Abstract
Objectives
Glucose monitoring in diabetes is changing overtime with a constant development of new devices for continuous glucose monitoring (CGM). Aim of this observational, prospective study was to evaluate the clinical performance of a novel intermittently scanned CGM system, the Glunovo Flash in a cohort of patients with type 1 diabetes.
Methods
A total of 45 patients with T1D followed at the Endocrinology Unit of the ASST-FBF-Sacco (Milan) were enrolled. All patients were habitual CGM users and were asked to wear simultaneously the Glunovo Flash system and their habitual CGM device for 14 days. A comparison of CGM glucose metrics was performed. Patients’ opinions on the new device were also collected.
Results
Thirty-five patients completed the study period of two weeks (7 habitual real time CGM users, 28 habitual intermittently scanned CGM users). Mean Time In Range resulted significantly higher with the novel studied sensor respect to intermittently scanned CGM comparator. No differences were found considering other glucose metrics. A positive correlation was found between the Time In Range recorded by Glunovo Flash and intermittently scanned CGM comparators as well as for Time Above Range, Glucose Management Indicator, Time Below Range and Coefficient of Variation. No correlations were found between glucose metrics recorded by Glunovo Flash and real time CGM comparators. Patients reported a positive experience of use with the new sensor but some elements appeared improvable.
Conclusions
The CGM device Glunovo Flash for patients with diabetes shows similar performance to other intermittently scanned CGM systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Both intermittently scanned continuous glucose monitoring (isCGM, also named Flash Glucose Monitoring) and real time continuous glucose monitoring (rtCGM) can improve glycemic outcomes and quality of life in children, adolescents and adults with type 1 diabetes (T1D) compared to self-monitoring of blood glucose [1, 2]. CGM provides a complete overview of patients’ glucose trends and time spent in defined glucose ranges [3], being an irreplaceable tool in clinical practice. Intermittently scanned continuous glucose monitoring, particularly, has greater convenience and ease of use and it can ensure successful management of multiple dose insulin therapy also in type 2 diabetes [4, 5]. Moreover, emerging evidence demonstrated CGM usefulness also in hospitalized, complicated and frail subjects with increased risk of adverse outcomes related to uncontrolled blood glucose levels [6,7,8,9]. An effective utilization of continuous glucose monitoring technology depends on different factors such as patient’s acceptance and usability but also analytical, clinical and perceived accuracy [10,11,12]. Several new CGM devices are commercialized, available and reimbursed by the health systems [13]. Nevertheless, only the earliest and most used ones (i.e., Dexcom, Medtronic Minimed Guardian, Abbott Freestyle Libre, Senseonis Eversense) have a considerable amount of published data and scientific evidences to support their use. Glunovo Flash is a new CGM system for intermittently scanned monitoring with some unique features, such as a 14 days lasting sensor to be applied on the abdomen, a 36 months lasting reusable transmitter and frequency of glucose recording of 3 min. It is endowed of hypoglycemia and hyperglycemia threshold alarms and it has a smartphone app as a receiver (Iris Flash app). First Glunovo Flash generation (i3) needed two daily calibrations by capillary blood glucose, while the second generation (P3F), currently delivered, is factory calibrated. Preliminary unpublished data showed a mean absolute relative difference of nearly 10% (https://www.a-ps.it/wp-content/uploads/2022/01/glunovo_flash_brochure_2021_EN.pdf.). The aim of this study was to evaluate the glycemic outcomes registered with the use of the Glunovo Flash CGM system and patients’ usage experience as compared to other devices routinely used for blood glucose monitoring (rtCGM or isCGM) in a cohort of patients with T1D.
Methods
To investigate the Glunovo Flash system performance in a clinical setting we designed an observational, prospective, single arm, single center study. Consecutive adult patients with T1D habitual CGM users on multiple dose insulin therapy or continuous subcutaneous insulin infusion were screened during regular diabetes care consultations at the Endocrinology Unit of the ASST-FBF-Sacco, Milan. Pregnancy, breastfeeding, acute inflammatory states, ongoing use of acetaminophen or documented allergic reaction to any glucose monitoring system material were considered exclusion criteria. The primary objective of the study was to the head-to-head comparison between glycemic outcomes registered by the Glunovo Flash system and other sensors (habitual CGM device, used as comparator). Secondary objective was the evaluation of patients’ experience and reactions to the new device. Eligible patients simultaneously used the Glunovo Flash system for 14 days (corresponding to the duration of the sensor) and the CGM comparator. They performed capillary blood glucose measurements depending on clinical need. Enrolled patients were trained to the technical use of the study sensor and to the exclusive clinical utilization of glucose data obtained from habitual devices. The study design (two different sensor worn simultaneously) did not allow validated questionnaires administration. Therefore, free comments of subjects were collected and ranked in order of frequency. Data were downloaded after two weeks of simultaneous utilization of the two sensors from dedicated platforms (Glunovo share, Dexcom Clarity, CareLink™ System and LibreView digital diabetes). The study was approved by local Ethical Committee and all the participants signed a consent form. Data are presented as % or mean ± SD. A sample t-test analysis has been performed for primary endpoint continuous variable. Correlation analyses were performed using the Pearson correlation coefficient. All data analysis were performed with Graph-Pad Prism version 8.0; GraphPad Software, Inc., San Diego, CA. Two-tailed P values of less than 0.05 were considered statistically significant.
Results
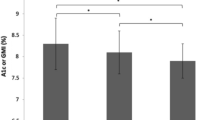
Between June 2022 and June 2023, we screened 52 patients and 45 expressed the intention to participate. Mean age was 49.5 ± 13.9 years, mean duration of diabetes 19.9 ± 14.7 years, mean BMI 25.3 ± 3.7 Kg/m2, mean HbA1c 7.2 ± 0.9%. 10 cases were considered drop out for sensor wear shorter than 2 days because of technical issues, spontaneous removal by the patient or cutaneous reactions. Thirty-five subjects with these characteristics completed the study period of two weeks. Two patients were on continuous subcutaneous insulin infusion. Seven were habitual users of a rtCGM device (one Minimed Guardian 4, six Dexcom G6), twenty-eight of a isCGM device (fourteen Freestyle Libre 1, fourteen Freestyle Libre 2 with activated alarms).
Glycemic outcomes
Glunovo Flash data analysis of the whole cohort revealed (mean values) a glucose management indicator of 7.0 ± 1.3%, time in range of 69.5 ± 22.7%, coefficient of variation of 31.3 ± 10.1%, time below range of 2.6 ± 3.7%, time above range of 27.5 ± 23.9. Subgroup analysis of rtCGM and isCGM users with comparison between Glunovo Flash and habitual sensor data are detailed in Table 1. A strong positive correlation was found between glucose management indicator, time in range, time above range and time below range recorded by Glunovo Flash and isCGM comparator (Fig. 1). A positive correlation was also found between coefficient of variation. No correlation was found between glucose metrics recorded by Glunovo Flash and rtCGM comparator.
Correlation analysis of data obtained with study sensor (Glunovo Flash) and isCGM Comparator. TIR (%) obtained with Glunovo system show direct correlation with TIR (%) obtained with isCGM comparator (A), as well as TBR (%) among the two systems (B), TAR (%) (C) and GMI (%) (D) Abbreviations: isCGM intermittent scanning Continuous Glucose Monitoring, TIR Time In Range 70–180 mg/dL, TBR Time Below 70 mg/dL, TAR Time Above Range, GMI Glucose Management Indicator
Patient perspectives
Most of patients felt no difference in sensor wear and experienced similar skin issues respect to the CGM comparator. Sensor duration, quality design, usability and alarm settings were considered positive features of Glunovo Flash system. On the other hand, patients reported a worse experience with system receiver app and some of them experienced fewer accuracy respect to the comparator. Spontaneous patients’ comments clustered in different items are listed and ranked by frequency in Table 2.
Discussion
This study evaluates the clinical performance of a novel intermittently scanned continuous glucose monitoring system, the Glunovo Flash system, in patients T1D. Different studies have demonstrated that continuous glucose monitoring is a cost-effective strategy in the management of T1D reducing diabetes-related complications and hospitalization, and it will likely replace self-monitoring of blood glucose in near future [14,15,16]. Therefore, studies evaluating new CGM systems accuracy and usability are of particular interest. Glunovo real time CGM (rt-CGM) performance has been evaluated in a multicenter accuracy study in patients with T1D and T2D, demonstrating a satisfying mean absolute relative difference over 14 days, with mean value of 10.30 ± 4.86% [17]. To date no studies on the Glunovo Flash system are present in literature, despite its current clinical use in some countries. In this study all patients were already using another CGM system and were asked to keep it while wearing the study sensor. We compared the glucose metrics recorded by Glunovo Flash with those obtained by Abbott isCGM FreeStyle Libre 1 and Libre 2, Dexcom G6 rtCGM and Medtronic Guardian 4 rtCGM. As expected, most of the metrics did not differ between the study sensor and comparators. Surprisingly, mean time in range (70–180 mg/dL) resulted significantly higher with the novel studied sensor respect to isCGM comparator and not statistically but clinically higher respect to rtCGM. Nevertheless, a strong correlation was found between the time in range values of study sensor and isCGM comparator, as well as time below range, time above range and glucose management indicator (Fig. 1). No correlation was found comparing time in range of study sensor with rtCGM, likely for the small size of the group considered (7 subjects). These results point out the need of further investigate and compare different systems performance in larger cohorts of patients, to give an exhaustive interpretation to these data. To date there are few head-to-head studies comparing isCGM and rtCGM systems used simultaneously in the same patients [18]. Accuracy data with comparison between sensor readings and standardized reference (i.e., Yellow Springs Instrument) are also needed. Besides objective comparisons, we also investigated patients’ personal experience and perception with the system, as they constitute a key factor for the subsequent therapeutic adherence [12]. Altogether, patients felt no differences in sensor wearing, with almost similar skin issues respect to the CGM comparator. About 9% of subjects experienced skin issues, but no major adverse skin reactions were reported. Among patients complains, some reported a worse experience with the Glunovo Flash app receiver respect to the comparator ones in terms of frequent transmitter signal losses or difficulties in sensor-transmitter matching. On the other hand, sensor duration of 14 days, alarm settings for hypo- and hyper-glycemia thresholds, frequent glucose values recordings and quality design were considered positive features. Regarding perceived accuracy, 5 of the 38 patients who completed the study experienced fewer accuracy respect to the comparator. This could happen with all CGM systems and patients should be trained to give right interpretation to such discrepancies. Strengths of the study are the homogeneity of the sample of subjects enrolled, the simultaneous utilization of two CGM systems and its real-life setting. Limitations include a rather low number of patients enrolled and short follow-up. In conclusion, Glunovo system seems to be a reliable isCGM device for patients with diabetes showing a good performance correlation with the most used isCGM. Some data discordance about Time In Range must be further investigated in larger studies and correlated to accuracy data. Patients’ issues should be traced and recorded by the digital diabetes platforms according to international guidelines [19].
Data availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Sandig D et al (2020) Continuous glucose monitoring in adults with type 1 diabetes: real-world data from the German/Austrian prospective diabetes follow-up registry. Diabetes Technol Ther 22:1–11. https://doi.org/10.1089/dia.2020.0019
Cherubini V et al (2020) Time in range in children with type 1 diabetes using treatment strategies based on Nonautomated insulin delivery systems in the real world. Diabetes Technol Ther 22:509–15. https://doi.org/10.1089/dia.2020.0031
Battelino T et al (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International consensus on time in range. Diab Care 42:1593–603. https://doi.org/10.2337/dci19-0028
Haak T et al (2017) Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 8:55–73. https://doi.org/10.1007/s13300-016-0223-6
Bergenstal RM et al (2022) Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Complications 36(3):108106. https://doi.org/10.1016/j.jdiacomp.2021.108106.
Finn E et al (2023) Real-world accuracy of CGM in inpatient critical and noncritical care settings at a safety-net hospital. Diabetes Care 10:dc230089. https://doi.org/10.2337/dc23-0089
Gallieni M et al (2021) Continuous glucose monitoring in patients with type 2 diabetes on hemodialysis. Acta Diabetol 58(8):975–81. https://doi.org/10.1007/s00592-021-01699-6
Rossi A et al (2022) One year of hybrid closed loop on peritoneal dialysis: a case report. Acta Diabetol 59(7):985–8. https://doi.org/10.1007/s00592-022-01880-5
La Rocca E et al (2000) Patient survival and cardiovascular events after kidney-pancreas transplantation: comparison with kidney transplantation alone in uremic IDDM patients. Cell Transpl 9(6):929–32. https://doi.org/10.1177/096368970000900621
Freckmann G et al (2019) Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices. J Diabetes Sci Technol 13(3):575–83. https://doi.org/10.1177/1932296818812062
Kovatchev BP et al (2015) Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther 17(3):177–86. https://doi.org/10.1089/dia.2014.0272
Smith MB et al (2019) Human factors associated with continuous glucose monitor use in patients with diabetes: a systematic review. Diabetes Technol Ther 21(10):589–601. https://doi.org/10.1089/dia.2019.0136.
Gavin JR et al (2023) Continuous glucose monitoring impact and implications of real-world evidence: past, present, and future. Diabetes Technol Ther 25(S3):S5-S13. https://doi.org/10.1089/dia.2023.0057
Jiao Y et al (2022) A systematic review: cost-effectiveness of continuous glucose monitoring compared to self-monitoring of blood glucose in type 1 diabetes. Endocrinol Diabetes Metab 5(6):e369. https://doi.org/10.1002/edm2.369
Pitocco D, Working group of Diabetes and Technology AMD-SID-SIEDP et al (2022) Health care organization and use of technological devices in people with diabetes in Italy: results from a survey of the Working Group on Diabetes and Technology. Nutr Metab Cardiovasc Dis 32(10):2392–8. https://doi.org/10.1016/j.numecd.2022.07.003
Lin R et al (2021) Continuous glucose monitoring: a review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med 38(5):e14528. https://doi.org/10.1111/dme.14528
Meng R et al (2021) Performance evaluation of the Glunovo® continuous blood glucose monitoring system in Chinese participants with diabetes: a Multicenter, Self-Controlled Trial. Diabetes Ther 12(12):3153–65. https://doi.org/10.1007/s13300-021-01171-2
Bonora B et al (2016) Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest 39(12):1391–9. https://doi.org/10.1007/s40618-016-0495-8
Holt RIG et al (2021) The management of type 1 diabetes in adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 64(12):2609–52. https://doi.org/10.1007/s00125-021-05568-3
Acknowledgements
We thank the Fondazione Romeo and Enrica Invernizzi for outstanding support.
Funding
The study was supported by an unconditioned grant Alpha Pharma Service SRL. The funding source had no impact to the design of the study, interpretation of results and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Ethics Statement: the study has been approved by the ethics committee of the ASST Fatebenefratelli-Sacco, Luigi Sacco Hospital, Milan and was carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Subject of the case gave written consent to the study.
Conflict of interest
On behalf of all authors the corresponding author states that they have no other conflict of interest related to the work submitted for publication. No financial interests are directly or indirectly related to the work submitted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rossi, A., Rossi, G., Montefusco, L. et al. A new glucose monitoring system for the intermittent monitoring of interstitial glucose values in patients with diabetes mellitus. J Diabetes Metab Disord (2024). https://doi.org/10.1007/s40200-024-01488-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40200-024-01488-2