Abstract
Background
The pharmacokinetic properties of the immediate-release (IR) and the recently developed controlled-release (CR) formulation of pregabalin are dose proportional. Pregabalin IR can be taken with or without food.
Objectives
This analysis characterizes the effect of food on pregabalin CR. The objectives of this analysis were: (1) to evaluate the effect of administration time and fat or caloric content of an accompanying meal on the pharmacokinetic properties of a single dose of pregabalin CR (330 mg) relative to a single dose of pregabalin IR (300 mg); (2) to evaluate the pharmacokinetic properties of a single dose of pregabalin CR administered fasted relative to a single dose of pregabalin CR administered immediately after food; and (3) to determine the safety and tolerability of single-dose administration of pregabalin CR and IR with and without food.
Methods
The effect of food on the pharmacokinetic properties of pregabalin CR was determined in five phase I, open-label, single-dose, crossover studies (24–28 participants/study). Caloric and fat content of meals were varied and treatments were administered in the morning, at midday, or in the evening. Blood samples were collected up to 48 h post-dose. Pharmacokinetic parameters were estimated from plasma concentration–time data using standard noncompartmental methods. Adverse events were monitored throughout all studies.
Results
One hundred and twenty-eight healthy participants (19–54 years of age) received pregabalin. Peak plasma concentrations (C max) were lower for CR than the respective pregabalin IR doses, and time to C max occurred later. When pregabalin CR was administered with food at midday or in the evening, total exposures [area under the plasma concentration–time curve from time zero extrapolated to infinite time (AUC∞)] were equivalent for pregabalin CR and IR formulations regardless of fat or caloric content. When pregabalin CR was administered with an 800–1,000 calorie medium-fat breakfast, AUC∞ was equivalent for pregabalin CR and IR. Bioequivalence criteria for comparison of pregabalin CR after a low- or medium-calorie breakfast relative to pregabalin IR were not met; however, bioavailability of the pregabalin CR vs. IR formulation was relatively high (75–86 %). When pregabalin CR was administered fasted, the AUC∞ was 70–78 % of the AUC∞ of pregabalin CR administered with food and bioequivalence criteria were not met. Additionally, the AUC∞ of the pregabalin CR formulation administered fasted was 62–69 % of that of pregabalin IR administered fasted and bioequivalence criteria were not met. Single-dose pregabalin CR and IR were well tolerated in all studies, with no serious or severe adverse events reported.
Conclusion
Time of day of administration and the fat and caloric content of the accompanying meal had minimal overall effect on the pharmacokinetic properties and bioavailability of the pregabalin CR formulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Time of day of administration and the fat and caloric content of the accompanying meal have minimal overall effect on the pharmacokinetic properties and bioavailability of pregabalin controlled-release (CR) 330 mg |
A single dose of pregabalin CR 330 mg was well tolerated in healthy volunteers, with adverse events mild to moderate in severity |
The favorable pharmacokinetic profile of the pregabalin CR formulation when administered with food supports its development for the clinical practice setting |
1 Introduction
The immediate-release (IR) formulation of the α2δ ligand pregabalin (Lyrica®; Pfizer Inc, New York, NY, USA) is marketed in over 100 countries [including the USA and countries in the European Union (EU)] for several indications including partial seizures (USA [1] and EU [2]), neuropathic pain (EU [2]), neuropathic pain associated with diabetic peripheral neuropathy, spinal cord injury, and postherpetic neuralgia (USA [1]), fibromyalgia (USA [1]), and generalized anxiety disorder (EU [2]). Pregabalin IR is rapidly and highly absorbed, does not bind to plasma proteins, has negligible metabolism, and shows linear pharmacokinetics across the therapeutic dose range [3, 4].
Depending on the indication, pregabalin IR is administered twice or three times a day, with a maximum recommended dose of 600 mg/day [1, 2]. It is thought that reducing multiple dosing to once-daily dosing could improve adherence in long-term care [5]. To potentially achieve a once-daily dosing regimen for pregabalin, a controlled-release (CR) formulation that is administered after a meal has been developed. Initial observations have shown that pregabalin CR 300 mg administered following a medium-fat 600–750 calorie (medium-calorie) evening meal is equivalent to pregabalin IR 300 mg with respect to total exposure (Studies 1174 and 1206, Pfizer Inc, data on file). However, as the relative bioavailability of the 300-mg pregabalin CR formulation averaged 85–91 % that of pregabalin IR, the strength of the pregabalin CR tablet was increased by 10 % to 330 mg per tablet in the studies reported herein.
When administered with food, the rate of pregabalin IR absorption decreases [6], but there is no clinically significant effect on the extent of absorption with food; therefore, pregabalin IR can be taken without regard to meals [1, 2]. However, the effect of food on the pharmacokinetic properties of the pregabalin CR formulation, beyond that of a medium-fat, medium-calorie meal, is unknown. The studies reported here aimed: (1) to evaluate the effect of the time of day of administration and the fat or caloric content of the accompanying meal on the pharmacokinetics and the equivalence of the extent of absorption of a single dose of pregabalin CR 330 mg relative to a single dose of pregabalin IR 300 mg; (2) to evaluate the pharmacokinetics and equivalence of the extent of absorption of a single dose of pregabalin CR 330 mg administered under fasted conditions relative to a single dose of pregabalin CR 330 mg administered immediately after food; and (3) to determine the safety and tolerability of single-dose administration of the pregabalin CR and IR treatments.
2 Methods
Five studies investigating the pharmacokinetic properties of pregabalin CR 330 mg compared with pregabalin IR 300 mg were conducted in healthy adult volunteers between April 2010 and June 2011. All studies are registered on ClinicalTrials.gov under the following identifiers: Study 1, NCT01321671 (study sponsor identifier, A0081228); Study 2, NCT01291524 (A0081238); Study 3, NCT01257529 (A0081227); Study 4, NCT01080612 (A0081239); and Study 5, NCT01270815 (A0081188).
Studies 1, 2, and 4 were conducted by New Haven Clinical Research (New Haven, CT, USA) and Studies 3 and 5 by Quintiles Phase One Services (Overland Park, KS, USA). The final protocols and informed consent documentation were reviewed and approved by the ethics committees of either Integreview (Austin, TX, USA) or the Midlands Institutional Review Board (Overland Park, KS, USA). Participants provided written informed consent before entering a study. All studies were conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines.
2.1 Study Design
These five studies were phase I, single-dose, open-label, randomized, crossover studies. Study 1 was a three-way crossover study, while Studies 2–5 were four-way crossover studies. Participants received, in a randomized sequence, a single dose of pregabalin CR 330 mg or pregabalin IR 300 mg in each period. There was at least a 7-day washout between each period of treatment in all studies.
The meals served at the time of pregabalin administration were standardized within studies and were categorized by either calorie or fat content. Meals defined as low calorie contained 400–500 calories, medium-calorie meals contained 600–750 calories, and high-calorie meals contained 800–1,000 calories, each with approximately 30 % of the calories coming from fat. Meals defined as low, medium, or high fat contained approximately 15, 30, or 50 % fat, respectively; these meals contained 800–1,000 calories. All meals contained approximately 15–20 % protein. Study 1 compared pregabalin CR administered in the evening with food (taken following a medium-calorie meal) or under fasted conditions with pregabalin IR administered in the evening under fasted conditions. Study 2 compared pregabalin CR administered following a low- or medium-calorie evening meal and pregabalin CR administered 4 h after a medium-calorie evening meal (i.e., fasted, at bedtime) with pregabalin IR administered in the evening under fasted conditions. Study 3 compared pregabalin CR administered with a low-, medium-, or high-fat evening meal with pregabalin IR administered with a medium-fat evening meal. Study 4 compared pregabalin CR administered with a low-, medium-, or high-calorie breakfast with pregabalin IR administered in the morning under fasted conditions. Study 5 compared pregabalin CR administered with a low-, medium-, or high-calorie midday meal with pregabalin IR administered midday under fasted conditions.
2.2 Participants
All participants were required to be in good health, which was verified at screening by medical history, physical examination, urine drug test, safety laboratory panel, electrocardiogram, and vital signs (blood pressure and pulse rate). Participants aged 18–55 years, with a body mass index (BMI) of 17.5–30.5 kg/m2 and a total body weight of >50 kg were eligible for entry. Participants were required to abstain from caffeine-containing products for 24 h prior to the start of dosing and until collection of the final pharmacokinetic sample of each study period.
Key study exclusion criteria consisted of estimated creatinine clearance of <60 mL/min; the use of tobacco or nicotine-containing products in excess of the equivalent of five cigarettes/day; and the use of prohibited medication, including prescription or nonprescription medication (other than contraceptives or hormone-replacement therapy) in the 7 days or five half-lives (whichever was longer) prior to receiving the first dose of study medication. Herbal supplements had to be discontinued ≥28 days prior to the first dose of study medication. As an exception, the use of acetaminophen (≤1 g/day) was permitted if required.
2.3 Medication Dosing Schedule
Study medication was administered at approximately the same time of day for a given participant across all treatment periods, except in Study 2 where the pregabalin CR dose given at bedtime was administered 4 h later than other doses in the study. When administered with food, administration occurred within approximately 5 min following completion of the meal. Study medication was administered with water and swallowed whole.
For evening dosing with food, participants fasted for 5.5 h before the evening meal and abstained from all food and drink (except water) until breakfast the following morning. For morning dosing with food, participants fasted for 8 h prior to and for 4 h following breakfast. For midday dosing with food, participants fasted for 5.5 h prior to and for 6 h following lunch. For pregabalin CR and IR dosing under fasted conditions, participants fasted for 4–6 or 5–8 h prior to and for 4–8 or 2–4 h following dose administration, respectively. It should be noted that for studies with evening medication administration, breakfast was not controlled for and some study participants may not have eaten breakfast.
2.4 Pharmacokinetic Sampling
Blood samples were taken before each dose of study medication (Time 0). For pregabalin CR, samples were also taken at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, 24, 36, and 48 h post-dose. For pregabalin IR, samples were taken at 0.33, 0.67, 1, 1.5, 2, 4, 6, 9, 12, 16, 24, 36, and 48 h post-dose.
2.5 Sample Handling and Determination of Plasma Pregabalin Concentration
All blood samples were collected into tubes containing sodium heparin, and the plasma was stored frozen at −20 °C until analyzed. Plasma samples were analyzed for pregabalin concentrations at WuXi App Tec (Shanghai, China) using a validated, sensitive, and specific high-performance liquid chromatography-tandem mass spectrometric method; the methodology was identical to that described by Alebic-Kolbah [7], with the exception of the anticoagulant used, as Alebic-Kolbah [7] used lithium heparin.
The lower limit of quantification (LLOQ) for pregabalin was 0.0250 µg/mL and the upper limit of quantification (ULOQ) was 10 µg/mL. Clinical specimens with plasma pregabalin concentrations below the LLOQ were reported as being below the LLOQ, and those with concentrations above the ULOQ were adequately diluted into calibration range. Assay precision, expressed as the between-day percent coefficients of variation (% CV) of the mean estimated concentrations of quality control (QC) samples, was ≤8.2 % across these five studies for low QC (0.06 µg/mL), medium-low QC (0.60 µg/mL), medium-high QC (2.00 µg/mL), high QC (7.50 µg/mL), and dilution QC (20.00 µg/mL) concentrations. The between-day assay accuracy, expressed as the percent relative error for QC concentrations, was in the range of −8.3 to +3.3 % across these five studies for the low, medium, high, and dilution QC samples.
2.6 Statistical Analyses
Pharmacokinetic parameters for pregabalin CR and IR were calculated for each participant for each treatment using noncompartmental analysis of concentration–time data. Concentrations below the LLOQ were treated as zero for the analysis. The observed C max and the time of maximum observed plasma concentration (t max) were recorded as observed. The area under the plasma concentration–time curve from time 0 extrapolated to infinite time (AUC∞) was calculated using the linear/log trapezoidal method. The terminal half-life was calculated according to the formula Loge(2)/k el (where k el is the terminal phase rate constant calculated by a linear regression of the log-linear concentration–time curve).
Natural log-transformed (without dose normalization) AUC∞ and C max were analyzed using a mixed-effect model with sequence, period, and treatment as fixed effects and participant within sequence as a random effect. Estimates of the adjusted mean differences (Test–Reference) and corresponding 90 % confidence intervals (CI) were obtained from the model.
When comparing pregabalin CR doses with pregabalin IR doses, bioequivalence of the Test formulation to the Reference formulation was concluded if the 90 % CIs for AUC∞ were contained within 80–125 %. When comparing pregabalin CR treatments with other pregabalin CR treatments, bioequivalence of the Test formulation to the Reference formulation was concluded if the 90 % CIs for both C max and AUC∞ were contained within 80–125 %.
2.7 Safety Evaluations
Study investigators recorded all observed or volunteered adverse events (AEs), the severity of the events, and the relationship to the study treatment. AEs included adverse drug reactions, illnesses with onset during the study, and exacerbation of previous illnesses. Any clinically significant changes in physical examination findings and abnormal objective test findings were also recorded as AEs.
3 Results
3.1 Participants
A total of 128 participants (96 men, 32 women) received pregabalin across the five studies (Table 1). Participants were 19–54 years of age, with a total body weight of 53–107 kg and a BMI of 18–31 kg/m2.
3.2 Pregabalin Pharmacokinetics: Descriptive and Statistical Summary
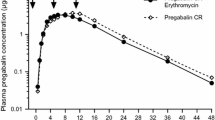
Mean plasma pregabalin concentration–time profiles for each study are presented in Fig. 1 and observed pregabalin pharmacokinetic parameter values are summarized descriptively in Table 2. Plots of individual and median parameter values by treatment are presented in Fig. 2 for AUC∞ and Fig. 3 for C max. Overall, single-dose C max was lower for pregabalin CR treatments than the comparable single-dose pregabalin IR treatment, and t max occurred later (Figs. 1 and 3; Tables 2 and 3). Overall, when pregabalin CR was administered following a meal, the total exposure (AUC∞) was similar for the pregabalin CR and IR formulations (Fig. 2; Table 2). When pregabalin CR was administered in the morning with a low- or medium-calorie breakfast (Study 4), the AUC∞ decreased and the % CV increased compared with pregabalin IR administered fasted (Table 2). For pregabalin CR administered fasted vs. with food, the AUC∞ decreased by 22–30 % (Studies 1 and 2) and t max occurred 2–3 h earlier (Table 2). Variability across all five studies was low for most treatments with % CV values for AUC∞ between 12 and 36 and for C max between 16 and 29 (Table 2). The terminal half-life values were similar for all doses across the five studies (Table 2).
Mean plasma pregabalin concentration–time profiles. Pregabalin was administered at a dose of 330 mg [controlled release (CR)] or 300 mg [immediate release (IR)]. Accompanying meals were categorized by either calorie or fat content. Low kilocalorie (kcal) = 400–500 calories/meal, medium (med) kcal = 600–750 calories/meal, and high kcal = 800–1,000 calories/meal (in each instance with 30 % of calories coming from fat). Low fat = 15 % fat content, med fat = 30 % fat content, and high fat = 50 % fat content (in each instance in a meal of 800–1,000 calories)
Individual and median pregabalin area under the plasma concentration–time curve from time zero extrapolated to infinite time (AUC∞) values by treatment. Open circles represent individual participant values. Scatter plot shows median and 25–75 % quartiles. Pregabalin was administered at a dose of 330 mg [controlled release (CR)] or 300 mg [immediate release (IR)]. Accompanying meals were categorized by either calorie or fat content. Low kilocalorie (kcal) = 400–500 calories/meal, medium (med) kcal = 600–750 calories/meal, and high kcal = 800–1,000 calories/meal (in each instance with 30 % of calories coming from fat). Low fat = 15 % fat content, med fat = 30 % fat content, and high fat = 50 % fat content (in each instance in a meal of 800–1,000 calories). Study 1: A CR, med kcal, evening; B CR, fasted, evening; C IR, fasted, evening. Study 2: D CR, low kcal, evening; E CR, med kcal, evening; F CR, fasted, bedtime (4 h after a med kcal evening meal); G IR, fasted, evening. Study 3: H CR, low fat, evening; I CR, med fat, evening; J CR, high fat, evening; K IR, med fat, evening. Study 4: L CR, low kcal, morning; M CR, med kcal, morning; N CR, high kcal, morning; O IR, fasted, morning. Study 5: P CR, low kcal, midday; Q CR, med kcal, midday; R CR, high kcal, midday; S IR, fasted, midday
Individual and median pregabalin peak plasma concentration (C max) values by treatment. Open circles represent individual participant values. Scatter plot shows median and 25–75 % quartiles. Pregabalin was administered at a dose of 330 mg [controlled release (CR)] or 300 mg [immediate release (IR)]. Accompanying meals were categorized by either calorie or fat content. Low kilocalorie (kcal) = 400–500 calories/meal, medium (med) kcal = 600–750 calories/meal, and high kcal = 800–1,000 calories/meal (in each instance with 30 % of calories coming from fat). Low fat = 15 % fat content, med fat = 30 % fat content, and high fat = 50 % fat content (in each instance in a meal of 800–1,000 calories). Study 1: A CR, med kcal, evening; B CR, fasted, evening; C IR, fasted, evening. Study 2: D CR, low kcal, evening; E CR, med kcal, evening; F CR, fasted, bedtime (4 h after a med kcal evening meal); G IR, fasted, evening. Study 3: H CR, low fat, evening; I CR, med fat, evening; J CR, high fat, evening; K IR, med fat, evening. Study 4: L CR, low kcal, morning; M CR, med kcal, morning; N CR, high kcal, morning; O IR, fasted, morning. Study 5: P CR, low kcal, midday; Q CR, med kcal, midday; R CR, high kcal, midday; S IR, fasted, midday
The relative bioavailability of pregabalin CR administered with food vs. pregabalin IR (with food or fasted) generally met bioequivalence criteria with respect to total pregabalin exposure. The 90 % CIs for the ratios (pregabalin CR/IR) of adjusted geometric mean AUC∞ values were contained within the 80–125 % range for all pregabalin CR treatments, with the exception of pregabalin CR administered with a low- or medium-calorie breakfast (Study 4) (Table 3). Pregabalin CR administered fasted (either in the evening following a 6-h fast or at bedtime following a 4-h fast) was not equivalent to pregabalin IR (fasted) with respect to AUC∞ (Table 3, Studies 1 and 2). Results for the comparison between pregabalin CR treatments are summarized in Table 3. When pregabalin CR was administered with food midday or in the evening, bioequivalence criteria (90 % CI for AUC∞ and C max contained fully within the 80–125 % range) for various combinations of calorie intake and fat were met. However, bioequivalence of pregabalin CR administered with food and pregabalin CR administered fasted (Studies 1 and 2) was not met, as the 90 % CI of AUC∞ and C max fell outside the 80–125 % range (Table 3).
3.3 Safety Evaluations
Single doses of pregabalin CR and IR were well tolerated in all five studies. No participants experienced serious or severe AEs. No deaths occurred during any of these studies. One participant (Study 4) discontinued treatment because of an AE of scrotal pain that was considered to be treatment related, and one participant (Study 5) discontinued treatment because of an AE of elevated blood pressure that was not considered to be treatment related.
The majority of AEs in these five studies were considered treatment related. Across the five studies, the most common all-causality AEs with pregabalin CR were dizziness (n = 37, 28.9 %), fatigue (n = 28, 21.9 %), somnolence (n = 26, 20.3 %), and euphoric mood (n = 15, 11.7 %) (Table 4). With pregabalin IR, the most common all-causality AEs were dizziness (n = 41, 33.6 %), somnolence (n = 14, 11.5 %), fatigue (n = 13, 10.7 %), and euphoric mood (n = 11, 9.0 %) (Table 4).
4 Discussion
The purpose of these five phase I, randomized, open-label studies was to evaluate the pharmacokinetic properties of single-dose pregabalin CR (330 mg) administered in the morning, at midday, or in the evening following meals of different caloric or fat content relative to pregabalin IR (300 mg). An additional objective was to evaluate the pharmacokinetics and bioequivalence of pregabalin CR administered fasted relative to pregabalin CR administered immediately after food.
Slow and sustained absorption occurred with pregabalin CR with median t max values of 5–12 h compared with 1.5 or 4 h with pregabalin IR administered fasted or with food, respectively. C max values for pregabalin CR treatments were 39–69 % of the values of the respective pregabalin IR treatments. For pregabalin CR administered with food at midday or in the evening, the relative bioavailability of pregabalin CR was ≥86 % (90 % CI for AUC∞ in the 80–125 % range) that of pregabalin IR, and varying the caloric or fat content of meals had minimal impact on bioavailability. Therefore, equivalence in the extent of absorption of pregabalin CR vs. pregabalin IR following the midday or evening meal was demonstrated. Overall, the AUC∞ decreased and the % CV increased when pregabalin CR was administered fasted or following a morning meal. When pregabalin CR was administered following an 800–1,000 calorie morning meal, total exposures were equivalent for pregabalin CR and IR. Bioequivalence criteria were not met when pregabalin CR was administered after a low- or medium-calorie breakfast, although bioavailability of pregabalin CR vs. the IR formulation was relatively high (75–86 %). One possibility for the reduced bioavailability observed with a low- or medium-calorie breakfast is a shortened absorption window owing to diurnal variability of gastrointestinal motility [8–11]. Pregabalin exposure was lower for the pregabalin CR vs. the IR formulation when pregabalin CR was administered fasted. When pregabalin CR was administered fasted (evening or bedtime), bioavailability was 62–68 % that of pregabalin IR.
Single doses of pregabalin CR 330 mg and pregabalin IR 300 mg were generally well tolerated across all five studies, with AE profiles similar to those previously reported for pregabalin IR across the therapeutic dose range in different patient populations [1, 2]. A pooled analysis of patient data from 13 clinical trials of pregabalin for peripheral neuropathic pain has shown that dizziness and somnolence are the two most common AEs experienced with pregabalin IR. These AEs typically emerge within the first 1–2 weeks of treatment and resolve 1–2 weeks later; resolution is not dependent on treatment cessation [12]. It should be noted that none of the five studies reported here were powered for the evaluation of AEs, although determining the safety and tolerability of pregabalin CR was an objective in all five studies.
The pharmacokinetic properties of pregabalin CR have been reported to be dose proportional over the therapeutic dosing range [13]. Taken together with the observations presented herein, the favorable pharmacokinetic profile of pregabalin CR when administered with food, plus the retention of several important pharmacokinetic properties of pregabalin IR (e.g., negligible metabolism and no plasma-protein protein binding [4], renal elimination as >98 % unchanged drug [4], and lack of significant drug–drug interactions [1, 14, 15]), suggest that the pregabalin CR formulation could be developed for use in a once-daily dosing regimen for clinical practice.
5 Conclusions
Time of day of administration and the fat and caloric content of the accompanying meal have minimal overall effect on the pharmacokinetic properties and bioavailability of pregabalin CR (330 mg). Furthermore, a single dose of pregabalin CR 330 mg was well tolerated in healthy volunteers, with AEs mild to moderate in severity. Overall, the favorable pharmacokinetic profile of the pregabalin CR formulation when administered with food supports its development for the clinical practice setting.
References
Lyrica (pregabalin) [package insert]. Pfizer Inc, New York. 2013. http://labeling.pfizer.com/ShowLabeling.aspx?id=561. Accessed 11 Jun 2014.
Lyrica EU product information. Pfizer Limited, Sandwich. 2009. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf. Accessed 11 Jun 2014.
Bockbrader HN, Radulovic LL, Posvar EL, et al. Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol. 2010;50:941–50.
Corrigan BW, Pool WF, Posvar EL, et al. Metabolic disposition of pregabalin in healthy volunteers [abstract PI-68]. Clin Pharmacol Ther. 2001;69:P18.
Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–34.
Bockbrader HN, Burger P, Knapp L, Corrigan BW. Population pharmacokinetics of pregabalin in healthy subjects and patients with chronic pain or partial seizures. Epilepsia. 2011;52:248–57.
Alebic-Kolbah T. Pregabalin: development and validation of an LC-MS/MS method for pediatric studies [abstract TP542]. J Am Soc Mass Spectrom. 2012;23:109.
Kellow JE, Borody TJ, Phillips SF, et al. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386–95.
Kumar D, Wingate D, Ruckebusch Y. Circadian variation in the propagation velocity of the migrating motor complex. Gastroenterology. 1986;91:926–30.
Wilson P, Perdikis G, Hinder RA, et al. Prolonged ambulatory antroduodenal manometry in humans. Am J Gastroenterol. 1994;89:1489–95.
Soffer EE, Thongsawat S, Ellerbroek S. Prolonged ambulatory duodeno-jejunal manometry in humans: normal values and gender effect. Am J Gastroenterol. 1998;93:1318–23.
Freynhagen R, Serpell M, Emir B, et al. A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract. 2013. doi:10.1111/papr.12146.
Chew ML, Alvey CW, Plotka A, et al. Pregabalin controlled-release pharmacokinetics in healthy volunteers: analysis of four multiple-dose randomized clinical pharmacology studies. Clin Drug Investig. 2014. doi:10.1007/s40261-014-0221-2.
Brodie MJ, Wilson EA, Wesche DL, et al. Pregabalin drug interaction studies: lack of effect on the pharmacokinetics of carbamazepine, phenytoin, lamotrigine, and valproate in patients with partial epilepsy. Epilepsia. 2005;46:1407–13.
Bockbrader HN, Burger P, Knapp L. Pregabalin effect on steady-state pharmacokinetics of carbamazepine, lamotrigine, phenobarbital, phenytoin, topiramate, valproate, and tiagabine. Epilepsia. 2011;52:405–9.
Acknowledgments
The studies described in this paper were sponsored by Pfizer Inc, who was involved in the study design, the collection, analysis, and interpretation of the data, the writing of the report, and the decision to submit the paper for publication. Medical writing support was provided by Lorna Forse, PhD, of Engage Scientific Solutions and funded by Pfizer Inc.
Conflict of interest
Marci L. Chew, Anna Plotka, Christine W. Alvey, Verne W. Pitman, Tanja Alebic-Kolbah, and Joseph M. Scavone are all full-time employees of Pfizer Inc and hold stock in Pfizer Inc. Howard N. Bockbrader was an employee of Pfizer Inc at the time these studies were conducted and holds stock in Pfizer Inc.
Ethical statement
All studies were conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines. All participants provided written informed consent before entering a study. The final protocols and informed consent documentation were reviewed and approved by the ethics committees of either Integreview (Austin, TX, USA) or the Midlands Institutional Review Board (Overland Park, KS, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chew, M.L., Plotka, A., Alvey, C.W. et al. Pharmacokinetics of Pregabalin Controlled-Release in Healthy Volunteers: Effect of Food in Five Single-Dose, Randomized, Clinical Pharmacology Studies. Clin Drug Investig 34, 617–626 (2014). https://doi.org/10.1007/s40261-014-0211-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0211-4