. Single-dose pharmacokinetics of methylphenidate extended-release administered as intact capsule or sprikles versus methylphenidate immediate-release tablets (Ritalin®) in healthy adult volunteers. J Child Adolesc Psychopharmacol. 2014 (in press).
Greenhill LL. Pharmacologic treatment of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 1992;15:1–27.
Patrick KS, Markowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharmacol. 1997;12:527–46.
Stein MA, Blondis TA, Schnitzler ER, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics. 1996;98:748–56.
Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. 1999;38:503–12.
Acknowledgments
Dr. Adjei is Executive Director of Product Development at Rhodes Pharmaceuticals L.P. and was Study Director for this study. This study was conducted at Frontage Laboratories, Exton, PA, USA. The authors acknowledge the contribution of Lisa Diamond, PhD, for her contribution to the conduct of this study. Medical writing support was provided by Linda Wagner and Malcolm Darkes, medical writers at Excel Scientific Solutions, and funded by Rhodes Pharmaceuticals L.P.
Conflicts of interest
Dr. Adjei is the Executive Director of Product Development at Rhodes Pharmaceuticals L.P. and was study director for this study. Dr. Kupper is an employee of Rhodes Pharmaceuticals L.P. Dr. Teuscher is a consultant for Rhodes Pharmaceuticals L.P. Dr. Wigal is an advisor board member/consultant/speakers bureau member for Eli Lilly, Ironshore, Neos, NextWave, Noven, NuTec, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shionogi, Shire, and Tris and has received grant and research support from Eli Lilly, Forest Laboratories, the National Institutes of Health, NextWave, Noven, NuTec, Rhodes Pharmaceuticals L.P., Shire, and Sunovion. Dr. Sallee is advisory board member/consultant/speakers bureau member for Ironshore, Neos, NextWave, Impax Labs, Otsuka Pharmaceutical Development and Commercialization, Purdue, Rhodes Pharmaceuticals L.P., Shionogi, and Shire, and has received grant and research support from the National Institutes of Health, Rhodes Pharmaceuticals L.P., and Shire. Dr. Sallee has an equity interest in and is member board of directors for P2D Bioscience Inc. Dr. Childress is an advisory board member/consultant/speakers bureau member for Bristol-Myers Squibb, Ironshore, NextWave, Novartis, Pfizer, Shionogi, and Shire, and has received research support from Arbor, Bristol-Myers Squibb, Forest Research Institute, Johnson & Johnson Pharmaceutical Research & Development, Lilly USA, Neos, Neurovance, NextWave, Novartis, Noven, Otsuka, Pfizer, Rhodes Pharmaceuticals L.P., Sepracor, Shionogi, Shire, Sunovion, Theravance, and Tris. Dr. Kollins has received research support and/or consulting fees from the following sources: Akili Interactive, Alcobra, Arbor, Atentiv, the Environmental Protection Agency, the National Institutes of Health (National Institute on Drug Abuse, National Institute of Environmental Health Sciences), Neos, Otsuka, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, and Tris. Dr. Greenhill has received research support from the National Institutes of Health (National Institute on Drug Abuse) and Shire and is on the advisory board for BioBehavioral Diagnostics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adjei, A., Kupper, R.J., Teuscher, N.S. et al. Steady-State Bioavailability of Extended-Release Methylphenidate (MPH-MLR) Capsule vs. Immediate-Release Methylphenidate Tablets in Healthy Adult Volunteers. Clin Drug Investig 34, 795–805 (2014). https://doi.org/10.1007/s40261-014-0234-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0234-x