Abstract
Chelant-assisted phytoextraction has widely been exploited as a feasible option for removing heavy metals from the contaminated soils. Some synthetic chelants have shown promising performances for this option, but they have also revealed several negative environmental consequences. This study has sought to investigate the feasibility of two biodegradable eco-friendly chelants, namely methylglycinediacetic acid (MGDA) and N,N-Bis(carboxymethyl)-L-glutamic acid (GLDA), as compared to the resistant ethylenediaminetetraacetic acid (EDTA), in enhancing phytoextraction of Zn and Pb from a contaminated calcareous soil. For this purpose, a greenhouse experiment was carried out comparing the growth and metal absorption of maize (Zea mays L.) grown on soils treated with EDTA, MGDA, and GLDA chelants at 2, 4 and 8 mmol kg− 1 levels. Results showed that the heavy metal uptakes by the plant shoots generally increased with increasing the chelant application level. Pb uptake by maize shoots increased from 10.6 mg plant− 1 in control to 416, 398, and 416 mg plant− 1 in the soils treated with 8 mmol kg− 1 MGDA, GLDA, and EDTA, respectively. The corresponding increases in Zn uptake were from 100.9 mg plant− 1 to 798.9, 718.9, and 530.4 mg plant− 1 in the MGDA-, GLDA-, and EDTA-amended soils, respectively. Moreover, the amounts of water-extractable, and thereby potentially leachable, Pb and Zn in the post-harvest soil were considerably greater in the soil treated with EDTA than those treated with MGDA and GLDA. Therefore, MGDA and GLDA would be potential alternatives to environmentally-persistent EDTA for enhanced metal phytoextraction from contaminated soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is an important sink for heavy metals discharged into the environment by various natural and anthropogenic sources [1]. Once entered into the soil environment, metals may be transferred to the other ecosystem components, i.e., groundwater and crops, posing a threat to human health [2]. Therefore, toxic metals must either be immobilized in or removed from the contaminated soils to minimize their absorptions by crops and/or transfer into groundwater.
Metal-contaminated soils may be subjected to various engineering remediation techniques, including excavation, solidification, flotation, stabilization, washing, electro-remediation, etc. [3]. However, these techniques are usually expensive, labor-intensive, highly invasive, and may cause secondary pollution of the environment that make them impracticable, especially when large areas or volumes of soils are contaminated [4]. The plant-based (phytoremediation) techniques have therefore been considered as the economically viable and environmentally-friendly methods not only to remediate contaminated soils but also to improve the soil quality parameters [5]. Phytoextraction is a type of phytoremediation method which consists of the absorption and translocation of metals by plant roots into the shoots that can then be harvested and eventually burned to produce energy and metal-enriched ash [6].
Candidate plants for the phytoextraction practice should exhibit such distinctive properties as fast growth potential, deep roots, and ability to absorb metals and transfer them to the shoots [7]. Regardless of the plant features, efficient metal phytoextraction also depends on the bioavailability of metals, which is usually low in neutral and alkaline soils [8]. Plant roots are able to release chelating agents with metal-binding abilities which enhance solubility, and thereby, availability of metals [9]. The availability of metals for the roots can be enhanced dramatically further in the phytoextraction practice through soil-addition of some synthetic chelants which are capable of forming stable soluble complexes with metal ions [10, 11]. The chelants have been revealed to detach metals from the soil solid particles into the soil liquid phase where they are absorbed by the plant roots [12]. These agents also facilitate metal transport from the soil solution into the xylem and improve metal transport from roots to shoots of plants [13].
A number of synthetic chelants such as ethylenediaminetetraacetic acid (EDTA), ethylenediamine-N,N0-bis(2-hydroxyphenyl) acetic acid (EDDHA), and diethylenetriamine pentaacetic acid (DTPA) have been employed in chelant-assisted phytoextraction, among which EDTA is the most widely used [14,15,16]. The increased metal absorption by plants in the presence of EDTA ligand has been reported in several studies and attributed to the formation of metal-EDTA complexes which are readily absorbed by plants [17,18,19]. From 1.3 to 161 folds increases in Pb accumulation by different plant species grown in contaminated soils have been reported as a result of EDTA addition, depending on the soil properties as well as time and method of EDTA application [20]. However, EDTA and its metal complexes have very slow optical, chemical, and biological decompositions [21, 22]. The persistence of EDTA and metal-EDTA chelates in the soil and their negative effects on sensitive plants and microorganisms are main disadvantages of phytoextraction assisted by this chelant [23].
To avoid the negative impacts of EDTA and other persistent chelants in the soil environment, the use of easily biodegradable and eco-friendly alternatives such as ethylenediamine-N,N’-disuccinic acid (EDDS) and nitrilo triacetic acid (NTA) has been proposed in chelant-assisted phytoextraction. For instance, Luo et al. [24] showed that EDDS was more successful than EDTA in enhancing Cu and Zn concentrations in beans and corn. Meers et al. [23] also reported that absorption of soil Cd and Cu by sunflower was higher when EDDS, rather than EDTA, was applied as a chelating agent. More recently, L-glutamic acid-N,N-diacetic acid (GLDA) and methylglycinediacetic acid (MGDA) have been introduced as biodegradable alternatives to persistent chelants [25]. GLDA has been introduced by AkzoNobel Functional Chemicals for commercial use, whose manufacture is based on the monosodium glutamate made from the fermentation of corn sugars [26]. Almost 60% of the L-GLDA decomposes in 28 days [27]. MGDA produced by BASF [28] under the commercial name of Trilon® M is another biodegradable chelant that has been recognized as a readily biodegradable material according to OECD [29] criteria.
Although many reports exist on EDTA-induced phytoextraction, limited information is available so far on the potential role of MGDA in enhancing bioavailability and uptake of metals by plants. To the best of our knowledge, application of GLDA as a chelant to assist metal uptakes by plants has not been reported. The aims of the present study were, therefore, to assess the effects of MGDA and GLDA as compared to EDTA, to enhance Zn and Pb absorption by Zea mays L. grown in a metal-contaminated calcareous soil.
Materials and methods
Site description
A composite soil sample, consisted of 10 sub-samples, was taken from 0–30 cm depth of an abandoned farmland (32° 31’ 02” N 51° 33’ 20” E) at a distance of 500 m from the Irankouh mine cone, Isfahan, Iran. The Bama Company has been exploiting the Zn/Pb deposits in the Irankouh mine as one of the largest Pb/Zn mines in the country since 1962 [30].
Because of the exploitation and processing activities and dust distribution, surrounding soils in a large area are potentially prone to metal contamination [31], though soils naturally contain high Zn and Pb concentrations in some regions [32]. Sepahanshahr city and other residential areas located in the vicinity of the mining facilities are suffering from the consequences of the mining activities [33].
Soil analysis
The composite soil sample was bulked and homogenized, a portion of which was air-dried, crushed, passed through a 2-mm mesh, and used for physico-chemical analyses. Soil pH and electrical conductivity (EC) were measured in soil:water (1:2) extracts with a glass electrode and a conductivity meter, respectively. Organic carbon [34], total nitrogen [35], and cation exchange capacity (CEC) [36] were also measured in the soil subsamples. The particle size distribution was determined by the pipette method [37] and water holding capacity (WHC) of the soil was measured at − 33 kPa potential using a pressure plate apparatus [38]. Pseudo-total Pb and Zn concentrations were measured via digesting soil samples in 6N HNO3 [39], and bioavailable metal concentrations were estimated using the DTPA-CaCl2-TEA extraction method [40]. Metal concentrations in the extracts were measured using a Perkin-Elmer Aanalyst 200 Flame Atomic Absorption Spectrophotometer (FAAS). The detection limits for Zn, Pb, Fe, and Mn were 0.02, 0.01, 0.03 and 0.01 mg L− 1, respectively. Some of the properties and metal concentrations of the soils are listed in Table 1.
Greenhouse experiment
A portion of the soil sample was air-dried, sieved (< 4 mm), and used for a pot experiment. Sub-samples of 2-kg (dry weight basis) were transferred into PVC pots and sown with five seeds of maize (Zea mays L. cv. SC704). Seedlings were thinned to three plants per pot a week after sowing. Plants were grown in a temperature-controlled greenhouse chamber (temperature 32 ± 3 °C, day/night cycle 13/11 h). Irrigation of pots was carried out by daily weighing until obtaining 80% capacity of the field. After 90 days of plant growth, the treatments were imposed. The dissolved chelating agents were applied to the soil surfaces at rates of 0 (control), 2, 4, and 8 mmol kg− 1. The experimental layout was a completely randomized design with three replicates.
Plant analyses
Plant shoots were harvested 7 days after chelant additions [41]. The harvested materials were rinsed with de-ionized water and dried in an oven at 65 ºC for 48 h. Subsequently, the samples were weighed and ground in an electric mill. The ground samples were then dry-ashed at 550 ◦C for 4 h and digested on a hot plate with 2 M HCl. The extracts were then filtered through Whatman 42 filter papers and brought to a final volumes of 50 ml with distilled water [42]. Finally, concentrations of Zn, Pb, Fe, and Mn in the extract were measured using the FAAS.
Metal concentrations in maize shoots were calculated and expressed as mg metal kg− 1 dry weight (mg kg− 1 DW). Metal uptake was calculated by multiplying metal concentration in shoot dry matter by the total weight of dry matter produced by each plant using the following formula:
The soil-to-plant transfer factor (TF) was obtained as the ratio of metal concentration in plant shoot divided by the pseudo-total metal concentration in soil.
Extraction of EDTA, MGDA, and GLDA from plant material was carried out according to Epstein et al. [43]. Briefly, 2 mL of 50% (v/v) ethanol was added to 0.1 g pulverized shoot dried matter, heated at 80 °C for 15 min, and centrifuged at 3000 g for 20 min. The extraction was repeated 3 times and the supernatants from each extraction were combined. Samples were filtered through a 0.22-µm nylon membrane filter (Ultrafree-MC, Millipore) prior to analysis. The filtrates (100 µl) was mixed with 400 µl of 6.5 mM FeCl3 in 7.1 M CH3COOH solution and HPLC grade water to a total volume of 1 mL. Finally, the samples were analyzed using a HP-1090 high-performance liquid chromatograph (HPLC) equipped with a photodiode array detector. A symmetry C18 column (particle size 5 p; 250 mm × 4.6 mm id; Waters Associates Inc., Manchester, UK) was used at 25 °C for the chromatographic separation. The mobile phase comprising 5 mmol L− 1 NH4H2PO4 (pH 2.4) was delivered at a flow rate of 0.5 mL min− 1. The injection volume was 20 µL, and measurements were carried out at a wavelength of 254 nm [44].
Post-harvest soil solution extraction
Soil samples were taken from the pots immediately after the harvest and analyzed for water-soluble metal concentrations by extraction with deionized water at soil-to-water ratio of 1:5 [45]. After shaking for 2 h at 120 rpm, the samples were centrifuged and filtered to collect the supernatants and analyzed for metal concentrations by the AAS. Speciation modeling was performed using geochemical computer program PHREEQC (version 2.18.00) for calculation of the dominant metal species in soil solution.
Statistical analysis
Statistical data analysis was conducted using SAS V9.0 software and drawing diagrams via Excel 2013. One-way ANOVA was conducted to assess whether significant (P < 0.05) differences existed among the chelants with respect to their effects on growth and metal absorption of the maize plants. Mean comparisons were performed according to the LSD test in a completely randomized design.
Results
Effects of the chelants on maize shoot growth
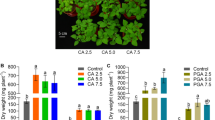
Figure 1 shows the effects of chelants on maize shoot dry weight (DW). As can be seen, the addition of the chelants significantly (P < 0.05) increased shoot DW of maize compared to that of the control. All three chelants induced nearly the same enhancing effect on maize shoot DW at 2 and 4 mmol kg− 1 application levels. At 8 mmol kg− 1 chelant concentration, however, MGDA induced significantly (P < 0.05) higher shoot DW than EDTA, while GLDA performed intermediately between the other two chelants. No visible toxicity symptoms appeared in the maize plants after the chelant addition, except for necrotic patches observed in a few plant leaves at the highest application dose of EDTA.
Chelant effects on shoot Zn content
Compared to the control, application at all levels of MGDA and GLDA increased the Zn concentration in harvested maize biomass while EDTA significantly affected that only at the 4 and 8 mmol kg− 1. Moreover, MGDA and GLDA were more successful than EDTA in increasing the Zn concentration in maize shoots at all application levels (Fig. 2). The greatest enhancing effect on the shoot Zn concentration was achieved when 8 mmol kg− 1 of the chelants was applied. MGDA and GLDA had nearly the same effect on shoot Zn concentration at all 3 application rates (Fig. 2A). For example, shoot Zn concentration of the control plants (without chelant addition) was 184 mg kg− 1, which increased to 277 and 281 mg kg− 1 when 8 mmol kg− 1 of MGDA and GLDA were added to the soil, respectively.
The effectiveness of chelant-assisted phytoextraction was assessed by the total amount of Zn removed from the soil and transferred to the plant shoot since the success of phytoextraction is dependent on harvested shoot biomass as well as shoot Zn concentration. The results showed that the applied chelants significantly (P < 0.05) enhanced the total amount of Zn taken up by the harvested maize biomass at all three application levels (Fig. 3). The highest Zn uptake was observed at the MGDA and GLDA levels of 8 mmol kg− 1 which were significantly (P < 0.05) higher than that of the maize shoots grown in the EDTA-amended soil at the same application level. Total shoot Zn uptake in the control plant was 100.9 mg plant− 1 which significantly (P < 0.05) increased by 7.92, 7.12, and 5.26-folds when 8 mmol kg− 1 of MGDA, GLDA, and EDTA were applied to the soil, respectively. There were no statistical differences among the enhancing effect of the chelants on shoot Zn uptake at 2 and 4 mmol kg− 1 application rates (Fig. 2).
Chelant effects on shoot Pb content
Effects of chelating agents on Pb concentration in the maize shoots are shown in Fig. 2. The chelants, at all application levels, increased Pb concentration of the maize shoots in the order EDTA > GLDA ~ MGDA. For instance, Pb concentration in the harvested biomass of the control plants (without chelant addition) was 18 mg kg− 1, which increased to 143, 155, and 187 mg kg− 1 when 8 mmol kg− 1 of MGDA, GLDA, and EDTA were added to the soil, respectively.
Figure 3 shows the total Pb taken up by the harvested maize biomass as affected by the chelant treatments. The chelants significantly (P < 0.05) increased total uptake of Pb into shoots compared to that of the control, except for MGDA and GLDA at the lowest application level (2 mmol kg− 1). Addition of 8 mmol kg− 1 MGDA, GLDA, and EDTA were the most effective treatments leading to 39.2, 37.5, and 39.2 folds increases in total Pb uptake by plant shoots, respectively, and there were no significant differences among the chelants with this respect (Fig. 3).
Metal solubility in post-harvest soils
Figure 4 shows the soluble Zn and Pb concentrations in the post-harvest soil. Both Pb and Zn mobilization by the chelants were dose-dependent, with the highest dose (8 mmol kg− 1) mobilizing the highest metal amounts, suggesting that no saturation of metal complexation occurred within the range of chelants used in this experiment. The water-soluble Zn in the unamended soil was 1.4 mg kg− 1. However, when 8 mmol kg− 1 of MGDA, GLDA, and EDTA was added, the water-extractable Zn significantly (P < 0.05) increased to 15.3, 15.7, and 60 mg kg− 1, respectively, corresponded to 9.9, 10.2, and 41.8 times increases compared to that of the control. The use of chelants also significantly (P < 0.05) increased the solubility of Pb in the soil compared to the control. For instance, adding 8 mmol kg− 1 of MGDA, GLDA, and EDTA increased soluble Pb concentration by 6.16, 6.75, and 12.29 times, respectively, compared to that of the control (Fig. 4).
Discussion
Plant growth
The higher plant growth observed in this study in the presence of the chelants can directly reflect the plant’s enhanced nutritional status or its improved resistance to metal-induced stresses. For instance, it has been well known that metal-chelates in plants generally have lower negative effects on cell processes than the free ionic metals do [46, 17]. Liu et al. [47] reported that the Pb-induced decrease in pigments contents in Sedum alfredii H. was alleviated by the EDTA treatment. Ruley et al. [48] also showed that applications of EDTA, DTPA, HEDTA, NTA, and citric acid alleviated the negative effects of free Pb on the growth and photosynthetic activity on Sesbenia durmmondii grown in a contaminated soil. Similarly, González et al. [49] showed that the application of 6 and 10 mmol plant− 1 of MGDA significantly increased the biomass produced by Oenothera picensis plants grown in a copper-contaminated soil.
Moreover, Pb-induced oxidative stresses are prevented by chelating agents. Huang et al. [50], for example, reported that the presence of EDTA reduced Pb-induced peroxidation of lipids and induction of anti-oxidative enzymes in Sedum alfredii H. Ruley et al. [51] reported that chelants mitigate Pb-induced oxidative stress by modulating anti-oxidative enzyme activities in Sesbania drummondii seedlings. The protective role of EDTA against Pb-induced toxicity in Vicia faba was also demonstrated by Shahid et al. [15] and attributed to decreasing free Pb2+ concentration in the plant via Pb–EDTA complex formation.
It is also possible that the growth-promoting effects of the chelants might have been due to the enhanced nutrient absorption of the plants [52, 53]. For instance, the application of the chelants in the current study at all doses significantly (P < 0.05) increased Fe and Mn absorption by the plants (Figs. 2 and 3). Maize plants grown in the absence of the chelating agents had a mean shoot Fe concentration of 14.7 mg kg− 1 which increased to 70.7, 69.3, and 80.0 mg kg− 1 in the presence of 8 mmol kg− 1 MGDA, GLDA, and EDTA, respectively (Fig. 2). Soil amendment of 8 mmol kg− 1 MGDA, GLDA, and EDTA also increased Mn concentration in harvested maize biomass from 6.0 mg kg− 1 in the control to 130.0, 129.3, and 154 mg kg− 1, respectively (Fig. 2). Fässler et al. [52] also reported that the application of EDDS to the growth medium had a positive effect on the growth of sunflower, and they attributed it to the enhanced Fe absorption and translocation from the roots to the shoots. Chaturvedi et al. [54] reported that application of EDTA led to enhanced mobilization and uptake of micronutrients by Raphanus sativus L. and Brassica oleracea L. Release of phosphates bound by soil colloids induced by chelating ligands has also been reported as a possible mechanism to increase plant available phosphorus in the soil [55].
Metal phytoextraction
Zea mays L. is of interest as a plant to be used for concurrent energy crop production and phytoextraction because it produces a high biomass yield and accumulates substantial quantities of metals, particularly when induced by the addition of chelants [56]. The results obtained in this study revealed that MGDA, GLDA, and EDTA applications at 2, 4, and 8 mmol kg− 1 significantly enhanced metal absorption by maize plants (Fig. 2).
One mechanism by which the applied chelants could promote metal absorption by maize plant is by increasing the metal transfer from the soil solid phase to the root surfaces. Complex formation of the soil metals with the chelants at the soil-water interface and subsequent metal detachments into the soil solution increases the total metal concentrations in the soil solution, thus enhancing the metal transportations through diffusion or mass flow toward the roots [57]. An alternative mechanism could be that the metal-EDTA complexes, unlike free metal ions, are not bound to the carboxyl groups or polysaccharides on the rhizodermal cell surface, thereby leading to a more easily transport of the complexes through the cortex to the xylem vessels [15].
In the current study, a close relationship was found between the concentrations of metals and chelants in the maize shoots (Fig. 5). This finding may reveal that the metal chelates were directly absorbed by the roots and transferred to the shoots. While we do not know of any studies which have focused on the effects of MGDA and GLDA on metal uptake by plants, some studies have been tried on the mechanisms of EDTA-induced metal absorption and translocation in plants. Epstein et al. [43] and Schaider et al. [58], for example, demonstrated that Pb-EDTA was the main form of Pb absorbed and translocated to the shoots in Brassica juncea. Luo et al. [59] also found a significant positive correlation between Pb and EDTA concentrations in mustard shoots.
It is assumed that metal chelates, upon absorption, pass through the root apoplasm, flow to the xylem, and transfer to the shoot by the evapotranspiration pressure [49, 20]. Binding of metals to the extracellular sites of the apoplast and cell walls is reduced when they are absorbed in complexed form, and thereby the translocation of the metals toward the root xylem is facilitated [60]. Speciation estimations suggest that in the chelant-treated soils almost all Pb and Zn ions were complexed by the chelants. Formation of anionic metal-ligand complexes in the soil solution can explain the higher Pb absorption and translocation by the maize plants in the presence of the chelants. Chelation of metals by ligands can also be described to decrease possible metal precipitation in roots as insoluble salts [20].
The Casparian strip at the root endodermis presumably blocks water flow in the apoplastic pathway. However, it has been suggested that Pb-EDTA uptake by plant roots was restricted to the region between 3 and 140 mm from the root tip, where the cell walls suberization had not yet taken place [61]. The apoplastic transport of the Pb-EDTA complexes could also take place through breaks in the Casparian strip, where lateral roots are initiated and the suberized region has yet to be reformed [62]. Any physical damage of the roots can also increase the absorption of the metal-EDTA complex [57].
The soil-to-plant transfer factor (TF), expressed as the ratio of metal concentration in plant shoot divided by the pseudo-total metal concentration in soil, is an index for assessing the transfer potential of a metal from soil to harvestable plant biomass [63]. In this study, the TF value of Zn and Pb in the control system (without chelant addition) were 0.32 and 0.07, respectively. The obtained results are in agreement with the previous studies, suggesting that maize plants are more favorable for accumulating Zn than Pb [64, 65]. The TF values of 0.82 and 0.07 have also been reported for Zn and Pb, respectively, in maize plants grown in a metal-contaminated soil [64]. Moreover, chelant additions affected more drastically the TF value of Pb than that of Zn. For instance, the TF value of Zn increased from 0.32 in control to 0.49, 0.49, and 0.42 in the soils treated with 8 mmol kg− 1 MGDA, GLDA, and EDTA, respectively. These represent 1.53, 1.53, and 1.31 folds increases, respectively, over the control. The TF value of Pb, however, increased from 0.07 in the control system to 0.58, 0.63, and 0.76, representing 7.3, 9.0, and 10.8 folds increases, respectively, in the soils amended with 8 mmol kg− 1 MGDA, GLDA, and EDTA.
Post-harvest metal concentrations in soil solution
The results revealed that the water-soluble concentrations of Pb and Zn were significantly raised in the chelant-treated soils as compared to those of the control (Fig. 4). Chelating agents desorb metals from the soil solid particles by forming stable soluble complexes. Speciation calculations confirmed that in the soils treated with MGDA, GLDA, and EDTA ligands, almost all Pb ions were chelated as Pb-MGDA−, Pb-GLDA2−, and Pb-EDTA2−, respectively. The dominant Zn species in the MGDA-, GLDA-, and EDTA-treated soils were ZnGLDA− and ZnHGLDA−, and Zn-EDTA2−, respectively. The formation of these negatively-charged chelates prevented free metal ions from binding to the soil particles.
In addition, the amounts of water-extracted Pb and Zn from soil treated with EDTA were significantly higher than those treated with GLDA and MGDA (Fig. 4). At the highest applied level, for example, EDTA induced soil solution Zn concentration of 60 mg kg− 1, while equal doses of GLDA and MGDA caused mobilization of 15.7 and 15.3 mg kg− 1 of Zn. This could be due to the fact that EDTA forms much more stable complexes with metals than GLDA and MGDA do [66]. Also, metal-GLDA and metal-MGDA complexes in the soil may be degraded faster than metal-EDTA complexes. In chelant-assisted phytoextraction practices, the higher the soluble metal concentration in the post-harvest soil, the more it may be leached and transferred into the ground- and surface-water bodies. Chen et al. [67] showed that about 3.5% and 13.7% of soil Pb and Zn, respectively, were leached from the contaminated soil columns after the application of 5.0 mmol kg− 1 of EDTA. Therefore, toxic metals are more vulnerable to leaching and more readily affect soil organisms in EDTA-treated soil than those treated with MGDA and GLDA.
Conclusions
Application feasibility of MGDA and GLDA, as eco-friendly alternatives of EDTA, for enhancing Pb and Zn uptake of maize (Zea Maize L.) from soil were tested in a greenhouse experiment. Measurements of Pb and Zn in shoots of maize plants revealed that MGDA and GLDA were superior to, or comparable with, EDTA in terms of phytoextraction improvement. In contrast to EDTA, however, the new ligands caused significantly lower postharvest soil metal concentrations, which can decrease the risk of adverse environmental effects due to reduced metal mobilization and persistence in the postharvest soil. The results of this study, therefore, support the use of MGDA and GLDA as promising chelants for enhanced environmentally-friendly phytoextraction of metals from contaminated soils. Application of GLDA and MGDA also significantly increased Fe and Mn availability to maize plants which are crucial for proper plant growth and, hence, phytoremediation efficiency. Furthermore, addition of these green chelants to soils can be implemented for biofortifcation of crops with Zn, Fe, and Mn.
References
Soriano A, Pallarés S, Pardo F, Vicente A, Sanfeliu T, Bech J. Deposition of heavy metals from particulate settleable matter in soils of an industrialised area. J Geochem Explor. 2012;113:36–44.
Micó C, Recatalá L, Peris M, Sánchez J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere. 2006;65(5):863–72.
Hasegawa H, Rahman IMM, Rahman MA. Environmental remediation technologies for metal-contaminated soils. Berlin: Springer; 2016.
Hakeem K, Sabir M, Ozturk M, Mermut AR. Soil remediation and plants: prospects and challenges. Cambridge: Academic; 2014.
Hernández-Allica J, Becerril J, Zárate O, Garbisu C. Assessment of the efficiency of a metal phytoextraction process with biological indicators of soil health. Plant Soil. 2006;281(1–2):147–58.
Fangueiro D, Kidd PS, Alvarenga P, Beesley L, de Varennes A. Strategies for soil protection and remediation. In: Duarte AC, Cachada A, Rocha-Santos T, editors. Soil pollution. Cambridge: Academic; 2018. p. 251 – 81.
Robinson BH, Mills TM, Petit D, Fung LE, Green SR, Clothier BE. Natural and induced cadmium-accumulation in poplar and willow: Implications for phytoremediation. Plant Soil. 2000;227(1–2):301–6.
Quartacci M, Argilla A, Baker A, Navari-Izzo F. Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere. 2006;63(6):918–25.
Parker D, Reichman S, Crowley D. Metal chelation in the rhizosphere. In: Wright SF, Zabeb RW, editors. Root and soil management: interactions between roots and the soil. Madison: American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; 2005. pp. 57–93.
Song J, Zhang H, Duan C, Cui X. Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress. J For Res. 2018;29(6):1497–505. https://doi.org/10.1007/s11676-017-0579-0.
Khan I, Iqbal M, Shafiq F. Phytomanagement of lead-contaminated soils: critical review of new trends and future prospects. Int J Environ Sci Technol. 2019;16(10):6473–88. https://doi.org/10.1007/s13762-019-02431-2.
Leštan D, Luo C-l, Li X-d. The use of chelating agents in the remediation of metal-contaminated soils: a review. Environ Pollut. 2008;153(1):3–13.
Huang JW, Chen J, Berti WR, Cunningham SD. Phytoremediation of lead-contaminated soils: role of synthetic chelates in lead phytoextraction. Environ Sci Technol. 1997;31(3):800–5.
Evangelou MW, Ebel M, Schaeffer A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere. 2007;68(6):989–1003.
Shahid M, Austruy A, Echevarria G, Arshad M, Sanaullah M, Aslam M, et al. EDTA-enhanced phytoremediation of heavy metals: a review. Soil Sediment Contam. 2014;23(4):389–416.
Gul I, Manzoor M, Silvestre J, Rizwan M, Hina K, Kallerhoff J, et al. EDTA-assisted phytoextraction of lead and cadmium by Pelargonium cultivars grown on spiked soil. Int J Phytoremediation. 2019;21(2):101–10. https://doi.org/10.1080/15226514.2018.1474441.
Shahid M, Pinelli E, Pourrut B, Silvestre J, Dumat C. Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol Environ Saf. 2011;74(1):78–84.
Sun Y-b, Zhou Q-x, An J, Liu W-t, Liu R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma. 2009;150(1–2):106–12.
Zia MH, Meers E, Ghafoor A, Murtaza G, Sabir M, Zia-ur-Rehman M, et al. Chemically enhanced phytoextraction of Pb by wheat in texturally different soils. Chemosphere. 2010;79(6):652–8.
Shahid M, Pinelli E, Dumat C. Review of Pb availability and toxicity to plants in relation with metal speciation; role of synthetic and natural organic ligands. J Hazard Mater. 2012;219:1–12.
Nörtemann B. Biodegradation of EDTA. Appl Microbiol Biotechnol. 1999;51(6):751–9. https://doi.org/10.1007/s002530051458.
Oviedo C, Rodríguez J. EDTA: the chelating agent under environmental scrutiny. Quim Nova. 2003;26(6):901–5.
Meers E, Ruttens A, Hopgood M, Samson D, Tack F. Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere. 2005;58(8):1011–22.
Luo C, Shen Z, Li X. Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere. 2005;59(1):1–11.
Begum ZA, Rahman IM, Tate Y, Sawai H, Maki T, Hasegawa H. Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants. Chemosphere. 2012;87(10):1161–70.
Seetz J, Stanitzek T, editors. GLDA: the new green chelating agent for detergents and cosmetics. SEPAWA Congress and European Detergents Conference Proceedings; 2008.
Kołodyńska D. Application of a new generation of complexing agents in removal of heavy metal ions from different wastes. Environ Sci Pollut Res. 2013;20(9):5939–49.
BASF. Technical Information (Ti/EVD 1418 e): Trilon® M types: BASF2007.
OECD. Biodegradability. DOC Die-Away method (301A). Guideline for testing chemicals. Paris: Organisation for Economic Cooperation and Development; 1992.
Seifabad MC, Zavareh SB. Excavation method in Goushfill mine. Int J Res Eng Technol. 2013;2(3):225.
Arabyarmohammadi H, Darban AK, van der Zee SE, Abdollahy M, Ayati B. Fractionation and leaching of heavy metals in soils amended with a new biochar nanocomposite. Environ Sci Pollut Res. 2018;25(7):6826–37.
Ghaderian S, Hemmat G, Reeves R, Baker A. Accumulation of lead and zinc by plants colonizing a metal mining area in Central Iran. J Appl Bot Food Qual. 2007;81(2):145–50.
Dayani M, Mohammadi J. Geostatistical assessment of Pb, Zn and Cd contamination in near-surface soils of the urban-mining transitional region of Isfahan, Iran. Pedosphere. 2010;20(5):568–77.
Nelson D, Sommers L. Total carbon, organic carbon, and organic matter. In: Sparks DL, editor. Methods of soil analysis part 3—chemical methods. vol methodsofsoilan3. Madison: Soil Sci. Soc. Am. and Am. Soc. Agr.; 1996. p. 961–1010.
Bremner J. Nitrogen-total. In: Sparks DL, editor. Methods of soil analysis part 3—chemical methods. Madison: SSSA and ASA; 1996. pp. 1085–121.
Sumner M, Miller W. Cation exchange capacity and exchange coefficients. In: Sparks DL, editor. Methods of soil analysis. Part 3. Madison: SSSA and ASA; 1996. pp. 1201–29.
Gee G, Or D. Particle-size analysis. In: Dane JH, Topp GC, editors. Methods of soil analysis. Part 4. Vol. 598. Madison: SSSA; 2002. pp. 255–93.
Cassel D, Nielsen D. Field capacity and available water capacity. In: Klute A, editor. Methods of soil analysis: Part 1—Physical and mineralogical methods. Madison: SSSA and ASA; 1986. pp. 901–26.
Zheljazkov VD, Nielsen NE. Effect of heavy metals on peppermint and cornmint. Plant Soil. 1996;178(1):59–66.
Lindsay WL, Norvell WA. Development of a DTPA soil test for zinc, iron, manganese, and copper 1. Soil Sci Soc Am J. 1978;42(3):421–8.
Wenger K, Gupta S, Schulin R. The value of nitrilotriacetate in chelate-assisted phytoremediation. Dev Soil Sci. 2008;32:679–95.
Estefan G, Sommer R, Ryan J. Methods of soil, plant, and water analysis. Beirut: International Center for Agricultural Research in the Dry Areas (ICARDA); 2013.
Epstein AL, Gussman CD, Blaylock MJ, Yermiyahu U, Huang JW, Kapulnik Y, et al. EDTA and Pb—EDTA accumulation in Brassica juncea grown in Pb—amended soil. Plant Soil. 1999;208(1):87–94.
Miah S, Rahman IM, Takemura M, Fukiage S, Mashio AS, Maki T, et al. Determination of multiple chelator complexes in aqueous matrices using ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry. Talanta. 2019;194:980–90.
Yu H, Wang J, Fang W, Yuan J, Yang Z. Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice. Sci Total Environ. 2006;370(2–3):302–9.
Piechalak A, Tomaszewska B, Barałkiewicz D. Enhancing phytoremediative ability of Pisum sativum by EDTA application. Phytochemistry. 2003;64(7):1239–51.
Liu D, Li TQ, Jin XF, Yang XE, Islam E, Mahmood Q. Lead induced changes in the growth and antioxidant metabolism of the lead accumulating and non-accumulating ecotypes of Sedum alfredii. J Integr Plant Biol. 2008;50(2):129–40.
Ruley AT, Sharma NC, Sahi SV, Singh SR, Sajwan KS. Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ Pollut. 2006;144(1):11–8.
González I, Cortes A, Neaman A, Rubio P. Biodegradable chelate enhances the phytoextraction of copper by Oenothera picensis grown in copper-contaminated acid soils. Chemosphere. 2011;84(4):490–6.
Huang H, Li T, Tian S, Gupta D, Zhang X, Yang X-e. Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Bioresour Technol. 2008;99(14):6088–96.
Ruley AT, Sharma NC, Sahi SV. Antioxidant defense in a lead accumulating plant, Sesbania drummondii. Plant Physiol Biochem. 2004;42(11):899–906.
Fässler E, Evangelou MW, Robinson BH, Schulin R. Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere. 2010;80(8):901–7.
Yu F, Li Y, Li F, Li C, Liu K. The effects of EDTA on plant growth and manganese (Mn) accumulation in Polygonum pubescens Blume cultured in unexplored soil, mining soil and tailing soil from the Pingle Mn mine, China. Ecotoxicol Environ Saf. 2019;173:235–42.
Chaturvedi R, Favas P, Pratas J, Varun M, Paul MS. EDTA-Assisted Metal Uptake in Raphanus sativus L. and Brassica oleracea L.: Assessment of Toxicity and Food Safety. Bull Environ Contam Toxicol. 2019;103(3):490–5. https://doi.org/10.1007/s00128-019-02651-9.
Edwards CL. Effect of Synthetic chelating agent application to soils on phosphorus availability: Virginia Tech; 2013.
Meers E, Van Slycken S, Adriaensen K, Ruttens A, Vangronsveld J, Du Laing G, et al. The use of bio-energy crops (Zea mays) for ‘phytoattenuation’of heavy metals on moderately contaminated soils: a field experiment. Chemosphere. 2010;78(1):35–41.
Jiang M, Liu S, Li Y, Li X, Luo Z, Song H, et al. EDTA-facilitated toxic tolerance, absorption and translocation and phytoremediation of lead by dwarf bamboos. Ecotoxicol Environ Saf. 2019;170:502–12.
Schaider LA, Parker DR, Sedlak DL. Uptake of EDTA-complexed Pb, Cd and Fe by solution- and sand-cultured Brassica juncea. Plant Soil. 2006;286(1):377–91. https://doi.org/10.1007/s11104-006-9049-8.
Luo C, Shen Z, Li X, Baker AJ. The role of root damage in the chelate-enhanced accumulation of lead by Indian mustard plants. Int J Phytoremediation. 2006;8(4):323–37.
Tandy S, Schulin R, Nowack B. The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere. 2006;62(9):1454–63.
Tanton T, Crowdy S. Water pathways in higher plants: II. Water pathways in boots. J Exp Bot. 1972;23(3):600–18.
Collins RN, Merrington G, McLaughlin MJ, Knudsen C. Uptake of intact zinc-ethylenediaminetetraacetic acid from soil is dependent on plant species and complex concentration. Environ Toxicol Chem. 2002;21(9):1940–5.
Wang G, Su M-Y, Chen Y-H, Lin F-F, Luo D, Gao S-F. Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ Pollut. 2006;144(1):127–35.
Máthé-Gáspár G, Anton A. Phytoremediation study: Factors influencing heavy metal uptake of plants. Acta Biol Szeged. 2005;49(1–2):69–70.
Wuana R, Okieimen F, Imborvungu J. Removal of heavy metals from a contaminated soil using organic chelating acids. Int J Environ Sci Technol. 2010;7(3):485–96.
Begum ZA, Rahman IM, Tate Y, Egawa Y, Maki T, Hasegawa H. Formation and stability of binary complexes of divalent ecotoxic ions (Ni, Cu, Zn, Cd, Pb) with biodegradable aminopolycarboxylate chelants (dl-2-(2-carboxymethyl) nitrilotriacetic acid, GLDA, and 3-hydroxy-2, 2′-iminodisuccinic acid, HIDS) in aqueous solutions. J Solution Chem. 2012;41(10):1713–28.
Chen Y, Li X, Shen Z. Leaching and uptake of heavy metals by ten different species of plants during an EDTA-assisted phytoextraction process. Chemosphere. 2004;57(3):187–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masoudi, F., Shirvani, M., Shariatmadari, H. et al. Performance of new biodegradable chelants in enhancing phytoextraction of heavy metals from a contaminated calcareous soil. J Environ Health Sci Engineer 18, 655–664 (2020). https://doi.org/10.1007/s40201-020-00491-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00491-y