Abstract
Aim
This study investigates the effects of minocycline (an inhibitor of microglial activation) administration on the expression level of spinal BDNF and DREAM proteins in diabetic neuropathic pain (DNP) rats.
Methods
The rats were divided into four groups (n = 16): non-diabetic control, diabetic control and diabetic rats receiving minocycline (80 μg/day or 160 μg/day). The diabetic rat model was induced by intraperitoneal injection of streptozotocin (60 mg/kg STZ). Tactile allodynia was assessed on day-0 (baseline), day-14 (pre-intervention) and day-22 (post-intervention). Minocycline at doses of 80 μg and 160 μg were given intrathecally from day-15 until day-21. On day-23, formalin test was conducted to assess nociceptive behaviour response. The spinal expression of OX-42 and level of BDNF and DREAM proteins were detected by immunohistochemistry and western blot analyses.
Results
Diabetes rats showed significant tactile allodynia and nociceptive behaviour. These were accompanied by augmented expression of spinal OX-42, BDNF and DREAM protein levels. Both doses of minocycline attenuated tactile allodynia and nociceptive behaviour and also suppressed the diabetic-induced increase in spinal expressions of OX-42, BDNF and DREAM proteins.
Conclusion
This study revealed that minocycline could attenuate DNP by modulating spinal BDNF and DREAM protein expressions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic neuropathic pain (DNP) is a chronic pain that could develop in diabetic patients due to misfiring of peripheral nerves of somatosensory system [1]. The occurrence of DNP is hyperglycaemia- related with local metabolic and microvascular alterations in both type I and type II diabetes mellitus (DM) [1, 2]. According to recent reports, approximately 30% of DM patients develop DNP which can be manifested as spontaneous pain, allodynia (pain to normally innocuous stimuli) and hyperalgesia (increased perception of pain to noxious stimuli) [3].
Hyperglycaemia is considered as a major pathophysiological factor in the development of DNP. However, the mechanism leading to DNP is not fully understood. Hyperglycaemia has been reported to activate some major pathways such as polyol pathway, advanced glycation end products (AGE), hexosamine flux, mitogen-activated protein kinase (MAPK), poly-ADP ribose polymerase (PARP) and cyclooxygenase-2 (COX-2). Activation of these pathways could initiate oxidative stress, release of various cytokines and eventually neuroinflammation [4].
Brain-derived neurotropic factor (BDNF) is one of the neurotrophins that ensures neuronal survival, growth and differentiation [5]. In the spinal cord, BDNF is distributed in primary sensory neurons of dorsal root ganglion and involved in the development of central sensitization in spinal dorsal horn [6, 7]. The effects of BDNF are mediated via its binding to the tyrosine kinase receptor B (TrkB) and subsequent activation of it’s downstream signalling pathways [8]. BDNF binds to neuronal TrkB receptors to form ligand-receptor complex which initiates the phosphorylation of TrkB and it’s tyrosine residues [9]. BDNF is also known as a potent modulator of synapses, thereby affecting short and long-term synaptic efficacy [10]. This endogenous neuromodulator is released from primary afferent terminals within the spinal cord in an activity-dependent manner and functions in regulating the nociceptive transmission in the spinal dorsal horn [5, 11]. Compelling evidences suggested the involvement of BDNF in spinal plasticity and central sensitization of physiological and pathological pain [11,12,13,14].
Downstream regulatory element antagonist modulator (DREAM) protein is a potent transcriptional repressor for prodynorphin gene, which is involved in pain processes [15, 16]. Prodynorphin gene consists of a consensus DNA sequence or downstream regulatory element (DRE) that is needed for direct association with the DREAM protein [17]. Cheng and colleagues [17] reported DREAM-deficient mice to display significant reduction in behavioural responses (analgesia) of acute thermal, mechanical, chemical and visceral pain. Furthermore, the DREAM-knockout mice also demonstrated an increase in spinal prodynorphin mRNA, but normal level of opioids, pro-opiomelanocortin, pro-enkephalin and c-fos mRNA levels. The enhanced mRNA levels of prodynorphin in the spinal cord of DREAM-knockout mice compared to the wild-type mice is believed to be accountable for the reduced pain behaviour in the DREAM- knockout mice. The increased mRNA of prodynorphin in this type of mice may lead to the moderate effect of long-term potentiation [18]. However, the role of DREAM protein in modulating pain processes especially in neuropathic pain such as DNP is still unknown.
Recent evidences linked microglia and other non-neuronal cells in the development of DNP. Activated microglia release a variety of neuromodulators, neuroactive substances and pro-inflammatory cytokines that are directly involved in the development of DNP [19, 20]. Minocycline, an inhibitor of microglia has been shown to attenuate the development of DNP possibly via anti-inflammatory and anti-oxidant mechanisms [19]. Despite many studies have implicated BDNF and DREAM proteins in DNP, nevertheless the role of these proteins in microglia-mediated DNP is still unknown. Therefore, in this study, we investigated the possible connection between BDNF and DREAM protein and microglial activation-mediated DNP.
Materials and methods
Experimental animals
Sixty-four adult Sprague-Dawley male rats (200–250 g, 12 weeks) were used in this study. The rats were randomly divided into 4 groups (16 for each): control group, diabetic group (PDN group), diabetic group treated with 80 μg minocycline (M 80) and diabetic group treated with 160 μg minocycline (M 160). The rats in each group were further divided into subgroups (8 for each) which were sacrificed for immunohistochemistry analysis for microglia expression or Western Blot analysis for BDNF and DREAM protein level. This study was conducted with the approval from animal ethics committee [USM/Animal Ethics Approval/2014 (91) (560)].
Induction of diabetes
All the rats fasted for at least 14 h before the induction of diabetes. Diabetic rat model was established by a single i.p. injection of streptozotocin (STZ) (Sigma-Aldrich, Germany) at a dose of 60 mg/kg body weight, freshly dissolved in citrate buffer (pH 4.5) as a vehicle. Ten percent (10%) sucrose solution was given to the rats on one day upon STZ injection to avoid the development of severe hypoglycaemia in the rats that could be lethal. Diabetes was confirmed 3 days after the STZ injection by assessment of blood glucose level. The blood glucose level was measured before the induction of STZ (day 0), after the induction of STZ (day 3), on the pre-intervention day (day 14) and on post-intervention day (day 22) using a strip-operated glucometer (Accu-chek Performa, Roche Diagnostics, Paris, France). Animals with the final blood glucose level ≥ 15 mmol/L were considered as diabetic.
Intrathecal drug administration by direct delivery method
The administration of minocycline 80 μg and 160 μg was carried out by direct intrathecal injection based on a procedure described by Lu and Schmidtko [21]. Selection of minocycline dosage was based on the previous study [22] and given at two doses to investigate the dose dependent effect.
Reagents and materials
STZ was purchased from Sigma-Aldrich from Germany; minocycline hydrochloride was purchased from International Laboratory, USA; rabbit polyclonal for β-actin, anti-CSEN, BDNF primary antibodies, goat anti rabbit and anti-mouse IgG secondary antibodies, ultrasensitive ABC rabbit and mouse staining kits and clarity western ECL substrate were purchased from Thermo Fisher Scientific, USA; mouse monoclonal [OX42] was bought from Abcam, UK.
Behavioural studies
Tactile allodynia by automatic von Frey apparatus
Tactile allodynia was measured in the rats using an automated von-Frey apparatus (Bioseb, USA). The rats were placed on a wire mesh floor in a quiet room and allowed to acclimatize for a minimum of 15 min before beginning the test. A semi-flexible filament weighing 0.099 g was applied to the mid-plantar region of the hind paw. The pressure was gradually increased until either a clear retraction, licking or jumping was observed. The force applied to the filament which evokes a hind paw withdrawal represented a maximal threshold and expressed in grams. The stimulation was conducted three times, and the average measurement was calculated. The stimulation using the filament was separated by 10 min interval from previous tactile stimulation of the paw [23]. On day 14 (pre-intervention day), DNP in rodents were confirmed when the reduction in noxious withdrawal thresholds by von-Frey test was more than 15% of the value obtained before STZ injection (day 0) [24].
Formalin test
The rats received an intraplantar injection of 50 μL of 5% formalin solution into dorsal surface of right hind paw and was immediately transferred to a Perspex testing chamber (26 cm ×20 cm × 20 cm). A mirror was placed beneath the floor directing base of the chamber at a 450 angle to allow an unobstructed view of the paw. Nociceptive response was recorded for 60 min using a video camera. The recorded response was assessed by two persons blinded to any treatment based on the pain score from 0 to 3, as follows:
-
0 = rats feel no pain at all (i.e. foot flat on the floor with all toes splayed)
-
1 = injected paw has little or no weight on it, with no toes splaying, indicating mild pain
-
2 = injected paw is elevated and the heel is not in contact with any surface, indicating moderate pain
-
3 = injected paw is licked, bitten or shaken, an indication of severe pain felt
The nociceptive responses were then tabulated every minute and averaged at 5-min intervals [25]. The nociceptive response was divided into two phases comprising of phase 1 (mean score from min 0 to 10) and phase 2 (mean score from min 15 to 60).
Immunohistochemistry for microglial expression
Seventy-two hours after the formalin test was conducted, the rats were deeply anaesthetized with sodium pentobarbitone (Alfasan, Netherlands) intraperitoneally. In our unpublished work, activated microglia was shown to be clearly expressed and localized to the portion of lamina I-IV in the spinal cord at three days after formalin injection. This finding has also been supported by Fu et al. [26]. It is the reason for sacrificing the rats at three days after formalin injection. Thoracotomy and perfusion fixation technique was performed to expose rats spinal cord according to our previous study [27]. The lumbar enlargement of L4-L5 spinal cord segment were sectioned (40 μm thickness) using a cryostat and sections were rinsed with Tris-buffered saline (TBS) twice for 5 min each and incubated overnight at 4 °C with primary mouse monoclonal antibody for OX-42 protein (microglia protein marker) diluted to 1:500 in buffer (mixture of TBS with 2% normal goat or horse serum and 0.2% Triton-X) followed by incubation with biotinylated horse anti-mouse IgG (for microglia protein) diluted at 1:200 in buffer (mixture of TBS with 2% normal goat/horse serum and 0.2% Triton-X) for 1 h with gentle agitation at room temperature. The sections were incubated with avidin-biotin-HRP (diluted 1:50 in TBS) for 1 h at room temperature and treated with diaminobenzidine (DAB) diluted 1:10 with stable peroxidase substrate buffer (1X) as a chromogen in the dark, until brown colouration of the solution was detected. The sections were then dehydrated with absolute ethanol for 15 min before being mounted with DPX mounting medium and cover slipped. Six random sections were captured using an image analyzer (Leica MPS 60, Japan) under 100 x lens magnifications. The total number of protein expression was counted manually from laminae I to VI and the average of six sections were taken from each rat from ipsilateral and contralateral sides of the spinal cord.
Western blot analysis
The BDNF and DREAM protein level in the spinal cord was determined by Western blot analysis. Seventy-two hours post-formalin injection, the rats were sacrificed by decapitation using a guillotine after deeply anaesthetized with an overdose injection of sodium pentobarbitone intraperitoneally. Lumbar enlargement of L4-L5 spinal cord segment was dissected out from the rats without a fixation process and separated into ipsilateral side by a cut at the midline of the spinal cord. Immediately after the removal, the tissue was deep-frozen in liquid nitrogen and kept at −80 °C until further assay. Total protein was extracted, quantified, prepared for SDS-PAGE, and detected by WB using indicated antibodies. The blot was examined using Clarity ™ Western ECL Substrate (Thermofisher Scientific, USA). The image was taken by an image analyzer Fusion FX Chemiluminescence Imaging apparatus (Vilber Lourmat, France). The integrated density values (IDV) of the BDNF, DREAM protein and β-actin proteins were measured using Spot Denso AlphaView™ software in the image analyzer. The mean relative intensity of fold change was measured by the formula below:
Statistical analysis
The data was analyzed using Statistical Package for Social Sciences (SPSS) software version 24. Changes in the nociceptive response by formalin test was analyzed by repeated measures ANOVA with within subject factor time (2 levels: phase 1 and phase 2) and between subject factor group (4 levels: the treatment groups) with the post-hoc LSD or Dunnett’s T3 tests. Meanwhile, blood glucose level and tactile allodynia were analyzed using repeated measures ANOVA with within subject factor time (4 levels: day 0, day 3, day 14 and day 22 for blood glucose), (3 level: day 0, day 14 and day 22 for tactile allodynia) and between subject factor group (4 levels: the treatment groups) with post-hoc Bonferroni test. Total numbers of microglial expression and mean relatives BDNF and DREAM protein level were analyzed using one-way analysis of variance (ANOVA) with the post-hoc LSD or Dunnett’s T3 tests. The results were expressed as a mean ± standard error of the mean (S.E.M.), and the level of significance was determined at p < 0.05.
Results
STZ injection increase blood glucose level
There was significant main effect of time in blood glucose level within the groups (F (7.516, 190.407) = 65.26), p < 0.001). Post-hoc Bonferroni test revealed a significant difference in blood glucose level between day 0 and 3 (p < 0.001). Meanwhile, no significant difference was detected between day 3 and 14 and between day 14 and 22. Moreover, the result of between groups effect reported the dramatically higher blood glucose level in all diabetic groups (PDN, M 80 and M 160) compared to control (p < 0.001) group on day 3, suggesting that the rats in diabetic groups were rendered diabetic post 60 mg/kg STZ injection. Treatment with lower and higher doses of minocycline started from day 15 until 21 did not give any effect as the blood glucose level remained high in all diabetic groups post minocycline treatments (Table 1).
Administration of minocycline increased noxious withdrawal threshold in diabetic rats
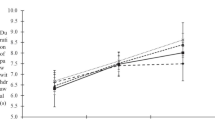
There was significant main effect of time within the groups for right (F (1.606, 126.353) = 38.321, p < 0.001) and left (F (1.858, 146.751) = 44.020, p < 0.001) hind paws. The repeated measures ANOVA also revealed a significant between groups effects in noxious withdrawal threshold at right (F (4.799, 123.16) = 26.018, p < 0.001) and left (F (6, 152) = 25.266, p < 0.001) hind paws throughout study period. Post-hoc Bonferroni test demonstrated that the noxious withdrawal threshold was lower (tactile allodynia increased) in all STZ-induced diabetic rats on day 14 (pre-intervention day) compared to control group (p < 0.001) (Fig. 1a and b). Intrathecal administration of minocycline at lower and higher doses for 1 week significantly caused higher noxious withdrawal threshold (tactile allodynia decreased) in minocycline treatment (M 80 and M 160) groups compared to PDN group (p < 0.001) on day 22 (p < 0.05 and p < 0.001 respectively) (Fig. 1a and b).
a Noxious withdrawal threshold of the rat’s right hind paw in the groups tested using von Frey test. Values are expressed as mean ± S.E.M (n = 16). **, p < 0.001 between PDN and control groups, #, p < 0.05 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups. b Noxious withdrawal threshold of the rat’s left hind paw in the groups tested using von Frey test. Values are expressed as mean ± S.E.M (n = 16). **, p < 0.001 between PDN and control groups, #, p < 0.05 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups, ¤, p < 0.05 between (M 160) and control groups
Administration of minocycline reduced nociceptive behaviour during phase 1 and 2 of formalin test
Nociceptive behaviour score by 5% formalin injections is divided into two phases of pain: Phase 1 represents the peripheral action whilst phase 2 represents the central action produced by the formalin injection. Phase 1 is determined from 0 to 5 min followed by an interphase stage at 10 min in which the nociceptive behaviour is reduced. Phase 2 is determined from 15 to 60 min which involves the enhanced nociceptive responses from the sensitized dorsal horn neurons. The overall pain behaviour score involving all phases of pain is shown in Fig. 2a for 60 min period. Meanwhile, the separation of each phase 1 and 2 are shown in Fig. 2b.
a Overall pain behaviour score in the groups (n = 16). b Nociceptive behaviour score during phase 1 and 2 of formalin test in all the groups. The values are expressed as mean ± S.E.M (n = 16). **, p < 0.001 between phase 1 and phase 2, *, p < 0.05 between phase 1 and phase 2. c Nociceptive behaviour score during phase 1of formalin test in all the groups. The values are expressed as mean ± S.E.M (n = 16). *, p < 0.05 between PDN and control groups, ##, p < 0.001 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups. d Nociceptive behaviour score during phase 2 of formalin test in all the groups. The values are expressed as mean ± S.E.M (n = 16). ##, p < 0.001 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups
The analysis by one-way repeated measures ANOVA revealed a significant within-subjects effect of time (phase 1 and 2) (F (1, 79) = 27.359, p < 0.001) and between groups effect (interaction between treatment groups and pain behaviour phases) (F (3, 76) = 50.132, p < 0.001). Post-hoc Bonferroni test demonstrated a significant higher nociceptive behaviour score in PDN group compared to control group (p < 0.05) especially in phase 1. Interestingly, the rats administered with minocycline at both doses (M 80 and M 160) had lower nociceptive behaviour score in both phase 1 and 2 compared to PDN and control groups (p < 0.001). No significant difference on nociceptive behaviour score was detected between the doses of minocycline in phase 1 and 2 (Fig. 2c and d).
Administration of minocycline reduced microglial expression on spinal cord of diabetic rat
In general, there was a difference in morphology of activated microglia in rat’s spinal cord between diabetic PDN rats compared to control rats. The activated microglia in PDN group was more pronounced and showed hypertrophic morphology, being higher in number and expression compared to control group at both ipsilateral (p < 0.05, Fig. 3) and contralateral (p < 0.001, Fig. 4) sides of spinal cord. Administration of minocycline at both doses (M 80 and M 160 groups) resulted in lower microglial expression compared to control and PDN groups ipsilaterally (p < 0.001, Fig. 3) and contralaterally (p < 0.001, Fig. 4). The effect is dose-dependent since the higher dose of minocycline resulted in (M 160 group) showed much lower microglial expression compared to the lower dose of minocycline used (M 80) group especially on the ipsilateral side of the spinal cord (p < 0.05) (Fig. 3).
a Total numbers of microglia positive neurons in all groups at ipsilateral side in lamina I-II of spinal cord region. A represents control group, B represents PDN group, C represents (M 80) group and D represents (M 160) group. Arrows indicate microglia positive neurons (magnification 200x objective lens). b Columns represent mean total numbers of microglia positive neurons. Values are expressed as mean ± S.E.M (n = 8 per group). *, p < 0.05 between PDN and control groups, ##, p < 0.001 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups, ¤, p < 0.05 between (M 80) and (M 160) groups
a Total numbers of microglia positive neurons in all groups at contralateral side in lamina I-II of spinal cord region. A represents control group, B represents PDN group, C represents (M 80) group and D represents (M 160) group. Arrows indicate microglia positive neurons (magnification 200x objective lens). b Columns represent mean total numbers of microglia positive neurons. Values are expressed as mean ± S.E.M (n = 8 per group). §, p < 0.05 between PDN and control groups, ##, p < 0.001 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups
Administration of minocycline reduced DREAM and BDNF protein level in spinal cord of diabetic rat
Spinal DREAM and BDNF proteins level were found to be higher in PDN group compared to control group (p < 0.05) (Figs. 5 and 6) as revealed by post-hoc Dunnett’s T3 test. The administration of minocycline at both doses (M 80 and M 160) groups were found to down regulate the DREAM (Fig. 5) and BDNF (Fig. 6) proteins level compared to control and PDN groups.
Mean relative DREAM protein level at ipsilateral side of spinal cord in groups. a An example of Western blot results for all groups at the ipsilateral side. The lower panel demonstrates the loading control (β-actin). b Quantification analysis of integrated density value represented by mean ± S.E.M for six separate experiments. The data were normalized by control group (n = 8 per group). **, p < 0.001 between PDN and control groups, #, p < 0.05 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups
Mean relative BDNF protein level at ipsilateral side of spinal cord in groups. a An example of Western blot results for all groups at the ipsilateral side. The lower panel demonstrates the loading control (β-actin). b Quantification analysis of the integrated density value represented by mean ± S.E.M for six separate experiments. The data were normalized by control group (n = 8 per group). *, p < 0.05 between PDN and control groups, ##, p < 0.05 between (M 80) and PDN groups, §§, p < 0.001 between (M 160) and PDN groups
Discussion
Intrathecal treatment with minocycline for seven days period increased the noxious withdrawal threshold on day 22 (post-intervention day) dose-dependently and suppressed nociceptive behaviour score during phase 1 and phase 2 in formalin test. These results are parallel with previously reported studies [19, 20, 28, 29]. This effect was probably due to inhibition of microglial activation that was found to be upregulated in the spinal cord of PDN group at three days after formalin injection [30, 31]. The activated microglia was shown by the hypertrophic cell body morphology with the shorter cellular processes as seen in the lumbar enlargement region of the spinal cord in PDN group. The upregulation of activated microglia may be associated with the occurrence of tactile allodynia and chemical hyperalgesia experienced by PDN group in this study. Administration of minocycline attenuated this tactile allodynia and chemical hyperalgesia accompanied by the reduction in the expression level of BDNF and DREAM protein in the spinal cord in this study. This suggests that minocycline attenuated tactile allodynia and chemical hyperalgesia by modulating the expression level of BDNF and DREAM proteins in diabetic rats in this study.
The efficacy of minocycline in preventing tactile allodynia and chemical hyperalgesia could be attributed to its suppression of microglial activation and modulation of DREAM and BDNF proteins expression level during the course of the neuropathic pain condition. Microglial activation is capable of inducing NR2B subunit of NMDA receptors activation via signalling pathways involving BDNF protein to maintain the occurrence of central sensitization [32]. Previous investigations reported no change [33] or an increase [34, 35] in BDNF protein level in the spinal cord of diabetic rats. The increase in BDNF protein level and expression could be associated with the increased microglial activation as seen in PDN group in this study. Tsuda et al. [36] reported that the activated microglia P2X4 receptors induce the synthesis and release of BDNF protein and this protein acts on KCC2 receptors to render gamma-amino butyric acid (GABA) effects of depolarization. In return, the released BDNF protein may then produce signals to lamina I neurons which cause exaggerated responses of pain that maintains the development of neuropathic pain [36]. The release of BDNF protein may also prolong the activation of microglia as this protein binds to TrkB receptors on the activated microglia itself [37]. We postulated that BDNF protein stimulates TrkB receptor, downregulates voltage-gated potassium (Kv) channels current activity, thereby increases the neuronal excitability [35] in diabetic rat model in this study. Theoretically, the inhibition of Kv channels activity may enhance the frequency of firing and broadening the action potential leading to the elevated Ca2+ influx, the release of neurotransmitter glutamate [35] and relieving Mg2+ block of NMDA receptors [38]. As a microglial activation inhibitor, minocycline indirectly suppress the production and expression of BDNF protein released by the activated microglia in the diabetic rats in this study. The inhibition of BDNF protein from the activated microglia may indirectly inhibit neuronal excitability leading to the suppression of allodynia and hyperalgesia by minocycline as proven in this study. The inhibition of BDNF protein expression by minocycline has also been reported by Zhou and colleagues [22].
Meanwhile, the increase in DREAM protein expression level in PDN group indicates the activation of NMDA receptors by noxious stimulation which allows the influx of Ca2+ into the neurons. This Ca2+ may directly bind to DREAM protein to cause its removal from DRE region. As a result of DREAM protein removal, transcription progresses and dynorphin protein is produced. In this study, the increased level of DREAM protein and postulated release of dynorphin protein in the spinal cord may have increased the synaptic plasticity and neuronal excitability that leads to the maintenance of DNP in PDN group. Rowjeska and colleagues [39] reported that minocycline attenuated the prodynorphin mRNA and endogenous dynorphin protein levels following sciatic nerve injury in rats. The inhibition of DREAM protein expression by minocycline suggests the contribution of activated microglia to the increased DREAM protein expression level in the spinal cord of diabetic rats. Therefore, this effect facilitates the pain transmission during the maintenance of diabetic neuropathic pain. The finding from immunohistochemistry analysis for microglial protein expression are consistent with the Western Blot analysis for expression level of BDNF and DREAM protein. Thus, we suggest that the finding from both experiments corresponded with one another since we used separate groups of animals for both experiments.
As a conclusion, we demonstrated in this study that minocycline ameliorated diabetic neuropathy via modulation of BDNF and DREAM protein level that are released by activated microglia within spinal cord. This study may shed light on the possible mechanism involved in the pathogenesis of diabetic neuropathy and may facilitate future researchers in developing medications to alleviate diabetic neuropathy and possibly other neuropathic pain conditions.
References
Wang D, Couture R, Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur J Pharmacol. 2014;728:59–66.
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93.
Tavakoli M, Malik RA. Management of painful diabetic neuropathy. Expert Opin Pharmacother. 2008;9:2969–78.
Sandireddy R, Yerra VG, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol. 2014;2014:1–10.
Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signalling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26(1):126–37.
Mannion R, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege J, et al. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96(16):9385–90.
Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and TrkB receptors. Trends Pharmacol Sci. 2003;24(3):116–21.
Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42.
Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J Cell Biol. 2003;163(2):385–95.
Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317.
Geng SJ, Liao FF, Dang WH, Ding X, Liu XD, Cai J, Han JS, Wan Y, Xing GG Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol 2010; 222(2): 256–266.
Nitta A, Murai R, Suzuki N, Ito H, Nomoto H, Katoh G, et al. Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicol Teratol. 2002;24(5):695–701.
Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–21.
Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21(5):570–9.
Carrion AM, Link WA, Ledo F, Mellström B, Naranjo JR. DREAM is a Ca2+−regulated transcriptional repressor. Nature. 1999;398(6722):80–4.
Constigan M, Woolf CJ. No DREAM, no pain: closing the spinal gate. Cell. 2002;108(3):297–300.
Cheng HYM, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, et al. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108(1):31–43.
Liliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, et al. Altered Aβ formation and long-term potentiation in a calsenilin knock-out. J Neurosci. 2003;23(27):9097–106.
Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661(1–3):15–21.
Ledeboer A, Sloane E, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83.
Lu R, Schmidtko A. Direct intrathecal drug delivery in mice for detecting in vivo effects of cGMP on pain processing. Methods Mol Biol. 2013;1020:215–21.
Zhang X, Xu Y, Wang J, Zhou Q, Pu S, Jiang W, et al. The effect of intrathecal administration of glial activation inhibitors on dorsal horn BDNF overexpression and hind paw mechanical allodynia spinal nerve ligated rats. J Neural Transm. 2012;119(3):329–36.
Zulazmi NA, Gopalsamy B, Farouk AAO, Sulaiman MR, Bharatham BH, Perimal EK. Antiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic pain. Fitoterapia. 2015;105:215–21.
Daulhac L, Maffre V, Mallet C, Etienne M, Privat A, Kowalski-Chavuvel A, et al. Phosphorylation of spinal N-methyl-D-aspartate receptor NR1 subunits by extracellular signal-regulated kinase in dorsal horn neurons and microglia contributes to diabetes-induced painful neuropathy. Eur J Pain. 2011;15(2):169 e1–e12.
Hayati AA, Zalina I, Myo T, Badariah A, Azhar A, Idris L. Modulation of formalin-induced fos-like immunoreactivity in the spinal cord by swim stress-induced analgesia, morphine and ketamine. Ger Med Sci. 2008;6:Doc05.
Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825(1–2):59–67.
Long I, Suppiah R, Ismail Z. The effects of pre-emptive administration of ketamine and norBNI on pain behavior, c-Fos, and prodynorphin protein expression in the rat spinal cord after formalin-induced pain is modulated by the DREAM protein. Korean J Pain. 2013;26(3):255–64.
Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560(2–3):142–9.
Sun JS, Yang YJ, Zhang YZ, Huang W, Li ZS, Zhang Y. Minocycline attenuates pain by inhibiting spinal microglia activation in diabetic rats. Mol Med Rep. 2015;12(2):2677–82.
Fu KY, Tan YH, Sung B, Mao J. Peripheral formalin injection induces unique spinal cord microglial phenotypic changes. Neurosci Lett. 2009;449(3):234–9.
Lin K, Lin T, Light CY, AR FKY. Peripheral formalin injury induces 2 stages of microglial activation in the spinal cord. J Pain. 2010;11(11):1056–65.
Chen W, Walwyn W, Ennes HS, Kim H, McRoberts JA, Marvizón CG. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur J Neurosci. 2014;39:1439–54.
Morgado C, Pereira-Terra P, Cruz C, Tavares I. Minocycline completely reverses mechanical hyperalgesia in diabetic rats through microglia-induced changes in the expression of the potassium chloride co-transporter 2 (KCC2) at the spinal cord. Diabetes Obes Metab. 2011;13(2):150–9.
Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J Neurochem. 1995;64(3):1231–7.
Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem. 2010;114(5):1460–75.
Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Invest. 2016;7:17–26.
Zhou LJ, Yang T, Wei X, Liu Y, Xin WJ, Chen Y, et al. Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain Behav Immun. 2011;25(2):322–34.
Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11(6):1164–74.
Rowjeska E, Makuch W, Przewlocka B, Mika J. Minocycline prevents dynorphin-induced neurotoxicity during neuropathic pain in rats. Neuropharmacology. 2014;86:301–10.
Acknowledgments
This study was supported by the Universiti Sains Malaysia research funding (RUI 1001/PPSK/812139) and Government of Malaysia Fundamental Research Grant Scheme (FRGS 203/PPSK/6171189).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in preparing this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ismail, C.A.N., Suppian, R., Aziz, C.B.A. et al. Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J Diabetes Metab Disord 18, 181–190 (2019). https://doi.org/10.1007/s40200-019-00411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-019-00411-4