Abstract
Background and Objectives
Statins (HMGCoA-reductase inhibitors) produce numerous non-lipid related, ‘pleiotropic’ effects. Our aim was to investigate whether simvastatin treatment affects serum levels of vascular calcification inhibitors, such as fetuin-A, osteoprotegerin (OPG) and osteopontin (OPN), in patients with coronary artery disease (CAD).
Methods
A total of 98 statin-free patients with angiographically proven, newly diagnosed CAD were treated with simvastatin (20–40 mg daily) for 6 months to target a low-density lipoprotein (LDL) level <100 mg/dL (the statin group [SG]). Thirty-five age- and sex-matched healthy individuals without any chronic metabolic or cardiovascular disease at baseline served as a healthy control group (HCG). Clinical, anthropometrical and metabolic parameters and serum fetuin-A, OPG, OPN and high-sensitivity C-reactive protein (hsCRP) levels were assayed at baseline in all participants and after 6 months only in SG patients.
Results
Compared with HCG subjects at baseline, SG patients exhibited higher serum levels of OPG (7.39 ± 2.94 pmol/L vs 2.47 ± 1.15 pmol/L, p < 0.001), OPN (60.99 ± 17.52 ng/mL vs 45.45 ± 10.26 ng/mL, p = 0.005) and hsCRP (4.66 ± 1.74 mg/L vs 1.58 ± 0.56 mg/L, p < 0.001) as well as lower serum levels of fetuin-A (0.222 ± 0.036 μg/L vs 0.839 ± 0.092 μg/L, p < 0001). Apart from significantly reducing plasma total cholesterol and LDL, simvastatin also reduced serum levels of fetuin-A (by ~62.6 %), OPG (by ~47.2 %), OPN (by ~44.6 %) and hsCRP (by ~45.3 %) (p < 0.05) in SG patients. In standard multiple regression analysis, the simvastatin-induced reduction in fetuin-A was independently associated with changes in total cholesterol (β = −0.289, p = 0.048) and LDL (β = −0.302, p = 0.032) (R 2 = 0.305, p = 0.040).
Conclusion
Patients with CAD showed derangements in serum levels of all vascular calcification inhibitors compared with those in healthy controls. Simvastatin treatment for 6 months significantly decreased serum fetuin-A, OPG and OPN levels, but the clinical relevance of this requires further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Osteopontin (OPN) and osteoprotegerin (OPG) constitute physiologically potent inhibitors of vascular calcification, highly expressed at macrophage- and foam cell-rich sites within atherosclerotic plaques [1]. Elevated serum levels of OPN and OPG have shown an independent association with cardiovascular diseases (CVDs) [2, 3]. Most, but not all, researchers have demonstrated considerable downregulation of OPN and OPG serum levels after statin (HMGCoA-reductase inhibitor) therapy, related to beneficial changes in carotid plaque morphology [4, 5].
In addition to OPN and OPG, fetuin-A, a liver-derived inhibitor of calcification, has been inversely associated with arterial stiffness [6] and cardiovascular morbidity and mortality [7]. Most, but not all, prospective studies have suggested low serum fetuin-A as a valid predictor of coronary artery disease (CAD) incidence, severity and related mortality [8–10]. Those discordant findings do not address the question of whether fetuin-A constitutes a causative factor or a bystander in CAD development. Moreover, the precise mechanisms of fetuin-A involvement in CAD remains elusive. Unfortunately, there are extremely limited data about the pharmaceutical modification of fetuin-A.
Statins are the most effective and well-tolerated agents for treating dyslipidaemia in CAD [11]. They are recognized as first-line therapy for primary and secondary prevention of CAD and have been shown to reduce cardiovascular morbidity and mortality in large trials [12]. Moreover, statins show ‘pleiotropic’ effects, like anti-oxidative and anti-inflammatory effects [13, 14]. All patients with CAD should be evaluated as candidates for statin therapy as part of a multidisciplinary approach to reduce cardiovascular risk.
In this short-term study, we assessed the effect of 6-month therapy with simvastatin on serum levels of fetuin-A, OPG and OPN in patients with newly diagnosed CAD.
2 Subjects and Methods
2.1 Subjects and Study Design
This prospective clinical study was conducted between January 2009 and March 2011. In our study we enrolled the following two groups:
(1) Statin group (SG): Statin-free patients with CAD were recruited to receive simvastatin therapy for 6 months. The simvastatin dose was gradually uptitrated (from 20 mg to 40 mg per day) targeting a low-density lipoprotein (LDL) level <100 mg/dL. Participants were considered eligible if they had just been diagnosed with stable CAD on coronary angiography. All participants underwent coronary angiography performed by experienced cardiologists. During analysis, investigators of the study were unaware of patients’ clinical and biochemical data. CAD was defined as angiographically proven stenosis of 50 % or more of the luminal diameter in a major epicardial coronary vessel, without a recent acute coronary syndrome (ACS). Eligible patients who underwent coronary angioplasty received a dual anti-platelet regimen (aspirin plus clopidogrel). The rest of the patients received constant anti-platelet monotherapy (aspirin or clopidogrel in the case of aspirin contra-indications) after angiography. The exclusion criteria were lipid-lowering therapy for at least 2 months before coronary angiography, liver impairment (alanine aminotransferase [ALT] >2.5 times higher than the upper normal limit), acute or chronic renal failure (creatinine level >2.0 mg/dL), acute cerebrovascular ischaemic attack or ACS within the preceding 12 months, evidence of alcohol abuse, hypothyroidism, osteoporosis, myopathy, significant body-weight changes during the last 2 months prior to study entry, malignancies, acute or chronic infectious disease and any kind of immune-mediated disease. Concomitant anti-hypertensive or hypoglycaemic medications were maintained unaltered during this study, unless it was deemed medically necessary for better glucose and arterial pressure control.
(2) Healthy control group (HCG): A cohort of 35 individuals without any chronic metabolic disease, CVD or overt cardiac-origin symptoms served as controls at baseline. Healthy volunteers were selected from a pool of visitors to our hospitals for preventive check-ups and they were matched for age and sex with CAD patients by a 2:1 ratio. The exclusion of CVD was based on a complete medical history, comprehensive physical examination, electrocardiography and echocardiography. Half of them had previously, within the last year, undergone a functional ischaemic test. Healthy controls were free from any long-term medication or any acute infection.
Instructions for smoking cessation were provided to both groups at baseline. The present study was conducted in compliance with the Declaration of Helsinki and was approved by the local ethics committee. The study procedure and goals were explained to the participants approved for entry into the study, who then signed an informed consent form.
2.2 Clinical and Echocardiographic Evaluation
A medical history of smoking habits, diabetes mellitus, hypertension and current medications was reported at baseline and at the end of the study. At the same time points, we measured blood pressure (BP) and body-mass index (BMI) in both groups. In particular, BP was measured twice, after keeping all participants in a sitting position for 15 min. There was a 5 min interval between the two measurements and the mean value was estimated for study purposes. The diabetes mellitus diagnosis was based on American Diabetes Association criteria [15]. Hypertension was considered when patients reported more than two home measurements of BP higher than 140/90 mmHg or when patients were already treated with antihypertensive agents. All the aforementioned measurements were obtained only at baseline for HCG subjects.
All participants underwent an echocardiographic examination (Vivid 5; General Electric, Columbus, OH, USA) to evaluate left ventricular morphology and systolic function at baseline (both groups) and after 6 months (only CAD patients).
2.3 Blood Biochemistry
Blood samples were drawn from the participants after an overnight fast at baseline and at the end of the study. Each sample was centrifuged at 5000 rpm for 5 min. 500 μL of each serum sample was sent for analysis of fasting plasma glucose (FPG) and lipids enzymatically (Chemwell 2910; Awareness Technology Inc., Palm City, FL, USA). Glycated haemoglobin (HbA1c) was determined by high-performance liquid chromatography (Menarini Diagnostics, Florence, Italy). The remainder of the serum samples were frozen and stored (at −80 °C) until analysis in the same assay with commercially available enzyme-linked immunosorbent assay (ELISA) kits: fetuin-A (BioVendor Laboratory Medicine, Inc., Brno, Czech Republic), OPG (Metra, San Diego, CA, USA) and OPN (R&D Systems Inc., Minneapolis, MN, USA) assessment. The intra- and inter-assay coefficients of variance were 3.9 % and 5.1 % for fetuin-A, 7 % and 6.8 % for OPG and 2.6 % and 5.7 % for OPN, respectively. We determined high-sensitivity C-reactive protein (hsCRP) using a nephelometric assay (BNII; Dade Behring, Marburg, Germany).
2.4 Statistical Analysis
The data were analyzed using the Statistical Package for the Social Sciences version 17 (SPSS Inc., Chicago, IL, USA). Frequencies, means, and standard deviations were used to describe the data whenever appropriate. Normality of distribution was assessed with the Kolmogorov–Smirnov test. Comparisons of all continuous variables within and between groups were performed by paired samples and student’s t tests, respectively. The paired sample Wilcoxon signed rank test and the chi-squared test were used for differences within and between proportions, respectively. The relationships of fetuin-A, OPG and OPN to age, total cholesterol, high-density lipoprotein (HDL), LDL, hsCRP and BMI were evaluated in the CAD group using Pearson correlation. Variables with significant correlations in univariate analysis were entered into standard multiple regression analysis, concerning fetuin-A, OPG and OPN as the dependent variable each time. A two-tailed p value <0.05 was considered to be statistically significant.
3 Results
3.1 Baseline Comparisons
Baseline results are depicted in Table 1. With the exception of smoking, there were no significant differences in demographic and biochemical characteristics, such as BMI and lipids, between the groups at baseline. Patients with CAD exhibited lower serum levels of fetuin-A compared with healthy controls (p < 0.05). Expectedly, they were characterized by higher baseline serum levels of hsCRP (p < 0.001), white blood cell count (p = 0.047), OPG (p < 0.001) and OPN (p = 0.005). Notably, among CAD patients, patients with 3-vessel disease showed significantly lower fetuin-A levels (0.115 ± 0.034 μg/L vs 0.473 ± 0.077 μg/L, p < 0.001) compared with the 1-vessel subgroup. The opposite was observed between the 3-vessel and 1-vessel subgroups for OPG (10.31 ± 3.03 pmol/L vs 5.88 ± 3.03 pmol/L, p < 0.001) and OPN (72.88 ± 22.58 ng/mL vs 48.91 ± 15.78 ng/mL, p = 0.011).
3.2 Follow-Up Results
A total of 98 patients with CAD were initially enrolled. Two patients treated with simvastatin were withdrawn from the study because of myalgia or liver enzyme elevation and one patient refused follow-up measurements. Ninety-five patients completed follow-up measurements and so their data were included in statistical analysis. A significant proportion of them (82 %) underwent either percutaneous or open surgical revascularization and since then, they were free from symptoms. Neither significant adverse events nor any cardiovascular complications were reported throughout the entire study period in the remaining participants. At follow-up, the vast majority of active smokers, within the SG, ceased smoking. In parallel, a total of six CAD patients initiated or modified their anti-hypertensive medications, while five diabetic patients uptitrated their anti-diabetic medications, during the study. Cardiac systolic function did not differ between the groups (p = 0.756) and remained adequate throughout the study.
Six months of simvastatin treatment significantly reduced concentrations of total cholesterol, LDL and triglycerides (p < 0.05) but produced no effect on serum levels of HDL cholesterol (p = 0.985). Because of the modification of anti-hypertensive medications, systolic BP was significantly reduced (p = 0.012). Similarly, the alteration in the anti-diabetic regimen conferred a significant downregulation in FPG and HbA1c levels (p < 0.01). The simvastatin-treated group had considerably reduced serum levels of hsCRP (p = 0.009), WBC (p = 0.042), fetuin-A (p < 0.001), OPG (p < 0.001) and OPN (p < 0.001). All the above results are presented in Table 2.
We further investigated the influence of traditional cardiovascular risk factors on serum concentrations of fetuin-A, OPG and OPN at baseline. There was no difference in serum levels of fetuin-A, OPG and OPN between diabetic and normoglycaemic subjects, between smokers and non-smokers, and between hypertensive and normotensive subjects (p > 0.05 for all) (data not shown). In parallel, revascularization procedures had only a slight influence on the final results in comparison with pharmaceutically-treated CAD patients (p > 0.05) (data not shown).
3.3 Correlations
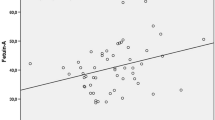
At baseline, we found considerable univariate correlations of vascular calcification inhibitors with other variables, within the SG (Table 3). In particular, fetuin-A was significantly correlated with OPG, OPN, total cholesterol and LDL (p < 0.05). In addition to fetuin-A, OPN was positively correlated with age and BMI and inversely with HDL (p < 0.05). Finally, baseline serum OPG levels correlated with age, hsCRP and BMI (p < 0.05).
We next searched the determinants of simvastatin-induced reductions in fetuin-A, OPG and OPN. In univariate analysis changes in total cholesterol and LDL were significantly related to fetuin-A changes. Those variables entered in the standard multiple regression analysis and their relationships with fetuin-A changes remained significant (β = 0.396, p = 0.045 and β = 0.348, p = 0.032, respectively) (R 2 = 0.305, p = 0.040). Moreover, the other two vascular calcification inhibitors were univariately correlated. Then in linear multiple regression analysis, the reduction in OPG was the only independent determinant of OPN reduction, and vice versa (β = 0.374, p = 0.017).
4 Discussion
In the present study, patients with CAD showed higher OPN and OPG and lower serum fetuin-A levels than healthy controls. Simvastatin treatment markedly decreased serum levels of all the above vascular calcification inhibitors. The effects of simvastatin on OPG and OPN are due to its ‘pleiotropic’ properties. However, the reduction in serum fetuin-A levels may be explained by the lipid-lowering potential.
In consistency with previous studies, our study confirmed the relationship of serum levels of both OPG [16] and OPN [17] to CAD. Moreover, we observed a gradual increment in OPG and OPN levels across the number of narrowed vessels. One could have hypothesized that the additional differences between groups, like glucose regulation and smoking habits, would have confounded our results. However, the absence of any relationship of novel biomarkers with those baseline characteristics restricts that possibility. Our results are of clinical importance, since they implicate the prognostic power of the latter variables as biomarkers of CAD presence and severity [17]. Another notable finding of the present study was the considerable downregulation of serum OPG and OPN levels after 6-month simvastatin therapy. Up to now, there have been conflicting results about the effects of statins on OPG and OPN, probably because of the wide range of clinical characteristics of studies’ cohorts [4, 18–21]. In agreement with our results, the majority of previous publications have documented the suppressive effects of statins on those biomarkers. Several theories have proposed the inhibition of either mevalonate entrance into the cholesterol synthesis pathway [22] or NF-κB activation [23] as potential mechanisms of statins’ negative effects on vascular calcification inhibitors. Unfortunately, our study was not designed to investigate the underlying mechanisms. Therefore, the observed independent relationship between OPG and OPN changes did not yield any firm conclusion as to how statins may affect OPG and OPN homeostasis. Regarding statins as the mainstay of atherosclerotic-related disease therapy, large-scale, long-term studies will shed more light on the interplay between statins and the aforementioned CVD biomarkers, indicating novel angioprotective mechanisms.
At baseline patients with CAD exhibited lower serum levels of fetuin-A. Importantly, patients with 3-vessel disease showed even lower serum fetuin-A levels than in patients with 1-vessel disease. Our findings are consistent with those of studies documenting the inverse relationship between serum fetuin-A levels and CAD presence and severity [9, 10]. We must underline that we excluded participants with renal impairment (creatinine <2 mg/dL), while differences in baseline characteristics, like diabetes, seem not to influence the concentrations of biomarkers. The cardioprotective role of fetuin-A has also been supported by other investigators who demonstrated low circulating fetuin-A as a negative predictor of ACS evolution [24]. The latter investigators considered fetuin-A as an anti-inflammatory glycoprotein and they hypothesized that low fetuin-A concentrations reflect a severe inflammatory burden related to unfavourable outcomes after ACS. On the other hand, limited data reporting the paradoxical positive association between high fetuin-A levels and CVDs put in dispute the athero-protective role of fetuin-A [25–27]. In other words, it is still unknown whether fetuin-A is an exacerbating or a protective factor in CVDs.
Up to now, there have been limited data about the pharmaceutical modulation of fetuin-A. In particular, a very small study has previously reported a significant reduction of serum fetuin-A levels in non-diabetic, high-risk patients after combined treatment with simvastatin and pioglitazone [28]. Similar results were observed in ten diabetic patients after 6-month treatment with pioglitazone [29]. The latter result was predominantly ascribed to the improvement of the hepatocellular lipid content and the consequent insulin-sensitizing effect. However, the relationship of fetuin-A to insulin resistance is still a subject of debate [30, 31]. To our knowledge, this is the first study to demonstrate the suppressive effect of a statin on serum fetuin-A in patients with CAD. Furthermore, the latter effect could be ascribed to the lipid-lowering action of simvastatin. The role of the atherogenic profile (total cholesterol, LDL) in fetuin-A regulation has been also observed by other investigators [32]. Therefore, our results raise a question about the interplay between fetuin-A and CVDs through lipid modulation. One could hypothesize a dual functionality of fetuin-A, where it could act as an atherogenic factor or as an anti-inflammatory agent. In the former case, the statin-induced lowering of fetuin-A reflects the counterregulatory anti-inflammatory mechanism of statins. In the latter case, fetuin-A reduction may mirror the repression of the anti-inflammatory mechanisms, after the depletion of the inflammatory burden by statins. Moreover, the existence of confounding factors, such as diabetes, microvascular complications and distinct stages of atherosclerosis, may distort the association of fetuin-A with CVD. Taking into account the above discrepancy, low serum fetuin-A levels do not justify the rationale for its causative or bystander role in atherosclerosis.
Our study had several limitations. Firstly, we included a limited number of patients and, therefore, our results need to be confirmed by a larger study. Secondly, despite the usage of a maximal dose of simvastatin in almost all patients, a significant portion of them did not achieve the LDL target. Therefore, it cannot be ruled out that the effect of statin therapy might have been greater if we had administered high doses of other statins, like rosuvastatin or atorvastatin. Thirdly, we cannot rule out the influence of changes in several pharmaceutical agents during the study, like anti-platelets, anti-hypertensive medications, etc. On the other hand, the imbalance between conservatively treated and interventionally treated patients prevented us from detecting a potential contribution of revascularization to vascular calcification inhibitors. Our study, by design, did not include 6-month follow-up information for the HCG. Perhaps this might have improved the interpretation of our results. Finally, because of the short duration of treatment, the question of whether long-term treatment may more prominently affect serum vascular calcification inhibitors accompanied with lower clinical outcomes rate remains unresolved.
5 Conclusion
Patients with CAD showed elevated serum levels of both OPG and OPN, while serum fetuin-A levels were significantly lower than those in healthy controls. Simvastatin treatment considerably reduced all the above vascular calcification inhibitors. The underlying mechanisms and the clinical effectiveness of statins through the modification of vascular calcification inhibitors require further investigation.
References
Golledge J, McCann M, Mangan S, et al. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004;35:1636–41.
Kiechl S, Schett G, Wenning G, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–80.
Abdel-Azeez HA, Al-Zaky M. Plasma osteopontin as a predictor of coronary artery disease: association with echocardiographic characteristics of atherosclerosis. J Clin Lab Anal. 2010;24:201–6.
Kadoglou NPE, Gerasimidis T, Kapelouzou A, et al. Beneficial changes of serum calcification markers and contralateral carotid plaques echogenicity after combined carotid artery stenting plus intensive lipid-lowering therapy in patients with bilateral carotid stenosis. Eur J Vasc Endovasc Surg. 2010;39:258–65.
Kadoglou NPE, Sailer N, Moumtzouoglou A, et al. Aggressive lipid-lowering is more effective than moderate lipid-lowering in carotid plaque stabilization. J Vasc Surg. 2010;51:114–20.
Pateinakis P, Papagianni A, Douma S, et al. Associations of fetuin-A and osteoprotegerin with arterial stiffness and early atherosclerosis in chronic hemodialysis patients. BMC Nephrol. 2013;14:122.
Mehrotra R. Emerging role for fetuin-A as contributor to morbidity and mortality in chronic kidney disease. Kidney Int. 2007;72:137–40.
Chen YC, Lin FY, Lin RH, et al. Relation between fetuin-a levels and fibroblast growth factor 23 with the severity of coronary artery disease measured by SYNTAX Scores. Am J Cardiol. 2013;112:950–3.
Zhao ZW, Lin CG, Wu LZ, et al. Serum fetuin-A levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes. Biomarkers. 2013;18:160–4.
Lim P, Moutereau S, Simon T, et al. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am J Cardiol. 2013;111:31–7.
Ginsberg HN, Stalenhoef AF. The metabolic syndrome: targeting dyslipidaemia to reduce coronary risk. J Cardiovasc Risk. 2003;10:121–8.
Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–57.
Lyngdoh T, Vollenweider P, Waeber G, et al. Association of statins with inflammatory cytokines: a population-based Colaus study. Atherosclerosis. 2011;219:253–8.
Kadoglou NP, Vrabas IS, Kapelouzou A, et al. Impact of atorvastatin on serum vaspin levels in hypercholesterolemic patients with moderate cardiovascular risk. Regul Pept. 2011;170:57–61.
Resnick HE, Harris MI, Brock DB, et al. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2000;23:176–80.
Ghaffari S, Yaghoubi A, Baghernejad R, et al. The value of serum osteoprotegerin levels in patients with angina like chest pain undergoing diagnostic coronary angiography. Cardiol J. 2013;20:261–7.
Tousoulis D, Siasos G, Maniatis K, Oikonomou E, Kioufis S, Zaromitidou M, Paraskevopoulos T, Michalea S, Kollia C, Miliou A, Kokkou E, Papavassiliou AG, Stefanadis C. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int J Cardiol. 2013;165:1924–8.
Mori K, Jono S, Emoto M, et al. Effects of pravastatin on serum osteoprotegerin levels in patients with hypercholesterolemia and type 2 diabetes. Angiology. 2010;61:86–91.
Tanaka N, Momiyama Y, Ohmori R, et al. Effect of atorvastatin on plasma osteopontin levels in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:e129–30.
Lazzerini PE, Capperucci C, Spreafico A, et al. Rosuvastatin inhibits spontaneous and IL-1β-induced interleukin-6 production from human cultured osteoblastic cells. Jt Bone Spine. 2013;80:195–200.
Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007;282:4983–93.
Kim JY, Lee EY, Lee EB, et al. Atorvastatin inhibits osteoclastogenesis by decreasing the expression of RANKL in the synoviocytes of rheumatoid arthritis. Arthritis Res Ther. 2012;14:R187.
Zhang J, Xu Y, Pan L, Chen T, et al. Effect of simvastatin on collagen I deposition in non-infarcted myocardium: role of NF-κB and osteopontin. Can J Physiol Pharmacol. 2010;88:1026–34.
Afsar CU, Uzun H, Yurdakul S, et al. Association of serum fetuin-A levels with heart valve calcification and other biomarkers of inflammation among persons with acute coronary syndrome. Clin Invest Med. 2012;35:E206–15.
Ix JH, Katz R, de Boer IH, et al. Fetuin-A is inversely associated with coronary artery calcification in community-living persons: the Multi-Ethnic Study of Atherosclerosis. Clin Chem. 2012;58:887–95.
Fisher E, Stefan N, Saar K, et al. Association of AHSG gene polymorphisms with fetuin-A plasma levels and cardiovascular diseases in the EPIC-Potsdam study. Circ Cardiovasc Genet. 2009;2:607–13.
Weikert C, Stefan N, Schulze MB, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–62.
Hanefeld M, Schaper F, Appelt D, et al. Effects of pioglitazone versus simvastatin on biomarkers of inflammation in patients on high cardiovascular risk. Horm Metab Res. 2011;43:980–3.
Mori K, Emoto M, Araki T, et al. Effects of pioglitazone on serum fetuin-A levels in patients with type 2 diabetes mellitus. Metabolism. 2008;57:1248–52.
Mori K, Emoto M, Yokoyama H, Araki T, et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care. 2006;29:468.
Himmetoglu S, Teksoz S, Zengin K, et al. Serum levels of fetuin a and 8-hydroxydeoxyguanosine in morbidly obese subjects. Exp Clin Endocrinol Diabetes. 2013;121:505–8.
Ishibashi A, Ikeda Y, Ohguro T, Kumon Y, Yamanaka S, Takata H, Inoue M, Suehiro T, Terada Y. Serum fetuin-A is an independent marker of insulin resistance in Japanese men. J Atheroscler Thromb. 2010;17:925–33.
Acknowledgments
This study was co-funded by the Operational Program ‘Competitiveness and Entrepreneurship’ and Regional Operational Programmes of the National Strategic Reference Framework (NSRF) 2007–2013 ‘SYNERGASIA’: ‘Collaborative Projects of Small and Medium Scale’.
Conflicts of interest
None of the authors has any potential conflicts of interest in relation to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT00306176.
Rights and permissions
About this article
Cite this article
Kadoglou, N.P.E., Kottas, G., Lampropoulos, S. et al. Serum Levels of Fetuin-A, Osteoprotegerin and Osteopontin in Patients with Coronary Artery Disease: Effects of Statin (HMGCoA-Reductase Inhibitor) Therapy. Clin Drug Investig 34, 165–171 (2014). https://doi.org/10.1007/s40261-013-0157-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0157-y