Abstract
Background and Objectives
Systemic lupus erythematosus (SLE) is a multisystem complex autoimmune disease that often mimics symptoms of other illnesses, which complicates the ability of healthcare providers to make the diagnosis. The objective of this study was to assess clinical outcomes, resource utilization, and costs between patients with earlier versus later SLE diagnosis.
Methods
Patients aged 18–64 years were identified from a large US commercial claims database between January 2000 and June 2010. Confirmed SLE diagnosis with a claims-based algorithm required either three or more claims for a visit to a rheumatologist on separate dates with an SLE diagnosis (International Classification of Diseases [ICD-9] code 710.0x), two or more claims for visits to a rheumatologist at least 60 days apart with SLE diagnoses, or two or more claims for visits to rheumatologist less than 60 days apart with SLE diagnoses with at least one dispensing for a typical SLE medication. SLE probable onset date was identified during the 12-month baseline period by the second claim for antinuclear antibody tests or prodromal symptoms of SLE. Patients were stratified into early or late diagnosis groups based on time between probable SLE onset and diagnosis (<6 months or ≥6 months, respectively). Each patient observation period began on the date of the first medical claim, with a diagnosis code for SLE that satisfied the inclusion criteria, and ended on the earliest date between health plan disenrollment and 30 June 2010. Patients in each group were propensity-score matched on age, gender, diagnosis year, region, health plan type, and comorbidities. Flare rates and resource utilization were compared post-diagnosis between groups using rate ratios. All-cause and SLE-related costs (adjusted to 2010 US dollars) per patient per month (PPPM) were calculated.
Results
There were 4,166 matched patients per group. Post-SLE diagnosis, the early diagnosis group had lower rates of mild (rate ratio [RR] 0.95; 95 % CI 0.93–0.96), moderate (RR 0.96; 95 % CI 0.94–0.99), and severe (RR 0.87; 95 % CI 0.82–0.93) flares compared with the late diagnosis group. The rates of hospitalizations (RR 0.80; 95 % CI 0.75–0.85) were lower for the early diagnosis group than the late diagnosis group. Compared with late diagnosis patients, mean all-cause inpatient costs PPPM were lower for the early diagnosis patients (US$406 vs. US$486; p = 0.016). Corresponding SLE-related hospitalization costs were also lower for early compared with late diagnosis patients (US$71 vs. US$95; p = 0.013). Results were consistent for other resource use and cost categories.
Conclusions
Patients diagnosed with SLE sooner may experience lower flare rates, less healthcare utilization, and lower costs from a commercially insured population perspective. This finding needs to be further explored within the context of background SLE disease activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients in the early systemic lupus erythematosus (SLE) diagnosis cohort had significantly lower rates of flares compared with patients in the late SLE diagnosis cohort. |
All-cause and SLE-related healthcare resource utilization and costs were significantly lower among patients in the early SLE diagnosis cohort compared with patients in the late SLE diagnosis cohort. |
The lower all-cause and SLE-related resource utilization and healthcare costs among patients in the early versus late SLE diagnosis cohorts was more evident over time (i.e. after ≥3 months post-SLE diagnosis). |
1 Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease that can damage several parts of the body, including skin, joints, and/or organs. Symptoms of the disease vary across individuals, affecting different parts of the body and flaring over time. Patients with SLE develop various immunological abnormalities and permanent organ damage [1]. SLE can be difficult to diagnose due to its multisystem involvement and lack of a single diagnostic test [2]. Serological tests for levels of various antibodies may assist in the diagnosis of SLE but are not individually conclusive. Adding to the complication of SLE diagnosis, other diseases can be confused with SLE, particularly in the early stages, due to similar physical or laboratory presentation [1]. These other diseases include undifferentiated connective tissue disease, primary Sjogren’s syndrome, primary antiphospholipid syndrome, fibromyalgia with positive antinuclear antibody (ANA), idiopathic thrombocytopenic purpura, drug-induced lupus, early rheumatoid arthritis, arthralgia, and Raynaud’s phenomenon [3].
The American College of Rheumatology (ACR) first outlined the criteria for classification of SLE in 1971, with revised versions in 1982 and 1997. Even with the introduction of ANA, anti-double stranded DNA (anti-dsDNA), and anti-Sm antibodies in 1982, the criteria only serve as a guideline in clinical practice as they lack the sensitivity and specificity for an accurate and timely diagnosis which may result in delay of initiation of appropriate medical therapy [3]. Using the 11 criteria set forth by the ACR, an SLE diagnosis is 95 % specific and 85 % sensitive if a patient fulfills four of the criteria in his or her medical history [1].
The complexity and uncertainty in definitively diagnosing SLE may result in considerable delay between the initial manifestations of disease, the establishment of a diagnosis, and the initiation of appropriate medical therapy. Although the time between onset of symptoms and diagnosis has decreased over the years [3–6], the delay or lack of treatment may increase the likelihood of organ damage due to persistent inflammatory disease activity. Thus, for patients who receive a diagnosis earlier after disease onset, inflammatory disease may be treated sooner and organ damage could potentially be minimized.
The average annual healthcare costs for patients with SLE have been estimated to range between US$12,000 and US$24,000 [7–12]. A recent study showed that the medical costs of the first year for patients newly diagnosed with SLE were US$19,178, while corresponding costs were estimated at US$15,487 for patients with existing SLE [12]. The economic burden of SLE is considerable and has been described in the literature; however, no study has evaluated the impact of the timing of SLE diagnosis on healthcare costs.
In light of these observations, and to address the question of whether earlier versus later diagnosis of SLE is associated with better clinical outcomes and lower healthcare resource utilization and costs, this study compared relevant clinical, resource use, and economic endpoints for two cohorts of patients—those with less than 6 months between manifestation of SLE symptoms and diagnosis, and those with 6–12 months between the onset of SLE symptoms and diagnosis.
2 Patients and Methods
2.1 Data Source
Health insurance claims from the Thomson Reuters MarketScan database were used to conduct the analysis. The MarketScan database combines two separate databases (i.e. the Commercial Claims and Encounters database and the Medicare Supplemental and Coordination of Benefits database) to cover all age groups, and contains claims from approximately 100 employers, health plans, and government and public organizations representing about 30 million covered lives in the US. All census regions are represented, but the South and North Central (Midwest) regions are predominant. The MarketScan data used in the current analysis covered the period from January 2000 to June 2010.
Data for the present study included health plan enrollment records, participant demographics, inpatient and outpatient medical services, and outpatient prescription drug dispensing records. Finally, the data included in the MarketScan database are de-identified and are in compliance with the Health Insurance Portability and Accountability Act of 1996 to preserve participant anonymity and confidentiality.
2.2 Study Design
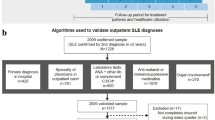
A retrospective longitudinal matched-cohort design was employed (Fig. 1). Each patient observation period began on the date of the first medical claim with a diagnosis code for SLE (index date) that satisfied the inclusion criteria and ended on the earliest date between health plan disenrollment and end of data availability (30 June 2010).
To be included in the study sample, the patients were required to meet the following criteria: (1) at least three claims for a visit to a rheumatologist on separate dates with an SLE diagnosis (International Classification of Diseases, Ninth Revision [ICD-9] code 710.0x), or at least two claims for a visit to a rheumatologist at least 60 days apart with an SLE diagnosis, or at least two claims for a visit to a rheumatologist on separate dates less than 60 days apart with an SLE diagnosis and at least one dispensing for lupus treatment medication including corticosteroids, antimalarials, immunosuppressants or cytotoxics, NSAIDs, and androgens; (2) be 18–64 years of age at the index date; and (3) have continuous health plan enrollment in the 12 months prior to the index date (baseline period). The use of this claims-based algorithm for the identification of patients with SLE has not been validated; however, a study has demonstrated that claims-based algorithms could identify patients with lupus nephritis with a very high predictive value using at least two claims for a visit to a specialist with an SLE diagnosis. Although the study focused primarily on lupus nephritis, the authors were also able to identify SLE patients with a very high predictive value using this algorithm [13].
SLE onset date was determined as the second diagnosis code for prodromal symptoms of SLE (e.g. malar rash, photosensitivity, non-erosive arthritis, pleuritis or pericarditis, and hematologic disorders) given by ACR guidelines or a claim for an ANA test [1]. The onset of SLE could have occured at any time during the 12-month baseline prior to the first SLE diagnosis. Diagnosis lag time was calculated as the time interval between SLE onset date and the first SLE diagnosis date. The diagnosis lag time was then used to define the cohort of patients with late SLE diagnosis and early SLE diagnosis.
Based on the literature reviewed by the authors during the study protocol development prior to conducting the study analyses, the early diagnosis of SLE cohort included patients with no more than 6 months between the SLE onset date and SLE diagnosis date, while the late diagnosis of SLE cohort comprised patients with 6–12 months between the SLE onset date and SLE diagnosis date. Data on the effect of early intervention for SLE are limited. In animal models, early intervention of treatment compared with treatment initiation after full symptoms of SLE are apparent has been more effective [14]. Among humans with SLE, a delay in renal biopsy (which is often followed by glomerulonephritis treatment) of more than 6 months has been associated with the development of adverse renal outcomes [15]. The mean time from onset to diagnosis has been reported to be 9 months for those diagnosed after 2000; [3] however, the authors emphasize that greater effort should be made to identify new biomarkers that would enable us to diagnose SLE sooner. Given the urge to diagnose SLE earlier and the limited data available, we used a 6-month cutoff to define early and delayed diagnosis.
2.3 Outcome Measures
All-cause and SLE-related hospitalizations, outpatient visits, emergency room (ER) visits, and corresponding costs along with SLE-related drug costs were analyzed to describe and compare resource utilization and healthcare costs between the late and early diagnosis groups. SLE-related costs were defined as hospitalizations and outpatient claims with a primary or secondary diagnosis for SLE. The costs represented the total gross payments to a provider, including deductibles, copayments, and coordination of benefits. Resource utilization and cost results were stratified at ≤3 months versus >3 months post-SLE diagnosis. The stratified analyses were conducted to distinguish between the different healthcare resource utilization and costs immediately following the SLE diagnosis and those following longer-term disease management.
SLE flare rates were also calculated after classifying flares by severity (mild, moderate, or severe) [16]. A mild flare was identified by initiation of (1) hydroxychloroquine or other antimalarial; (2) an oral corticosteroid with a prednisone-equivalent dose of ≤7.5 mg/day; or (3) non-immunosuppressive therapy (e.g. non-steroidal anti-inflammatory drugs [NSAIDs], androgens). Flares of moderate severity were identified by (1) initiation of an oral corticosteroid with a prednisone-equivalent dose of >7.5 mg/day and ≤40 mg/day; (2) initiation of immunosuppressive therapy (excluding cyclophosphamide); (3) an ER visit with primary diagnosis of SLE or for an SLE-related condition; or (4) an office visit for a new SLE-related condition, defined as no claim at baseline for that condition. Severe flares were identified by (1) initiation of an oral corticosteroid with a prednisone-equivalent dose of >40 mg/day; (2) initiation of cyclophosphamide; or (3) a hospital stay with a primary diagnosis of SLE or an SLE-related condition. A flare was assumed to last 30 days.
2.4 Statistical Analyses
The lack of randomization in such an observational study may lead to confounding across the early and the late diagnosis groups. The goal was to assemble a population, in which those with early SLE would be demographically similar to those with late SLE in addition to having similar co-morbidities that are not on the pathway of SLE (i.e. excluding SLE-related conditions and the level of SLE severity) at baseline. To ensure balanced subject characteristics, patients with early SLE diagnosis were matched 1:1 with patients with late SLE diagnosis based on propensity score calipers of 5 % and an exact matching on diabetes. The propensity score was generated using probability estimates from a logistic regression model including the following baseline characteristics: age, gender, year of SLE diagnosis, geographic region, health plan type, and the Charlson Comorbidity Index [17] (CCI) excluding SLE-related conditions based on the literature (see electronic supplementary material). The SLE-related conditions that were excluded from the CCI are cardiovascular diseases (i.e. myocardial infarction, congestive heart failure, peripheral vascular disease, and cerebrovascular disease), chronic pulmonary disease, rheumatic disease, and renal diseases [18–25]. The conditions that were included in the CCI are dementia, peptic ulcer disease, mild liver disease, moderate or severe liver disease, diabetes without chronic complications, diabetes with chronic complications, hemiplegia or paraplegia, any malignancy, metastatic solid tumor, and AIDS/HIV.
Demographic characteristics, SLE severity (mild, moderate, or severe; see Appendix for a description of the SLE Disease Severity Algorithm), the CCI (excluding SLE-related conditions) and its individual conditions separated in those included in the CCI and the SLE-related conditions not included, and other comorbidities during the baseline period were described and compared for patients with early and late SLE diagnosis. The mean and standard deviation (SD) were reported for continuous data and frequencies and proportions for categorical data. For comparison of variables between matched cohorts, baseline continuous variables were compared using paired t-tests, whereas baseline categorical variables were compared using the Pearson’s Chi-square test or the McNemar test. A two-sided p-value less than 0.05 was used to declare statistical significance.
Rate ratios (RRs), the ratio of the rate for the early diagnosis cohort divided by the rate for the late diagnosis cohort, were used to compare flare rates and all-cause and SLE-related resource utilization. The RRs were modeled using conditional Poisson regression models accounting for matched pairs and different lengths of observation among patients. To assess statistical significance relative to the null value of 1, 95 % confidence intervals (CIs) were calculated.
Costs were reported in 2010 US dollars on a per patient per month (PPPM) basis to adjust for the different lengths of follow-up among patients. The PPPM cost was calculated by dividing the costs incurred over the observation period by the person-time observed for each patient. Because costs are positive values that follow a non-normal distribution and also often have zero values, non-parametric methods were used to assess statistical significance: a permutation test with 1,000 replications was used to test the statistical significance of cost differences between the cohorts relative to the null value of 0.
To remove the potential impact of SLE patients who had a medium duration from onset to diagnosis, a sensitivity was also conducted for the cost analysis where we defined the early cohort as patients who had their onset in the 4 months before the index date, and the late cohort as patients who had their onset between months 8 and 12 prior to the index date.
All statistical analyses were performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Study Population
Figure 2 depicts the number of patients in the database eligible for inclusion in the study. There were 139,504 patients with at least one SLE diagnosis; among these, 29,973 had a confirmed diagnosis according to the criteria outlined in the Methods section. After further restriction of the population to patients who were aged 18–64 years at the time of diagnosis, without capitation-based health plans, and with at least 12 months of continuous baseline eligibility, 9,713 patients remained in the study population. The algorithm to determine SLE onset date resulted in approximately half of the patients being categorized in the early diagnosis cohort and half in the late diagnosis cohort. Among the 4,819 SLE patients in the early diagnosis cohort and 4,894 patients in the late diagnosis cohort, a total of 4,166 (86 %) early patients were matched with 4,166 late patients.
The characteristics of the matched cohorts are summarized in Table 1. The mean (median) duration between onset and the first SLE diagnosis date was 59 (40) days for the early group and 282 (288) days for the late group. The mean (median) observation period was 838 (635) days for the early group and 866 (671) days for the late SLE group. The mean ages of the matched cohorts were 45 years. Propensity score matching, based on age, gender, year of SLE diagnosis, geographical region, health plan type, and the CCI (excluding SLE-related complications) resulted in non-significant differences between the cohorts for these factors. Patients in the early diagnosis cohort were more likely to have mild SLE during the baseline period compared with patients in the late diagnosis cohort (64.9 vs. 55.3 %; p < 0.001).
3.2 Rate of Flares
Table 2 reports the rate of SLE flares. Patients in the early and late diagnosis cohort had 3.57 and 3.75 SLE flares of any severity per person-year, respectively, during the post-SLE diagnosis period. Patients in the early diagnosis cohort had significantly lower rates of flares compared with patients in the late diagnosis cohort [RRs, any severity: 0.95 (95 % CI 0.94–0.97); mild severity: 0.95 (95 % CI 0.93–0.96); moderate severity: 0.96 (95 % CI 0.94–0.99); severe: 0.87 (95 % CI 0.82–0.93)].
3.3 Healthcare Resource Utilization and Costs
Table 3 reports the healthcare resource utilization for matched SLE patients by timing of diagnosis. All-cause healthcare resource utilization was significantly lower among patients in the early diagnosis cohort. The RR for all-cause utilization for the early versus late diagnosis cohorts was 0.80 (95 % CI 0.75–0.85) for hospitalizations, 0.80 (95 % CI 0.79–0.80) for outpatient visits, and 0.76 (95 % CI 0.74–0.79) for ER visits. Similarly, SLE-related healthcare resource utilization was significantly lower among patients in the early diagnosis cohort. The RR for SLE-related utilization was 0.75 (95 % CI 0.68–0.82) for hospitalizations, 0.89 (95 % CI 0.88–0.90) for outpatient visits, and 0.67 (95 % CI 0.61–0.74) for ER visits. The lower all-cause and SLE-related hospitalization rates among patients in the early versus late diagnosis cohorts was more evident over time: at ≤3 months, all-cause hospitalization RR 0.99 (95 % CI 0.88–1.13) and SLE-related hospitalization RR 1.00 (95 % CI 0.85–1.17); >3 months, all-cause hospitalization RR 0.76 (95 % CI 0.70–0.81) and SLE-related hospitalization RR 0.66 (95 % CI 0.59–0.75).
Healthcare costs were significantly lower for the early compared with the late cohort (Table 4). Mean all-cause hospitalization costs PPPM were lower for the early diagnosis cohort than for the late diagnosis cohort (US$406 vs. US$486; p = 0.016). Corresponding results were similar for SLE-related hospitalization (US$71 vs. US$95; p = 0.013). Results were consistent for other cost categories. The mean (SD) number of days per all-cause hospitalization and SLE-related hospitalization during follow-up did not differ significantly between the early and late diagnosis cohorts (5.8 [5] vs. 5.4 [5], p = 0.973; and 5.9 [5] vs. 5.5 [6], p = 0.831, respectively). Results from the stratified cost analysis showed that all-cause hospitalization and SLE-related costs were not significantly different between cohorts in the first 3 months following SLE diagnosis, but were significantly lower in the early diagnosis cohort (except ER visits; p = 0.110) during the remaining period of follow-up.
For the sensitivity analysis where patients in the early SLE diagnosis cohort had their onset in the 4 months before the index date and the late SLE diagnoses cohort had their onset between months 8 and 12 prior to the index date (N = 3,132 patients in each cohort), the cost difference was increased compared with the main analysis. During the overall follow-up, mean all-cause hospitalization costs PPPM were significantly lower for the early diagnosis cohort compared with the late diagnosis cohort (US$396 vs. US$560; p = 0.001). Corresponding results were also significantly lower for SLE-related hospitalization (US$72 vs. US$106; p = 0.012). The cost differences were also increased for the other cost categories.
4 Discussion
This study, utilizing claims data from 8,332 patients with SLE diagnosis, was undertaken to characterize patients with short and long durations between the onset of SLE manifestation characteristics and SLE diagnosis, and to compare rates of SLE flares, healthcare resource utilization, and costs between these two cohorts of patients from a commercially insured population perspective. The algorithm to identify the SLE onset date within the claims was guided by ACR guidelines and clinical input over the course of the study. The main findings of this study indicate that early SLE diagnosis compared with late SLE diagnosis is associated with lower rates of SLE flares, healthcare resource utilization, and costs. SLE flare rates of any severity level are significantly lower among patients with a shorter time between SLE onset and SLE diagnosis. Both all-cause and SLE-related healthcare resource utilization for hospitalizations, outpatient visits, and ER visits is lower among patients with an early diagnosis of SLE. There are similar associations for healthcare cost results.
Early diagnosis may be associated with lower rates of flares, healthcare resource utilization, and costs because patients who are diagnosed early may be monitored and treated at an earlier timepoint in their disease course compared with patients with a late diagnosis. Earlier monitoring and treatment could potentially result in better health outcomes. Data on the effect of early intervention for SLE are limited, but some studies suggest that earlier treatment may yield improved outcomes. Corticosteroids and immunosuppressants are known to induce disease remission in most SLE cases [26], so detecting disease early may afford more time for introducing appropriate treatment and controlling disease. In a study of lupus nephritis, earlier detection of renal disease was associated with earlier treatment with prednisone and immunosuppressive agents and improved long-term prognosis [27]. Animal models have shown that successful treatments are most effective when introduced prior to the development of full symptoms of SLE [3].
Autoantibodies, such as ANA, may appear years before the clinical onset and diagnosis of SLE. These autoantibodies may induce tissue damage by way of generating deposits of immune material which can induce an inflammatory process. Clinical and immunological abnormalities can occur during this subclinical phase. Although the detection of ANA does not correlate completely with the onset of SLE, the more recent ability to detect ANA has aided the SLE diagnostic process in recent years and hence reduced the lag time between the onset and diagnosis of SLE over the years. Among patients diagnosed with SLE prior to 1980, the mean time between onset and diagnosis was 59 months. This mean time decreased to 28 months for patients diagnosed between 1980 and 1989, 15 months among patients diagnosed between 1990 and 1999, and 9 months for those diagnosed after 2000 [3]. Differences in delay in diagnosis before and after 1980 have been credited to ANA testing. The authors suggest that the average lag time of 9 months after 2000 is not soon enough and that great effort should be made to diagnose SLE earlier [3]. The current study concurs with the literature that the lag time should be reduced further since we have found significant difference in flares, resource use, and costs among patients with a 6- to 12-month lag compared with those with a lag of less than 6 months.
Inflammation caused by lupus can affect many areas of the body. Lupus can cause inflammation of the heart muscle, the arteries, and the heart membrane, and greatly increase the risk of heart attacks, stroke, and other cardiovascular diseases [18, 19]. Pulmonary vascular disease is also a common problem in patients with SLE [20]. Pulmonary complications of SLE are protean and include acute lupus pneumonitis, diaphragmatic dysfunction and shrinking lung syndrome, cavitating pulmonary nodules, pulmonary hypertension, pulmonary vasculitis, pulmonary embolism, alveolar hemorrhage, chronic interstitial pneumonitis, bronchiolitis obliterans, and opportunistic pulmonary infections or drug toxicity from immunosuppressive therapy [21]. Lupus can also cause serious damage to the kidneys [18]. Since these potential complications of SLE (cardiovascular diseases [i.e. myocardial infarction, congestive heart failure, peripheral vascular disease, and cerebrovascular disease], chronic pulmonary disease, rheumatic disease, and renal diseases) could be the result of a delayed SLE diagnosis, and therefore should not be adjusted, they were excluded from the CCI that was used to match the early and the late diagnosis cohorts in the current study. Other conditions related to SLE have also been identified in the literature, such as osteoporosis. Studies have identified low bone mineral density, which characterizes osteoporosis, in SLE patients when compared with age-matched controls, and the results of studies suggest a generalized reduction in bone mineral density that is evident in early SLE disease [22]. Hypothyroidism and hypercholesterolemia have also been described in the literature as potential complications of SLE [23–25].
The course of SLE is marked by periods of remission and exacerbation of symptoms during which organs can be irreversibly damaged [28]; hence, detecting disease and reducing the episodes of exacerbation by existing and new treatment modalities could improve survival. Results from prior studies on survival advantages following the introduction of therapy corroborate with those in the current study and support the idea that early detection may yield better clinical outcomes. The current study adds to this knowledge by demonstrating that earlier diagnosis may also be associated with better outcomes and lower healthcare costs. Of note, there are risks involved in early diagnosis or patients starting expensive and inconvenient therapies. Early diagnosis without concrete symptom validation may result in misdiagnosis. Newer treatments associated with greater retardation of lupus disease progression, fewer flares and relapses, less toxicity, and improved patient quality of life may provide a more favorable benefit over risk profile. In addition, these results should be explored in the context of background SLE disease activity.
This study has several limitations that should be considered. The claims-based algorithm used to identify SLE patients has not been validated. In an attempt to identify SLE patients, we have required SLE patients to have several visits to a rheumatologist with SLE diagnoses, and in some cases to be treated with lupus medication. A recent article published the identification and validation of lupus nephritis and SLE patients with a similar claims-based algorithm which had a very high predictive value, which suggests, although it cannot be confirmed, that our study also identified SLE patients. Also, claims databases may contain inaccuracies or omissions in coded procedures, diagnoses, or pharmacy claims; however, it would be unlikely that these have significantly impacted our results considering the large sample size. A general limitation of propensity score is that it can only account for observable factors. Despite these limitations, well-designed observational studies with appropriate statistical techniques adjusting for potential confounding factors through matching techniques provide valuable information with real-life scenarios and high generalizability.
5 Conclusions
Based on data from this large US commercially insured population, early diagnosis of SLE is associated with better clinical outcomes and reduced resource utilization and healthcare costs. Future studies should explore the risk-benefit analysis of earlier diagnosis and earlier institution of therapy.
References
Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 1999;42(9):1785–96.
How is lupus diagnoses. Lupus Foundation of America, Inc. http://www.lupus.org/answers/entry/diagnosing-lupus. Accessed 17 Dec 2013.
Doria A, Zen M, Canova M, Bettio S, Bassi N, Nalotto L, et al. SLE diagnosis and treatment: when early is early. Autoimmun Rev. 2010;10(1):55–60 Epub 2010 Sep 8.
Wallace DJ, Podell T, Weiner J, Klinenberg JR, Forouzesh S, Dubois EL. Systemic lupus erythematosus: survival patterns. Experience with 609 patients. JAMA. 1981;245:934–8.
Cervera R, Khamashta MA, Font J, et al. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine. 1993;72:113–24.
Ozbek S, Sert M, Paydas S, Soy M. Delay in the diagnosis of SLE: the importance of arthritis/arthralgia as the initial symptom. Acta Med Okayama. 2003;57(4):187–90.
Li T, Carls GS, Panopalis P, et al. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large Medicaid population. Arthritis Rheum. 2009;61:755–63.
Panopalis P, Yazdany J, Gillis JZ, et al. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum. 2008;59:1788–95.
Carls G, Li T, Panopalis P, et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med. 2009;51:66–79.
Yelin E, Trupin L, Katz P, et al. Impact of health maintenance organizations and fee-for-service on health care utilization among people with systemic lupus erythematosus. Arthritis Rheum. 2007;57:508–15.
Pelletier EM, Ogale S, Yu E, et al. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin Ther. 2009;31:2653–64.
Furst DE, Clarke A, Fernandes AW, et al. Resource utilization and direct medical costs in adult systemic lupus erythematosus patients from a commercially insured population. Lupus. 2013;22(3):268–78.
Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19(6):741–3.
Hahn B, Singh R. Animal models of SLE. In: Wallace DJ, Hahn BH, editors. Dubois’ Lupus Erythematosus 7th ed. 2007; p. 299–355.
Faurschou M, Starklint H, Halberg P, Jacobsen S. Prognostic factors in lupus nephritis: diagnostic and therapeutic delay increases the risk of terminal renal failure. J Rheumatol. 2006;33(8):1563–9.
Garris CP, Jhingran PM, Engel-Nitz NM, et al. Assessing systemic lupus erythematosus disease severity and disease flares: development of a claims-based algorithm. Arthritis Rheum. 2010;62 Suppl:757.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
Mayo Clinic. Lupus complications. http://www.mayoclinic.com/health/lupus/DS00115/DSECTION=complications. Accessed 17 Dec 2013.
Alliance for Lupus Research. Cardiovascular disease and lupus. http://www.lupusresearch.org/research/acr/cardiovascular.html. Accessed 17 Dec 2013.
Bhatt SP, Handa R, Gulati GS, et al. Peripheral vascular disease in systemic lupus erythematosus. Lupus. 2007;16:720–3.
Keane MP, Lynch JP 3rd. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax. 2000;55:159–66.
Sen D, Keen RW. Osteoporosis in systemic lupus erythematosus: prevention and treatment. Lupus. 2001;10:227–32.
Appenzeller S, Pallone AT, Natalin RA, Costallat LT. Prevalence of thyroid dysfunction in systemic lupus erythematosus. J Clin Rheumatol. 2009;15:117–9.
Pyne D, Isenberg DA. Autoimmune thyroid disease in systemic lupus erythematosus. Ann Rheum Dis. 2002;61:70–2.
Bruce IN, Urowitz MB, Gladman DD, Hallett DC. Natural history of hypercholesterolemia in systemic lupus erythematosus. J Rheumatol. 1999;26:2137–43.
Doria A, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med. 2006;119:700–6.
Esdaile JM, Joseph L, MacKenzie T, Kashgarian M, Hayslett JP. The benefit of early treatment with immunosuppressive agents in lupus nephritis. J Rhematol. 1994;21(11):2046–51.
Doria A, Rinaldi S, Ermani M, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. II: Role of clinical, immunological and psychological determinants. Rheumatology. 2004;43:1580–6.
Acknowledgments
This study was funded by GlaxoSmithKline (GSK), Collegeville, PA, USA. The funding from GSK was not contingent upon the study results. GSK and Human Genome Sciences (HGS) participated in the study design, results interpretation, and manuscript preparation, as reflected in the authorship by GSK and HGS employees, Alan Oglesby and Gregory Dennis, respectively. A human monoclonal antibody, belimumab (Benlysta), was developed by GSK and HGS, and was recently approved for SLE treatment in the US, Canada, and Europe. Caroline Korves, François Laliberté, Sapna Rao, and Mei Sheng Duh are employees of Analysis Group, Inc., which received a research grant from GSK for this study. Ellison Dial Suthoff and Robert Wei were employees of Analysis Group, Inc. at the time this study was conducted.
Parts of the manuscript were presented as posters at the Annual European League Against Rheumatism (EULAR) Congress, Berlin, Germany, 6–9 June 2012, and at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 17th Annual International Meeting, Washington, DC, USA, 2–6 June 2012.
Author Contributions
Study concept and design and data interpretation were primarily the work of Alan Oglesby, Mei Sheng Duh, François Laliberté, and Gregory Dennis, with assistance from the other authors. Caroline Korves, Sapna Rao, Ellison Dial Suthoff, and Robert Wei performed the data collection. Writing of the manuscript was shared by Caroline Korves, François Laliberté, and Mei Sheng Duh. Revision of the manuscript was shared by Alan Oglesby, Mei Sheng Duh, and Gregory Dennis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Systemic lupus erythematosus severity (as defined by Garris et al. [16])a
Moderate | Moderate Rx or moderate medical condition |
|---|---|
Rx | Oral corticosteroid dose ≥7.5 mg/day to <60 mg/day |
Immunosuppressive agent (excluding cyclophosphamide) | |
Medical condition | Cardiorespiratory: myocarditis, pericarditis, pleurisy/pleural effusion, vasculitis (excluding aortitis) |
Constitutional: hepatitis (non-viral) | |
Gastrointestinal: acute pancreatitis, lupus enterititis/colitis | |
Hematology: hemolytic anemia | |
Musculoskeletal: ischemic necrosis of bone | |
Neuropsychiatric: demyelinating syndrome/acute inflammatory demyelinating polyradiculoneuropathy, mononeuropathy/polyneuropathy, myelopathy, pseudotumor cerebri, seizure |
High | Intensive Rx or severe medical condition |
|---|---|
Rx | Oral corticosteroid dose ≥60 mg/day |
Cyclophosphamide | |
Rituximab | |
Medical condition | Cardiorespiratory: aortitis, arterial/ venous thrombosis, cardiac tamponade, pulmonary hemorrhage, stroke/transient ischemic attack |
Gastrointestinal: intestinal pseudo-obstruction | |
Neuropsychiatric: acute confusional state/psychosis, aseptic meningitis, cranial neuropathy | |
Ophthalmic: optic neuritis | |
Renal: end stage renal disease |
Rights and permissions
About this article
Cite this article
Oglesby, A., Korves, C., Laliberté, F. et al. Impact of Early Versus Late Systemic Lupus Erythematosus Diagnosis on Clinical and Economic Outcomes. Appl Health Econ Health Policy 12, 179–190 (2014). https://doi.org/10.1007/s40258-014-0085-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-014-0085-x