Abstract
Despite the widespread use of statins in the setting of high cardiovascular risk, many patients continue to experience clinical events. This highlights the need to identify additional therapeutic strategies for high-risk patients. Interest in the use of omega-3 polyunsaturated fatty acids to prevent cardiovascular disease has been high for several decades. Despite promising results from before the statin era, many clinical trials have produced disappointing findings regarding products containing conventional doses of omega-3 fatty acids. More recent clinical trials using high doses of omega-3 fatty acids in targeted populations have suggested potential benefit when targeting the risk driven by atherogenic dyslipidemia. We review the clinical implications of completed and ongoing trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
High dose EPA reduced cardiovascular events in the REDUCE-IT trial. |
This provides an opportunity to target high triglycerides in cardiovascular prevention. |
The ongoing STRENGTH trial (NCT02104817) is testing the benefit of triglyceride lowering with omega-3 carboxylic acid (eicosapentaenoic acid 75%, docosahexaenoic acid 25%). |
1 Introduction
Over the last three decades, randomized controlled trials have consistently demonstrated that lowering levels of low-density lipoprotein cholesterol (LDL-C) reduces cardiovascular event rates in primary and secondary prevention [1]. Early findings with statin agents have been extended to the use of high-intensity statins, ezetimibe [2], and proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors [3, 4], all demonstrating a direct relationship between incremental LDL-C lowering and cardiovascular benefit. As a result, treatment guidelines have increasingly emphasized the importance of intensive lipid lowering in approaches to cardiovascular prevention. However, despite widespread use of these agents, many cardiovascular events continue to occur [5]. This suggests an ongoing need to develop additional therapeutic strategies to further reduce cardiovascular risk.
2 Atherogenic Dyslipidemia and Cardiovascular Risk
Increasing evidence implicates a range of additional lipid and lipoprotein parameters, beyond LDL-C, in atherosclerosis and cardiovascular risk. Population studies demonstrate that both fasting and non-fasting triglyceride levels are associated with prospective cardiovascular risk [6]. This is supported by observations from Mendelian randomization that factors involved in the metabolism of triglyceride-rich lipoproteins are associated with cardiovascular risk [7, 8], implicating these particles in the causal pathway for atherosclerosis. Cellular studies suggest that triglyceride-rich lipoproteins promote inflammatory, oxidative, and thrombotic pathways in atherosclerosis [9,10,11], whereas transgenic mouse models aimed at increasing plasma triglycerides have an adverse effect on plaque burden [12].
Hypertriglyceridemia has become an increasingly prevalent risk factor by virtue of the rise in abdominal obesity and insulin resistance. This is evidenced by an increase in circulating triglyceride-rich lipoproteins, not only in the fasting state but also during the postprandial period. Following meals, chylomicron and remnant particles increase, with the latter thought to be particularly atherogenic. This is supported by reports that genetic polymorphisms associated with higher remnant cholesterol levels are also associated with cardiovascular risk [13] and that remnant cholesterol levels predict ongoing plaque progression in statin-treated patients [14]. It is therefore likely that any impact of triglyceride-rich lipoproteins in the atherosclerotic disease process is likely to become more problematic as a driver of cardiovascular risk.
In parallel, interest in the ability of high-density lipoproteins (HDLs) to protect against atherosclerosis has been considerable. Population studies demonstrate an inverse relationship between HDL cholesterol (HDL-C) and cardiovascular risk [15], although this risk is curvilinear, largely driven by the increased risk at low HDL-C levels [16]. Preclinical studies demonstrate that HDLs possess functional properties influencing cholesterol transport [17], inflammation [18], oxidation [19], and thrombosis [20] that may underscore the favorable effects of HDL-targeted interventions in animal models of atherosclerosis [21]. This is further supported by contemporary observations that quantitative and qualitative measures of HDL are associated with residual cardiovascular risk, even in the setting of intensive lipid lowering [22, 23]. Given that low HDL-C levels are commonly associated with hypertriglyceridemia, particularly in the setting of obesity and insulin resistance [24], this combined phenotype of atherogenic dyslipidemia presents a considerable challenge.
3 Therapeutic Approaches to Targeting Atherogenic Dyslipidemia
Lifestyle modification in terms of weight loss [25] and reduced alcohol consumption [26] can produce substantial triglyceride lowering. Fibrates are modest peroxisome proliferator-activated receptor (PPAR)-α agonists that are widely used for triglyceride lowering, with variable evidence of cardiovascular benefit in clinical outcomes trials. Early studies demonstrated benefit with gemfibrozil [27], but subsequent studies with other fibrates failed to demonstrate a clear reduction in cardiovascular events in statin-treated patients [28]. Meta-analyses of the fibrate trials reported a cardiovascular benefit that appears to be largely driven by their use in patients with baseline hypertriglyceridemia [29].
PPAR-γ agonists have been developed for glucose lowering in patients with type 2 diabetes mellitus (T2DM) because of their favorable effects on insulin sensitivity. They also modestly lower triglyceride levels [30], with favorable reductions in the triglyceride/HDL-C ratio associated with their ability to slow progression of coronary atherosclerosis in patients with T2DM [31]. Additional attempts to enhance PPAR therapeutics by increasing the potency of PPAR-α agonism or by developing dual PPAR-α/γ agonists have been disappointing, showing either no incremental benefit or toxicity [32]. Development of the selective PPAR-α modulator (SPPARM), pemafibrate, reduces triglycerides by 40–50% [33] and is currently undergoing evaluation in a large clinical outcome trial.
Niacin has a range of lipid-modifying effects, including raising HDL-C and lowering levels of both triglyceride and lipoprotein(a) [34]. However, its use has been limited by patients’ difficulty tolerating high doses and a lack of cardiovascular benefit in recent trials of statin-treated patients [35, 36]. While statins modestly lower triglyceride levels, guidelines advocate intensification of their use in high-risk patients with hypertriglyceridemia. A range of novel agents are currently being developed that target factors directly implicated in metabolism of triglyceride-rich lipoproteins (apolipoprotein C3 [37], angiopoietin-like proteins 3/4 [38]) based on genetic studies that causally associated these factors with atherosclerotic cardiovascular disease.
4 Omega-3 Fatty Acids
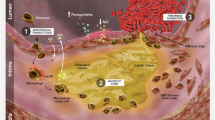
An additional approach to lowering triglyceride levels involves the administration of the polyunsaturated omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Considerable evidence from population studies has shown an inverse association between consumption of fatty fish or omega-3 fatty acids and cardiovascular disease. Mechanistic studies have demonstrated that omega-3 fatty acids possess several functional activities that may confer a protective influence against a range of cardiovascular disease states. These include altering cell membrane function, with favorable effects on cardiac rhythm, endothelial function, and the inflammatory, oxidative, and thrombotic pathways implicated in atherosclerosis [39]. Furthermore, omega-3 fatty acids favorably modulate triglyceride-rich lipoprotein metabolism [40] (Fig. 1). These activities directly correlate with achieved EPA/DHA levels within tissue, which supports observations that the protective effect of fatty fish and omega-3 fatty acids in population studies correlates with red blood cell EPA/DHA levels [41]. In the pre-statin era, the GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico)-Prevenzione study demonstrated that administration of low-dose prescription omega-3 fatty acids was associated with a reduction in cardiovascular events [42]. However, with the subsequent results of the major statin trials, attention shifted to the importance of LDL-C lowering, which became the cornerstone of cardiovascular prevention guidelines.
5 Contemporary Confusion with Omega-3 Fatty Acids
Many studies have evaluated the impact of omega-3 fatty acids regarding potential mechanistic effects and their influence on clinical outcomes (Table 1). These studies have been performed in a range of contemporary settings, including their potential effect on atherosclerotic disease, heart failure, and arrhythmia and have generally produced disappointing results. This has led to meta-analyses demonstrating no cardiovascular benefits from omega-3 fatty acids [43], prompting the popular media to suggest that use of these agents for putative effects on heart disease should be abandoned.
This has been further confirmed by two recent large clinical outcomes trials that similarly produced negative findings. ASCEND (A Study of Cardiovascular Events in Diabetes) compared the effects of marine n-3 fatty acids 840 mg (EPA 460 mg and DHA 380 mg) or matching olive oil placebo daily on cardiovascular events in 15,480 patients with diabetes but without clinically manifest atherosclerotic cardiovascular disease [44]. The n-3 fatty acid group demonstrated no benefit in lipid markers and a modest increase in red blood cell EPA/DHA content of 32.5%. After a mean follow-up of 7.4 years, this was associated with no significant difference in the primary cardiovascular endpoint of nonfatal myocardial infarction, nonfatal stroke, transient ischemic attack, or vascular death (8.9 vs. 9.2% in the fatty acid and placebo groups, respectively; p = 0.55).
The VITAL (Vitamin D and Omega-3) trial compared the effects of the same dose of n-3 fatty acids and matching placebo in 25,871 participants in a primary prevention study of men aged ≥ 50 years and women aged ≥ 55 years [45]. Similar to ASCEND, red blood cell EPA/DHA levels increased by 54% in the fatty acid group but did not reduce the composite incidence of myocardial infarction, stroke, or cardiovascular death (3.0 vs. 3.2% in the fatty acid and placebo groups, respectively; p = 0.24) during a median follow-up of 5.3 years. On face value, these studies would appear to reaffirm modern data suggesting that omega-3 fatty acids are not of cardiovascular benefit. However, they add to a large body of literature in which relatively small doses of omega-3 fatty acids, resulting in—at best—modest increases in tissue levels, were administered to a wide variety of patients. These data do, however, convey an important public health message that small-dose omega-3 fatty acids that can be easily purchased over the counter should not be used primarily with the objective of preventing heart disease. However, it remains to be determined whether administration of much higher omega-3 doses with the potential to substantially raise tissue EPA/DHA levels would be protective if they were administered to patients with potentially high modifiable cardiovascular risk.
6 JELIS
The first encouraging data to emerge from a large-scale clinical trial was from JELIS (Japan EPA Lipid Intervention Study) [46]. In this open-label study, 18,645 patients with a total cholesterol level > 6.5 mmol/L were randomized to treatment with EPA 1800 mg plus statin or statin only to evaluate the impact on cardiovascular event rates. During a mean follow-up of 4.6 years, reductions in LDL-C of 25% were observed in both treatment groups, whereas the EPA group demonstrated a greater, albeit modest lowering of triglyceride levels (− 9 vs. − 4%; p < 0.0001). Despite minimal lipid differences between the groups, patients treated with EPA demonstrated a 19% reduction in the primary composite endpoint of sudden cardiac death, myocardial infarction, unstable angina, or coronary revascularization (2.8 vs. 3.5%; p = 0.01). Subgroup analysis revealed significant reductions in cardiovascular events with EPA only in secondary prevention patients [47]. Subsequent analyses of JELIS have reported the greatest benefit with EPA administration in patients with baseline hypertriglyceridemia [48] and in those who achieved the highest red blood cell EPA levels [49]. The modest triglyceride-lowering benefit between the groups appears to be insufficient to explain the clinical outcome benefit and suggests that an alternative mechanism was most likely responsible for the favorable results. Nevertheless, these findings prompted more widespread use of EPA for cardiovascular disease prevention in Japan.
7 REDUCE-IT
REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial) endeavored to test the hypothesis that administration of high-dose EPA would be cardioprotective in high vascular risk patients with hypertriglyceridemia [50]. The study recruited 8179 patients with established cardiovascular risk or the presence of diabetes and other risk factors who were treated with a statin and had a fasting triglyceride level of 150–499 mg/dL. Patients were treated with icosapent ethyl 4 g or mineral oil placebo and followed-up for a median of 4.9 years. Administration of icosapent produced a greater reduction in triglyceride levels (− 18.3 vs. + 2.2%; p < 0.001), a smaller increase in LDL-C (+ 3.1 vs. + 10.2%; p < 0.001), and lower on-treatment C-reactive protein (1.8 vs. 2.8 mg/L; p ≤ 0.001) compared with the placebo group. Unlike the previous studies, a much more robust increase of 358% in red blood cell EPA levels was observed in the icosapent group.
This increase was associated with a 25% reduction in the primary composite endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina (17.2 vs. 22.0% in the icosapent and placebo groups, respectively; p < 0.001). More than 12 months of treatment was required before evidence of event curve separation became apparent, and significant reductions in each component of the primary endpoint, including cardiovascular death (by 20%), were observed in the icosapent group. In contrast, all-cause mortality was not reduced, although this is not dissimilar from most contemporary large-scale cardiovascular outcomes trials. In general, icosapent was well-tolerated, although a greater rate of atrial arrhythmia (3.1 vs. 2.1%; p = 0.004) and a nonsignificant trend toward more serious bleeding (2.7 vs. 2.1%; p = 0.06) were noted in the icosapent group. A subsequent analysis of REDUCE-IT demonstrated a similarly robust decrease in the incidence of total events, highlighting the potential to substantially influence the high residual clinical risk in these patients [51].
Of particular interest was the observation of a similar degree of relative risk reduction with icosapent in patients with higher and lower triglyceride levels at study entry. To date, there is no evidence to suggest that the benefit observed in this study was derived via its triglyceride-lowering effects. The findings have not been explored in patients with different baseline EPA levels, which may provide further insights. While the mechanism for the benefit remains uncertain, the finding that the event curves did not separate for at least 12 months is consistent with several contemporary lipid-lowering trials and would suggest that it was not due to any potential antithrombotic effects of EPA. Several modest and potentially adverse biochemical effects were observed in the mineral oil placebo group. This is likely to have had a minimal impact on the event rate difference between the groups, which would have remained substantial, but it does highlight the challenge of selecting truly inert placebos in studies of omega-3 fatty acids.
8 STRENGTH
In parallel, STRENGTH (Long-Term Outcomes Study to Assess Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia; clinicaltrials.gov NCT02104817) is evaluating the impact on cardiovascular risk of administration of high doses of combination EPA and DHA [52]. Epanova is an omega-3 carboxylic acid (EPA 75%, DHA 25%) that has undergone removal of the ethyl ester to a free fatty acid formulation during the manufacturing process. This provides an advantage in terms of removing the need for pancreatic lipase hydrolysis, required to achieve optimal intestinal absorption with omega-3 ethyl ester preparations. This permits administration irrespective of the fat content of meals. The difference in bioavailability achieves tissue EPA levels comparable to those with pure EPA formulations, with increases in trough EPA levels of up to 400% and DHA of 70%. Phase II studies in patients with hypertriglyceridemia revealed that administration of Epanova 2–4 g/day resulted in reductions in triglyceride levels of up to 30% and non-HDL-C of up to 10% [53]. In general, Epanova was well-tolerated by patients in these studies, with a higher incidence of diarrhea, nausea, and eructation being reported.
The STRENGTH study aims to determine whether administration of Epanova 4 g/day results in fewer cardiovascular events than does a matching corn oil placebo. The study has randomized 13,086 patients deemed to be at high cardiovascular risk based on a diagnosis of (1) clinically manifest atherosclerotic cardiovascular disease, (2) diabetes with an additional risk factor, or (3) requiring high-risk primary prevention and on a stable diet and statin dose for at least 4 weeks, with LDL-C < 100 mg/dL and presence of atherogenic dyslipidemia (triglyceride level ≥ 180 to < 500 mg/dL and HDL-C < 42 mg/dL for men and < 47 mg/dL for women). At least 50% of patients were required to meet the secondary prevention definition. The study has 90% power to demonstrate a 15% reduction in the primary efficacy endpoint, the time to first occurrence of the combination of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, and hospitalization for unstable angina. The trial is ongoing and will continue until 1600 primary endpoints have been adjudicated, with an anticipated median treatment duration of 3 years.
It is important to note the similarities and differences between STRENGTH and REDUCE-IT. Both studies are the first of their kind to test the impact of very high doses of omega-3 fatty acids with demonstrated ability to substantially raise tissue EPA/DHA levels. The agents differ, with icosapent being pure EPA and Epanova being a mixture of EPA and DHA. While less EPA is administered in Epanova, a similar tissue EPA level is achieved by virtue of the difference in fatty acid preparation. Icosapent is a traditional ethyl ester requiring hepatic conversion, whereas Epanova is a carboxylic acid. As a result, Epanova does not require hepatic conversion and in phase II studies achieved red blood cell EPA/DHA levels comparable to those observed with icosapent. The impact on vascular events of DHA within high-dose omega-3 fatty acids is unknown, providing an additional important feature that STRENGTH will contribute to the omega-3 fatty acid literature. Both studies required the presence of atherogenic dyslipidemia for study entry but differ in how it is defined. REDUCE-IT simply required modest hypertriglyceridemia, whereas STRENGTH requires a triglyceride level between 180 and 500 mg/dL in addition to a low HDL-C level. Whether these differences will influence the potential modifiability of cardiovascular risk in these patients is unknown. REDUCE-IT benefits from a long average treatment duration, demonstrating an increasing benefit that extends throughout the study; how this will look in STRENGTH remains to be determined. Finally, the two studies are using different placebos, although this is unlikely to produce substantial differences between the ultimate trial results.
9 Clinical Implications
It is important to note that all such pharmacological interventions for cardiovascular prevention should be applied on a background of lifestyle modifications that include changes to the diet and to exercise and smoking cessation, which have the potential to reduce triglyceride levels in their own right. The results of these studies have provided several important insights regarding targeting hypertriglyceridemia in cardiovascular prevention. The data establish that triglyceride-rich lipoproteins play an important role in the causation of atherosclerosis and drive residual risk in statin-treated patients. Observations from trials of fibrates and EPA demonstrate that targeting patients with baseline hypertriglyceridemia is beneficial and underscores the modifiability of the risk in these patients. More recent studies of omega-3 fatty acids suggest it is necessary to use high doses with the potential to profoundly elevate tissue EPA/DHA levels to observe any cardiovascular benefit with these agents. This ultimately shifts omega-3 use for cardiovascular disease prevention from over-the-counter use to high-grade pharmaceutical use. While the lack of discernible relationship between triglyceride lowering and cardiovascular benefit with these agents suggests alternative mechanisms are likely to be responsible, it continues to emphasize the importance of targeting the patient with elevated triglyceride levels in order to reduce cardiovascular risk. Other development programs are evaluating the impact on lipid levels of omega-3 phospholipid concentrate derived from krill oil as an alternative treatment in this field [TRILOGY 1 (NCT03398005) and TRILOGY 2 (NCT03361501)].
10 Conclusion
The findings of several clinical trials in recent years have suggested that residual cardiovascular risk can be modified in many statin-treated patients. The use of existing biomarkers is providing the opportunity to tailor the right therapeutic intervention to the right patient. Triglyceride levels may be one of these biomarkers, and high-dose omega-3 fatty acids present a highly effective intervention.
References
Cholesterol Treatment Trialists Consortium, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107.
Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46:1225–8.
Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–56.
Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–73.
Triglyceride Coronary Disease Genetics Consortium, Emerging Risk Factors Consortium, Sarwar N, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–9.
Doi H, Kugiyama K, Oka H, et al. Remnant lipoproteins induce proatherothrombogenic molecules in endothelial cells through a redox-sensitive mechanism. Circulation. 2000;102:670–6.
Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109:1022–8.
Wang YI, Bettaieb A, Sun C, et al. Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress. PLoS One. 2013;8:e78322.
Mansouri RM, Bauge E, Gervois P, et al. Atheroprotective effect of human apolipoprotein A5 in a mouse model of mixed dyslipidemia. Circ Res. 2008;103:450–3.
Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–33.
Puri R, Nissen SE, Shao M, et al. Non-HDL cholesterol and triglycerides: implications for coronary atheroma progression and clinical events. Arterioscler Thromb Vasc Biol. 2016;36:2220–8.
Ko DT, Alter DA, Guo H, et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol. 2016;68:2073–83.
Hamer M, O’Donovan G, Stamatakis E. High-density lipoprotein cholesterol and mortality: too much of a good thing? Arterioscler Thromb Vasc Biol. 2018;38:669–72.
Barter PJ, Brewer HB Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–7.
Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–72.
Nicholls SJ, Dusting GJ, Cutri B, et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–50.
Li D, Weng S, Yang B, et al. Inhibition of arterial thrombus formation by ApoA1 Milano. Arterioscler Thromb Vasc Biol. 1999;19:378–83.
Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol. 2005;25:2416–21.
Khera AV, Demler OV, Adelman SJ, et al. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation. 2017;135:2494–504.
Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93.
Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506.
Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83:1025–31 (quiz 1205).
Brinton EA. Effects of ethanol intake on lipoproteins and atherosclerosis. Curr Opin Lipidol. 2010;21:346–51.
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–8.
Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61.
Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–84.
Goldberg RB, Kendall DM, Deeg MA, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–54.
Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–73.
Nicholls SJ, Uno K. Peroxisome proliferator-activated receptor (PPAR alpha/gamma) agonists as a potential target to reduce cardiovascular risk in diabetes. Diab Vasc Dis Res. 2012;9:89–94.
Arai H, Yamashita S, Yokote K, et al. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor alpha modulator (SPPARMalpha), in combination with statin treatment: Two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis. 2017;261:144–52.
Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp(a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med. 1989;226:271–6.
HTC Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12.
A-H Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67.
Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015;373:438–47.
Dewey FE, Gusarova V, Dunbar RL, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377:211–21.
Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–94.
Phillipson BE, Rothrock DW, Connor WE, Harris WS, Illingworth DR. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985;312:1210–6.
Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis. 2007;193:1–10.
Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999;354:447–55.
Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–34.
ASC Group, Bowman L, Mafham M, et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379:1540–50.
Manson JE, Cook NR, Lee IM, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32.
Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8.
Matsuzaki M, Yokoyama M, Saito Y, et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J. 2009;73:1283–90.
Sasaki J, Yokoyama M, Matsuzaki M, et al. Relationship between coronary artery disease and non-HDL-C, and effect of highly purified EPA on the risk of coronary artery disease in hypercholesterolemic patients treated with statins: sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). J Atheroscler Thromb. 2012;19:194–204.
Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18:99–107.
Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22.
Bhatt DL, Steg PG, Miller M, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–802.
Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–8.
Kastelein JJ, Maki KC, Susekov A, et al. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the EpanoVa fOr Lowering Very high triglyceridEs (EVOLVE) trial. J Clin Lipidol. 2014;8:94–106.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this review or prepare this manuscript.
Conflict of interest
SJN has received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx, and Sanofi-Regeneron and is a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi-Regeneron, and Novo Nordisk. AJN and SM have no conflicts of interest that are directly relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Nelson, A.J., Mirzaee, S. & Nicholls, S.J. High-Dose Omega-3 Fatty Acids in Cardiovascular Prevention: Finally Living Up to Their Potential?. Am J Cardiovasc Drugs 20, 11–18 (2020). https://doi.org/10.1007/s40256-019-00363-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-019-00363-3