Abstract
Overproduction of Nitric oxide (NO) and many other pro-inflammatory mediators are responsible for many pathological disorders in humans, including Alzheimer’s disease (AD). In this study, active fractions isolated from Khaya grandifoliola (Kg) were screened for their inhibitory activities against NO production in lipopolysaccharide (LPS)-activated microglia. Among the 5 fractions tested, Kg25 was the most active and showed potent inhibitory activity towards NO production. The fraction further showed inhibitory effect on iNOS’s mRNA expression and other major pro-inflammatory cytokines including TNFα and IL1-β. Study of the effect of Kg25 on p38MAPKinase and JNK3 showed that the fraction inhibits these signaling pathways known to be involved in cell inflammatory pathways. These observations were confirmed at the protein level with Kg25 inhibiting iNOS and p38MAPK protein expressions in N9 cells. Analysis of Kg25 composition by HPLC identified 3 main compounds, namely: 6 phenyl, 4-(1`oxyehylphenyl) hexane, Carbamic acid, (4-methly-1-phenyl)-1, phenyl, and Benzene, 1 1`-(oxydiethylidene) bis. The above mentionned compounds were further analyzed for their bioactivity against the p38MAPKinase and iNOS receptors using molecular docking. MolDock results showed that 1-phenylethyl N-(4-methylphenyl)carbamate (compound 2) possesses the highest binding affinity (for iNOS); and 1-(1-phenylethoxy)ethylbenzene (compound 3) (for pMAPK) respectively and both compounds interact well with the active site residues. Hence, these compounds could be considered as scaffolds for further development of lead- drugs targeting neuroinflammation in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medicinal plants are still considered to be one of the major source of lead compounds for the pharmaceutical industry (Molinski et al. 2009). Historically, natural products have always been considered an important source of therapeutic compounds for the development of drugs. For instance, artemisinin, isolated from the Chinese medicinal plant Artemisia annua, is a drug that possess the most rapid action of all current drugs against Plasmodium falciparum (Chattopadhyay et al. 2007). It was reported that of the 175 molecules approved for the treatment of cancer, 85 (48.6%) of these were either natural products themselves or derived directly from natural products (Newman and Cragg 2012).
On the other hand, compounds and extracts derived from natural products are considered too complex and dirty to be compatible with the new automated drug discovery methodologies developed nowadays (Li and Vederas 2009). This is the reason why the pharmaceutical industry has shifted towards high-throughput screening technologies over the past few decades. However, there has recently been a renewed interest in natural product research due to the failure of alternative drug discovery methods to deliver lead compounds in key therapeutic areas such as immunosuppression, neurodegenerative and metabolic diseases (Butler 2004). Therefore, the application of molecular docking and virtual screening to compounds isolated from medicinal plants could emerge as efficient, rapid and inexpensive techniques for the identification and optimization of potential hit compounds (Schneider 2010).
Inflammation is a self-defensive reaction aimed at eliminating or neutralizing injurious stimuli, and restoring tissue integrity (Abdipranoto-Cowley et al. 2009). For instance, microglial, the resident macrophages in the brain, are believed to play an active role in brain inflammatory, immune and degenerative processes (Minghetti and Levi 1998). Upon activation, microglia produce a variety of pro-inflammatory mediators, including nitric oxide (NO), reactive oxygen species (ROS), tumor necrosis factor (TNFα), interleukin-1β (IL-1β) and interleukin-6 (IL-6). Activated microglia, by secreting proinflammatory molecules, are potential sources of harmful elements in the brain despite the fact that they also serve immune surveillance functions against foreign antigens (Akiyama et al. 2000). A common feature of AD is the chronic immune activation of microglia (Abdipranoto-Cowley et al. 2009). AD involves elevated levels of a diverse range of proinflammatory molecules in the brain. These inflammatory molecules are produced principally by activated microglia, which are found to be clustered within and adjacent to the senile plaques. Moreover, long-term treatment of patients with non-steroidal anti-inflammatory drugs has been shown to reduce the risk and incidence of AD and delay disease progression (Wilkinson and El Khoury, 2012).
Both in vivo and in vitro studies have documented the ability of LPS-activated microglia to secrete a variety of cytokines, including IL-1β, IL-6, IL-10, interferon-α (INF-α), IFN-β, TNFα, and chemokines (Lu et al. 2010). These inflammatory products contribute to produce neuronal toxicity and death. The over-activation of microglia and release of pro-inflammatory cytokines may also lead to neuronal death, causing neuropathological changes in CNS diseases such as multiple sclerosis (Lu et al. 2010), Parkinson’s disease (Depino et al. 2003), AD (Aldskogius 2000) and AIDS dementia (Glass and Wesselingh 2001). Therefore, therapeutic approaches focusing on inhibition of the microglia-mediated inflammatory response offer new opportunities to intervene in these diseases.
Khaya grandifoliola (Kg) also called ''African mahogany'' is a medicinal plant from the Meliaceae family used in tropical areas of sub-Saharan Africa for the treatment of malaria and neurological troubles. The plant is mainly found in Southern, Central and Western Africa. In Cameroon, stem barks are mainly used by traditional physicians against jaundice and viral hepatitis. Previous studies on Kg revealed anti-malaria activities of limonoids (Bickii et al. 2000), anti-inflammatory and trypanocidal activities of the aqueous crude extract (Stephen et al. 2009). Furthermore, anti-inflammatory activity of the crude extract was also reported (Falodun et al. 2009). Previous studies from our research group showed that three constituents, namely, benzene, 1,1′-(oxydiethylidene)bis (1), carbamic acid, (4-methylphenyl)-, 1-phenyl (2), and 6-phenyl, 4-(1′-oxyethylphenyl) hexene (3) were identified from the active fraction Kg25 by GC–MS (Galani et al. 2016) and were responsible for the antiviral activities observed in vitro (Fig. 1).
2D structures of Compound-1 (1-(1-phenylethoxy)ethylbenzene), Compound 2 (1-phenylethyl N-(4-methylphenyl)carbamate) and Compound-3 (3-(1-phenylethoxy)hex-5-enylbenzene) identified by HPLC (Galani et al. 2016)
In the present study, Kg extract was used to study anti-inflammatory effects on N9 microglia, and the compounds identified from the most active fraction were designed using Molview software (3D) and docked against iNOS and p38 MAPK targets.
Material and methods
Plant materials and solvent extraction
The plant was harvested in 2010, in the Bamun department (West region of Cameroon). Specimen were identified by Prof Njayou Frederic Nico of the University of Yaoundé I, Cameroon, and deposited at the National Herbarium, Yaoundé, Cameroon (Voucher number: 23434YA). Afterwards, plants extracts were prepared following the method described by Prof Njayou (Njayou et al. 2016). Briefly, dried Kg barks were air dried, cut into small pieces and ground. One Kg of the powder was immersed and extracted in methylene chloride/methanol 1/1 v/v at room temperature for 7 days. The mixture was afterwards filtered, and cakes were extracted and filtered three more times to increase the extraction yield. The procedure was repeated until the solvent presented a clear color. The filtrate was concentrated under reduced pressure, and the crude extract obtained was freeze-dried, and stored at 4 °C until used. The crude extract was subjected to flash chromatography to obtain 5 fractions (Fig. 2).
Chemicals
Fetal bovine serum (FBS), antibiotics (streptomycin/penicillin), and RPMI medium were purchased from Gibco (Grand Island, NY, USA). Escherichia coli-LPS and 3-(4, 5- dimethylthiazol-2-yl) -2, 5- diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Baicalin (99%) was purchased from Carbosynth Ltd. (Compton, Berkshire, UK). Mouse cytokines primers (iNOS, TNFα, IL-6, and IL-1β) were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for western blotting (iNOS, p38MAPK and GADPH) were purchased from Abcam, USA.
In vitro cell culture
The microglia cell line N9 was used to determine the effects of Kg on inflammation in vitro. The cells were cultured in RPMI medium (Life Technologies) containing penicillin (100U/ml) and 10% fetal bovine serum. Cells were cultured at 37 °C in a humidified incubator at an atmosphere of 5% CO2. N9 cells were grown in 12-well plates at a density of approximately 1 × 105 cells per well. The plant compounds were dissolved in dimethyl sulfoxide (DMSO) and filtered through 0.45 µm cellulose membranes.
MTT assay for measuring cell proliferation
The cytotoxic effect of Kg crude extract and fractions was evaluated using the MTT assay. 3-(4, 5- dimethylthiazol-2-yl) -2, 5- diphenyltetrazolium bromide (MTT) is a pale-yellow substrate that is reduced by living cells to yield a dark blue formazan product. This process requires active mitochondria, and only dead cells do not reduce significant amounts of MTT. Cells were seeded in 12-well plates (1 × 105 cells/well) and incubated for 24 h. After this incubation period, they were treated with various concentrations of Kg (0.01, 0.1, 1, 10, and 100 μg/ml), Baicalin (used as positive control at 5 μg/ml) and LPS (1 μg/ml) at 37 °C in 5% CO2 for 24 h. After treatment, 100 μL of MTT (5 mg/ml) dissolved in RPMI was added to each well, followed by incubation for 3 h. The medium was aspirated, and the formazan crystals were dissolved in 500 μL of DMSO for 15 min. The optical density of each well was measured at 540 nm using a microplate reader.
Determination of nitric oxide production
Production of NO was determined by measuring the accumulated levels of nitrite, an indicator of NO in the supernatant after 24 h of LPS treatment with or without different concentrations of plant material and Baicalin. After pre-incubation of cells (1 × 105 cells) for 24 h, Baicalin (5 µg/ml), or Kg (0.05, 0.5, 5, and 50 μg/ml) were added, together with LPS (1 μg/ml). The cells were further incubated at 37 °C, 5% CO2 for 24 h. The quantity of nitrite in the culture medium was measured as an indicator of NO production. Amounts of nitrite, a stable metabolite of NO, were measured using Griess reagent (1% sulfanilamide and 0.1% naphthyl ethylene diamine dihydrochloride in 2.5% phosphoric acid). Briefly, 50 μl of cell culture medium was mixed with 100 μl of Griess reagent. Subsequently, the mixture was incubated at room temperature for 10 min and the absorbance at 570 nm was measured in a microplate reader. Fresh culture medium was used as a blank in every experiment. The quantity of nitrite was determined from a sodium nitrite standard curve.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
N9 cells were treated with Baicalin, Kg and LPS (1 μg/ml) for 24 h. Total RNA from LPS-treated microglia was prepared using the innuPREP RNA Mini kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s protocol. cDNA (1 μg/ml) was synthesized from 1 μg of total RNA and was used to perform RT-PCR. After initial denaturation for 2 min at 95 °C, thirty amplification cycles were performed for iNOS (1 min of 95 °C denaturation, 1 min of 60 °C annealing, and 1.5 min 72 °C extension), TNF-α (1 min of 95 °C denaturation, 1 min of 55 °C annealing, and 1 min 72 °C extension), IL-1β (1 min of 94 °C denaturation, 1 min of 60 °C annealing, and 1 min 72 °C extension), IL-6 (1 min of 94 °C denaturation, 1 min of 60 °C annealing, and 1 min 72 °C extension) and GADPH (1 min of 94 °C denaturation, 1 min of 60 °C annealing, and 1 min 72 °C extension). The primer sequences used for quantification of iNOS, TNF α, IL-1 β, IL-6, GADPH and the PCR conditions are described in the Table 1. PCR products were separated by 1.5% agarose gel electrophoresis containing 10 mg/ml ethidium bromide, photographed using UVsolo system (Whatman Biometra, Goettingen, Germany) and densitometric analysis was performed with the software BioDocAnalyze (Whatman Biometra). Results were calculated as levels of target mRNAs relative to those of GADPH.
P38 MAPKinase and JNK3 inhibition assays
The inhibition of p38 MAPKinase and JNK3 were realized as described by (Goettert et al. 2012). Briefly, 96-well plates were coated with ATF-2, overnight, at 4 °C. Blocking buffer was added at room temperature and the plates were incubated for 30 min before the addition of the kinase reaction mix containing the enzyme, with or without test compounds. The p38α and JNK3 reactions were carried out by using kinase (12 ng per well), ATP (100 μM), for 45 min at 37 °C. The ATF-2 phosphorylation was detected with a specific anti-phospho ATF-2 (Thr69/71) antibody (60 min at 37 °C). After each incubation time, the plate was washed three times with double distilled water. The optical density was measured after the addition of the substrate at 450 nm, using a plate reader. The inhibitors SB203580 and SP600125 were used as reference compounds for p38MAPK and JNK3 assays, respectively.
Western blot analysis
In order to study the effect of Kg25 on iNOS and p38MAPK protein expressions, N9 microglia stimulated or not with LPS were treated with Kg25. Different protein samples were obtained from cell lysates, and the protein concentrations were determined. Thereafter, the protein samples were separated by SDS-PAGE, transferred to a PVDF membrane and blocked with TBS containing 0.05% Tween-20 (TBST) and 5% nonfat milk (in TBST) for 1 h. After this incubation time, the membranes were incubated with primary antibodies (iNOS, p38MAPK and GADPH) overnight at 4 °C. On the following day, the membranes were washed 3 × 5 min with TBST and incubated with corresponding secondary antibodies for 1 h at room temperature. Finally, the protein samples were visualized using a chemiluminescence instrument (Biorad).
Docking tools
We have used Molview for 3D structure generation, PyMOL for structure preparation and data analysis, and MolDock workflow for virtual screening (VS).
Protein structure preparation
The 3D structure of the different receptors was retrieved from the protein data bank (PDB) (http://www.rcsb.org). We have chosen respectively: p38MAPK (PDB ID: 1OUK), and iNOS (PDB ID: 2NOS). The proteins had one or two polypeptides and were co-crystallized with the ligand. The targets were visually inspected, and reference ligand identified for each receptor. Components to be excluded from docking were removed (1OUK: we removed water molecules and S02 HETATOM, iNOS: we removed water molecules). Receptors were prepared for docking by removal of water molecules, ligands, cofactors and assigning bonds, bond order, hybridization and charges using the MVD software (Dubey and Dubey 2020).
Ligand preparation
Compounds were identified from PubChem chemical database. The structure was drawn using Molview software (Fig. 3) and energy minimization was performed using the MM2 force field. Three natural compounds were identified from K. grandifoliola after HPLC analysis. The 2D and 3D representations were generated using Molview. The structure files were stored along with reference ligands as.MOL formats. The missing charges and hybridization states of different compounds were assigned with the help of the MVD software.
Kg25 suppression of LPS induced iNOS and p38 MAPK proteins in N9 microglias. (Left): Representative images of the western blot showing protein expression of iNOS and p38MAPK. GADPH was used as housekeeping. (Right) Relative quantitation of iNOS and p38MAPK. Data are expressed as mean \(\pm\) SD (n = 3). *** P < 0.001 vs control group
Docking search algorithm and scoring functions
MVD uses PLP (Piecewise Linear Potential) algorithm as scoring function for computational screening. In this study, the MolDock simplex evolution search algorithm was used for docking. In practice, the number of receptor cavities was limited to four and using the cavity prediction wizard, the cavity with large volume was selected. Docking of compounds in different receptors was performed and the best poses generated were used based on the docking scores.
Parameters for scoring functions
MVD software uses two scoring functions: the MolDock score and the ReRank score, where the MolDock score is an E score (docking scoring function) defined as: E score = E inter + E intra.
E inter: Sum of ligand–protein interaction energy, ligand-water interaction energy and ligand-cofactor interaction energy (Heble et al. 2016).
E intra: internal energy of the ligand.
ReRank score provides an estimation of the ligand-receptor interaction strength (Madhuri et al. 2014).
Statistical analysis
All experiments were reiterated at least three times in triplicate. The results of multiple observations are expressed as the mean ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA) using Graph Pad Prism 5.0 for windows. For all statistical analyses, significance levels were set at P < 0.05.
Results
Effect of K. grandifoliola crude extract and fractions on cell viability
Kg at the tested concentrations (up to 100 µg/ml) did not significantly affect the cell viability, as it resulted in 95% cell viability (Fig. 4). The study of the effect of Kg extract and fractions on N9 cells revealed that the crude extract and the 5 fractions obtained did not show any cytotoxic effect on the cells. Moreover, observation of cell’s morphology did not show any change due to the treatment with LPS and extracts up to 48 h of treatment (data not shown).
Effect of K. grandifoliola crude extract A and fractions B on the viability of LPS-induced microglia. N9 cells 5.105cells/ml were incubated with the indicated concentrations of Kg, in the presence or absence of LPS 1 µg/ml for 24 h. The cell viability was then determined by MTT assay as described in the Materials and Methods
Inhibition of LPS-induced nitric oxide production by Kg crude extract and fractions.
To further assess whether Kg could activate microglia, we assessed NO production in N9 cells. The cells were treated with LPS (1 µg/ml) for 24 h and after this time, the nitrite concentration in the medium increased remarkably by about twofold (data not shown). After treating the cells with different concentrations of the samples together with LPS (1 µg/ml), a significant concentration-dependent inhibition of nitric oxide was detected in the medium. The results showed that the crude extract and the fractions inhibited NO production in a dose dependent manner (Fig. 5A and B). However, fraction Kg25 was the most efficient with an IC50 value of 8.545 µg/ml in comparison to the crude extract and the other fractions (Fig. 5C). For further experiments, this fraction was chosen because of its inhibitory effect on LPS-induced NO production on N9 cells.
Effect K. grandifoliola crude extract (A), fractions at 50 µg/ml (B) and fraction Kg25 (C) on Nitric Oxide production. N9 cells were grown in complete RPMI 1640 medium containing penicillin, streptomycin, and 10 per cent FCS at 37 °C and 5 percent CO2. 5.105 cells were seeded into 24-well cell culture plates and cultured for 24 h. Afterward, cells were harvested and centrifuged, and supernatants collected for the analysis of NO concentrations by a standard Griess assay. D: Kg25 reduced LPS-induced iNOS gene expression in microglia cell line. The cells were incubated without or with LPS 1 µg-ml together with the indicated concentrations of Kg25. After 24 h iNOS mRNA was quantified by RT-PCR. *P < 0.05, ***P < 0.01 compared with LPS group
Inhibitory effect of the fraction Kg25 on iNOS mRNA expression
We have obtained that Kg25 inhibited NO production from LPS-activated microglia. We now wanted to see if this inhibition was due to an inhibition of iNOS mRNA expression in these cells. For this purpose, N9 cells were incubated in the presence of increasing concentrations of the plant. The results shown in Fig. 5D indicated that after treatment with LPS (1 µg/ml), a significant decrease in iNOS mRNA levels was observed after 24 h of treatment. Moreover, Kg25 significantly inhibited iNOS mRNA levels at all the concentrations tested, as did Baicalin.
Inhibitory effect of the fraction Kg25 on pro-inflammatory cytokines mRNA expression
The effect of Kg25 on pro-inflammatory cytokines mRNA expression was also evaluated. The results obtained showed that 1 µg/ml LPS significantly increased the mRNA levels of TNFα and IL1-β. After treatment, Kg25 significantly inhibited mRNA expression of IL-1β at 0.5, 5, and 50 µg/ml (Fig. 6A), and TNFα at all concentrations tested (Fig. 6B).
Effect of Kg25 on LPS-induced expression of IL1β A and TNFα B mRNA in microglia. N9 cells were pretreated with different concentrations of Kg25, with or without LPS for a stimulation period of 24 h. Total RNAs were isolated, and mRNA levels of IL1β and TNFα were measured by RT-PCR. GADPH expression was used as an internal control. *P < 0.05, ***P < 0.01 compared with LPS group. 4C: Inhibition of JNK3 activity by Kg25 fraction. The inhibitory potential of Kg25 and Baicalin was evaluated using ELISA. SP600125 was used as reference compound
Inhibitory effect of the fraction Kg25 on p38 MAPKinase and JNK3
Kg25 and the sub-fractions obtained after fractionation were tested for their ability to inhibit p38MAPKinase and JNK3. The inhibitors SB203580 and SP600125 were used for p38MAPK and JNK3, respectively. The results of p38MAPK are presented in Table 2 and showed that Kg25 has a better IC50 value in comparison to Baicalin and the sub-fractions.
As indicated in Fig. 4C, Kg25 also significantly inhibited the activity of JNK3, even though an incredibly low inhibition was observed with Baicalin.
Expression of iNOS and p38MAPK proteins in N9 cells treated with Kg25
Our aim was to study the effect of Kg25 on iNOS and p38MAPK protein expression in N9 microglia with or without stimulation with LPS. iNOS and p38MAPK proteins expression were high in LPS treated cells but lower or highly inhibited at different concentrations after treatment with Kg (Fig. 3).
Docking of compounds identified from Kg25
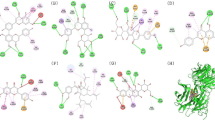
The 3D structures obtained with Molview are represented in Fig. 7. Compounds 1 and 2 were already identified in PubChem under ID 62,342 and 57,234, respectively. The results were obtained after docking the reference compounds identified from PDB database. Tables 3 and 4 give the binding energies obtained with test compounds and SB203580 which is the reference compound used in in vitro experiments.
After performing docking analysis, the results obtained in Tables 1, 2 and Fig. 7 indicated that the tested compounds fit perfectly at the active sites of 2nos and 1ouk, respectively. For 2nos (iNOS), the binding involved complex interactions with seven amino acids including Leu 2, Lys 6, Phe 5, Phe 65, Trp 19, Tyr 3 and Tyr 64. Moreover, the binding of compounds to 2nos receptor was stabilized by 2, 4 and 1 hydrogen bonds respectively for compounds 1, 2 and 3. The1 ouk receptor established 1 hydrogen bond with compound 1, 1 with compound 2 and 1 with compound 3. For SB203580, the reference inhibitor for 2nos and 1ouk, amino acids involved in the binding process included Arg 657, Asp 655, Glu 68, Lys 654, Phe 218, Ser 67, Lys 101, Ser 102, Ser 104 and Val 103, and the binding was stabilized with 4 hydrogen bonds for 2nos and 3 hydrogen bonds for 1ouk Tables 5 and 6.
The docking scores indicated that the highest binding for 2nos is obtained with compound 2 (MolDock score: – 77.099, ReRank score: – 62.866) and reference compound (MolDock score: – 91.145, ReRank score: – 76.593). As far as 1ouk is concerned, the highest score was obtained with compound 3 (MolDock score: – 94.926, ReRank score: – 80.559) with reference compound having (MolDock score: – 136.343, ReRank score: – 115.795).
Discussion
This study reports for the first time that a fraction purified from Kg has anti-inflammatory effects on N9 microglia in vitro. The fraction Kg25 was purified from the crude extract and showed inhibition of NO production and iNOS mRNA expression in LPS-activated microglia. Moreover, the fraction reduced mRNA expression of pro-inflammatory cytokines (TNFα and IL1-β). P38MAPK and NO inhibition assays showed that Kg25 has a better IC50 value in comparison to the other fractions. HPLC analysis of Kg25 performed in our research group identified 3 main compounds in the fraction, namely 3-(1-phenylethoxy)hex-5-enylbenzene, 1-phenylethyl N-(4-methylphenyl)carbamate, and 1-(1-phenylethoxy)ethylbenzene [36]. Docking studies with the 3 ligands showed that 1-phenylethyl N-(4-methylphenyl)carbamate, and 1-(1-phenylethoxy)ethylbenzene bind well in the binding site of p38MAPK and iNOS receptors, and this may explain the anti-inflammatory effect observed. In further experiment, Kg25 inhibitory effects on iNOS and p38MAPK protein expression was analyzed.
Microglia, also known as brain macrophages are the first immune cells to recognize invading pathogens and participate in both cell mediated and humoral immunity (Korns et al. 2011). When activated by an external stimuli, microglia upregulate the production of nitric oxide (NO) and various pro-inflammatory cytokines (Aktan, 2004). NO is an important cellular signaling transmitter of the immune system in response to pathogens, and is generated from the transformation of L-arginine to L-citrulline through the actions of an enzyme, the nitric oxide synthase (iNOS) (Aktan, 2004). NO is reported to mediate a diverse set of cellular functions, including vasodilatation (Groot et al. 2015) and elimination of pathogens by phagocytosis (Brandonisio et al. 2002). Moreover, NO has been identified as a major effector molecule in the destruction of cancer cells by macrophages (Blackwell et al. 2011). However, imbalances due to over-production of pro-inflammatory cytokines by microglia can be harmful to the body and cause pathological conditions including AD and rheumatoid arthritis (Ellwardt and Zipp 2014) (Kaur and Halliwell 1994). As seen in Fig. 4, the results showed that microglia activated by LPS (1 µg/ml) displayed significant induction of NO production in the culture medium. When incubated with Kg extract up to 50 µg/ml, we noticed a decrease in NO production from N9 cells with the crude extract and fractions. The treatment of LPS-activated microglia with the plant compounds almost completely suppressed NO production induced by LPS as seen in Fig. 4C. Because the fraction Kg25 had the best IC50 value in comparison with the crude extract and the 4 other fractions, this fraction was used for further analysis. Analysis of iNOS mRNA expression showed an inhibition at all the concentrations of Kg25 and the same with Baicalin, the reference compound used (Fig. 4D). This result suggests that inhibition of NO production by microglia is due to the reduction of iNOS mRNA expression and not to cell mortality as confirmed by MTT viability assay (Fig. 2). Direct inhibition of NO by many plant extracts and polyphenols through inhibition of iNOS expression was reported in many other studies (Cheng et al. 2014; Aktan 2004; Lechner et al. 2005).
Next, we determined the expression of pro-inflammatory cytokines by LPS-activated microglia. TNF-α is known as one of the major pro- inflammatory cytokines secreted by microglia upon the acute phase of inflammation (Matsuno et al. 2002). Compared to LPS-treated group, Kg25 was found to inhibit the expression of TNF-α from 9.560 ± 1.3 to 1.3 ± 2.1 based on RT-PCR analysis of TNF-α expression (Fig. 6B). The IL-1β cytokine secreted by activated microglia after stimulation by LPS is known to play a major role in neuroinflammation (Cai et al. 2014), while IL-6 is responsible for fever in autoimmune, infectious and non-infectious diseases (Iliopoulos et al. 2009). The induction of IL-1β mRNA expression in microglia was significantly increased upon treatment with LPS (Fig. 6A). Kg25 treatment of microglia seems to inhibit mRNA expression of IL1-β at all the concentrations tested (Fig. 6A).
With the aim of analyzing and purifying the Kg25 fraction, it was further subjected to silica gel chromatography. The 3 sub-fractions obtained were used, together with Kg25 to analyze the inhibition of p38MAPKinase. The family members of the mitogen-activated protein (MAP) kinases mediate a wide variety of cellular behaviors in response to extracellular stimuli (Zarubin and Han 2005). P38 kinase appears to be involved in the release of IL-1β (Lee et al. 2000) and the inhibition of p38MAPKinases is reported to be a therapeutic strategy against cancer and neuroinflammatory diseases (Lee et al. 2000). The results obtained in Table 2 showed that Kg25 IC50 = 2.71 µg/ml is higher than the IC50 of Baicalin IC50 = 3.43 µg/ml and the 3 sub-fractions (IC50 = 11.35, 51.58 and 14.17 for SF1, SF2 and SF3 respectively).
The cJun NH(2)-terminal kinase (JNK) signaling pathway is another signaling protein that contributes to inflammation and plays a key role in the metabolic response to obesity, including insulin resistance (Han et al. 2013). Kg25 also inhibited JNK3 as reported in Fig. 6C. This result, together with the results of NO inhibition by Kg25 and its sub-fractions shows that further fractionation of Kg25 decreases its inhibitory effect.
Analysis of Kg25 by HPLC identified 3 major compounds. These compounds are therefore considered to be potential hit compounds for the discovery of lead candidates for anti-inflammatory diseases. Indeed, despite considerable progress in genome- and proteome-based high-throughput screening methods and in rational drug design, natural products remain an important source of new leads for the drug market (Csermely et al. 2013).
Of the 3 compounds identified from Kg25 active fraction, compounds 1 and 2 are already known in the literature, whereas compound 3 is identified for the first time. Kg25 fraction showed prominent results in vitro on p38MAPK, and iNOS assays, respectively. Therefore, we have used molecular docking and virtual screening to have an idea of the effect of our compounds directly on the receptors and to have basic ideas on potential drug development issues. After generating the 3D structures of the compounds, we first had to remove water molecules which are not involved in the binding process. These molecules and others like S02 were therefore deleted to make computations easier and clear the binding pocket of possible molecules that could distort the pose search. In docking we typically search for molecules that can create multiple favorable contacts to the protein, and water molecules might confound this procedure. However, if it is known that one or more water molecules are involved in the binding of molecules to the target and its activity, those water molecules should be preserved. The docking results showed that compound 2 was the most efficient in inhibiting iNOS. The score obtained for iNOS inhibition with compound 2 was higher than the one obtained in a similar study investigating compounds from Phaseolus Vulgari against Nitric Oxide Synthase (Petchiammal et al. 2011). Based on these results, we suggest that compounds 2/3 may be the major compounds responsible for the anti-inflammatory activities observed in vitro with Kg25, because they bind well to the receptors investigated. The molecular interactions like hydrogen bonds, hydrophobic interactions, Van der Waal interaction and ionic bonds also play important roles. Visualization of the compound 2-iNOS complex showed 4 hydrogen bonds between the C–OH group of compound2 and the carboxy group of Arg193 and Phe 363. Moreover, the complex is stabilized by hydrophobic interactions with amino acids residues Tyr483, Met349, Ala191 and Val346.
Kg25 fraction showed many other activities on different cell models. The fraction was reported to have antioxidant and cytoprotective effects and induced nuclear translocation of Nrf2 in a human hepatocyte cell line (Njayou et al. 2015). At 100 µg/mL, Kg25 had a high inhibitory effect on HCV replication, comparable to that of 0.01 µM daclatasvir or 1 µM telaprevir (Galani et al. 2016). The results obtained in this study suggest that the fraction acts on N9 activated microglia by inhibiting the overproduction of NO and pro-inflammatory cytokines, through an inhibition of p38MAPKinase and JNK3 signaling pathways.
One of the most difficult tasks in drug design is to find out if the compounds/ligands are synthesizable. Our group has already synthesized Compounds 2 and 3 for further investigation against neuroinflammation Fig. 8.
Conclusion
The present study has revealed that Kg25, a fraction purified from Kg crude extract has anti-inflammatory activities on N9 microglia by inhibiting the NO and pro-inflammatory cytokines induced by LPS in vitro. Moreover, the fraction inhibited p38MAPKinase and JNK3 which are important proteins involved in inflammatory cell signaling pathways. + Docking of compounds identified in Kg25 showed that 1-phenylethyl N-(4-methylphenyl)carbamate and 1-(1-phenylethoxy)ethylbenzene (compound 3) bind well to p38MAPKinase and iNOS receptors, with binding scores comparable to their respective reference ligands. These results suggest that compounds 2 and 3 are potential lead compounds for the treatment of neuro-inflammatory related diseases.
Data availability
All data are available in the manuscript.
References
Abdipranoto-Cowley A, Jin SP, Croucher D, Daniel J, Henshall S, Galbraith S, Mervin K, Vissel B (2009) Activin A is essential for neurogenesis following neurodegeneration. Stem Cells 27:1330–1346. https://doi.org/10.1002/stem.80
Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K (2000) Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord 14(Suppl 1):S47–S53. https://doi.org/10.1097/00002093-200000001-00008
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653. https://doi.org/10.1016/j.lfs.2003.10.042
Aldskogius H (2000) Microglia–new target cells for neurological therapy. Lakartidningen 97:3358–3362
Bickii J, Njifutie N, Ayafor Foyere J, Basco LK, Ringwald P (2000) In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae). J Ethnopharmacol. https://doi.org/10.1016/S0378-8741(99)00117-8
Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS (2011) NF-{kappa}B signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol 187:2740–2747. https://doi.org/10.4049/jimmunol.1101495
Brandonisio O, Panaro M, Fumarola I, Sisto M, Leogrande D, Acquafredda A, Spinelli R, Mitolo V (2002) Macrophage chemotactic protein-1 and macrophage inflammatory protein-1 alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin Experim Med 2:125–129. https://doi.org/10.1007/s102380200017
Butler MS (2004) The role of natural product chemistry in drug discovery. J Nat Prod 67:2141–2153. https://doi.org/10.1021/np040106y
Cai Z, Hussain MD, Yan LJ (2014) Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 124:307–321. https://doi.org/10.3109/00207454.2013.833510
Chattopadhyay R, Mahajan B, Kumar S (2007) Assessment of safety of the major antimalarial drugs. Expert Opin Drug Saf 6:505–521. https://doi.org/10.1517/14740338.6.5.505
Cheng A, Yan H, Han C, Wang W, Tian Y, Chen X (2014) Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. Int J Biol Macromol 69:382–387. https://doi.org/10.1016/j.ijbiomac.2014.05.071
Csermely P, Korcsmáros T, Kiss HJM, London G, Nussinov R (2013) Structure and dynamics of molecular networks: a novel paradigm of drug discovery: a comprehensive review. Pharmacol Ther 138:333–408. https://doi.org/10.1016/j.pharmthera.2013.01.016
Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, del Rey A, Pitossi FJ, Oertel WH (2003) Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson’s disease. Eur J Neurosci 18:2731–2742. https://doi.org/10.1046/j.1460-9568.2003.03014.x
Dubey K, Dubey R (2020) Computation screening of narcissoside a glycosyloxyflavone for potential novel coronavirus 2019 (COVID-19) inhibitor. Biomed J. https://doi.org/10.1016/j.bj.2020.05.002
Ellwardt E, Zipp F (2014) Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp Neurol. https://doi.org/10.1016/j.expneurol.2014.02.006
Falodun A, Poh CF, Adelusi SA, Chemistry P, Pharmacy FOF, Of F, Medical B, Of C, Sciences H, Island W (2009) Phytochemical and anti-inflammatory evaluation of khaya grandifoliola stem bark extract. Int J PharmTech Res 1:1061–1064
Galani BRT, Sahuc ME, Sass G, Njayou FN, Loscher C, Mkounga P, Deloison G, Brodin P, Rouillé Y, Tiegs G, Séron K, Moundipa PF (2016) Khaya grandifoliola C.D.C: a potential source of active ingredients against hepatitis C virus in vitro. Arch Virol. https://doi.org/10.1007/s00705-016-2771-5
Glass JD, Wesselingh SL (2001) Microglia in HIV-associated neurological diseases. Microsc Res Tech 54:95–105. https://doi.org/10.1002/jemt.1124
Goettert M, Shaalan N, Graeser R, Laufer SA (2012) Development of a p38δ mitogen activated protein kinase ELISA assay for the quantitative determination of inhibitor activity. J Pharm Biomed Anal 66:349–351. https://doi.org/10.1016/j.jpba.2012.03.008
Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS (2015) The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593:3917–3928. https://doi.org/10.1113/JP270195
Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ (2013) JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339:218–22. https://doi.org/10.1126/science.1227568
Heble NK, Mavillapalli RC, Selvaraj R, Jeyabalan S (2016) Molecular docking studies of phytoconstituents identified in Crocus sativus, Curcuma longa, Cassia occidentalis and Moringa oleifera on thymidylate synthase An enzyme target for anti-cancer activity. J Appl Pharmaceut Sci https://doi.org/10.7324/JAPS.2016.601218
Iliopoulos D, Hirsch HA, Struhl K (2009) An Epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 139:693–706. https://doi.org/10.1016/j.cell.2009.10.014
Kaur H, Halliwell B (1994) Evidence for nitric oxide-mediated oxidative damage in chronic inflammation Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett 350:9–12. https://doi.org/10.1016/0014-5793(94)00722-5
Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL (2011) Modulation of macrophage efferocytosis in inflammation. Front Immunol. https://doi.org/10.3389/fimmu.2011.00057
Lechner M, Lirk P, Rieder J (2005) Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2005.04.004
Lee JC, Kumar S, Griswold DE, Underwood DC, Votta BJ, Adams JL (2000) Inhibition of p38 MAP kinase as a therapeutic strategy. Immunopharmacology 47:185–201. https://doi.org/10.1016/S0162-3109(00)00206-X
Li JW-H, Vederas JC (2009) Drug discovery and natural products: end of an era or an endless frontier? Science 325:161–5. https://doi.org/10.1126/science.1168243
Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, Wang Z, Wang JM, Le Y (2010) Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation 7:46. https://doi.org/10.1186/1742-2094-7-46
Madhuri M, Prasad C, Rao Avupati V (2014) In Silico Protein-Ligand Docking Studies on Thiazolidinediones as Potential Anticancer Agents. Int J Computer Appl. https://doi.org/10.5120/16597-6403
Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T, Yonezawa T, Saeki Y, Panayi GS, Pitzalis C, Kimura T (2002) The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): a study using a human RA/SCID mouse chimera. Rheumatology (Oxford) 41:329–337. https://doi.org/10.1093/rheumatology/41.3.329
Minghetti L, Levi G (1998) Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol 54:99–125. https://doi.org/10.1016/S0301-0082(97)00052-X
Molinski TF, Dalisay DS, Lievens SL, Saludes JP (2009) Drug development from marine natural products. Nat Rev Drug Discovery. https://doi.org/10.1038/nrd2487
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75:311–335. https://doi.org/10.1021/np200906s
Njayou FN, Amougou AM, Fouemene Tsayem R, Njikam Manjia J, Rudraiah S, Bradley B, Manautou JE, Fewou Moundipa P (2015) Antioxidant fractions of Khaya grandifoliola C.DC. and Entada africana Guill. et Perr. induce nuclear translocation of Nrf2 in HC-04 cells. Cell Stress Chaperones 20:991–1000. https://doi.org/10.1007/s12192-015-0628-6
Njayou FN, Kouam AF, Simo BFN, Tchana AN, Moundipa PF (2016) Active chemical fractions of stem bark extract of Khaya grandifoliola C.DC and Entada africana Guill. et Perr. synergistically protect primary rat hepatocytes against paracetamol-induced damage. BMC Complem Altern Med. https://doi.org/10.1186/s12906-016-1169-y
Petchiammal C, Waheeta Hopper. Virtual Screening and Molecular Docking Studies of Compounds from Phaseolus Vulgaris against Nitric Oxide Synthase. Int Confer Biosci Biochem Bioinform IPCBEE 5
Schneider G (2010) Virtual screening: an endless staircase? Nature reviews. Drug Discovery 9:273–276. https://doi.org/10.1038/nrd3139
Stephen UA, Abiodun F, Osahon O, Ewaen E (2009) Phytochemical analysis and antibacterial activity of Khaya grandifoliola stem bark. J Biol Sci. https://doi.org/10.3923/jbs.2009.63.67
Wilkinson K, El Khoury J (2012) Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimer’s Dis. https://doi.org/10.1155/2012/489456
Zarubin T, Han J (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res 15:11–18. https://doi.org/10.1038/sj.cr.7290257
Funding
This research was supported by the DAAD and the chemistry part co-funded by the International Foundation for Science. Authors would also like to thank Dr Galani Borris for his valuable contribution in the Chemical work of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Owona, B.A., Njayou, F.N., Mkounga, P. et al. Khaya grandifoliola active fraction as a source of therapeutic compounds for Alzheimer’s disease treatment: In silico validation of identified compounds. In Silico Pharmacol. 10, 11 (2022). https://doi.org/10.1007/s40203-022-00126-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40203-022-00126-0