Abstract
In this work, a series of Co-based ternary Co–Er–B bulk metallic glasses (BMGs) with excellent soft magnetic properties and high strength were developed, and the local atomic structure of a typical Co71.5Er3.5B25 metallic glass was studied through in situ high-energy synchrotron X-ray diffraction and ab initio molecular dynamics simulations. The results reveal that the BMG samples can be obtained in a composition region of Co68.5–71.5Er3.5–4B25–27.5 by a conventional copper-mold casting method. The Co–Er–B metallic glasses possess stronger atomic bond strengths and denser local atomic packing structure composed of a higher fraction of icosahedral-like clusters but fewer deformed body-centered cubic and crystal-like polyhedrons, and they exhibit slower atomic diffusion behaviors during solidification, as compared to Co–Y–B counterparts. The enhancement in structural stability and the retardation of atomic-ordered diffusion lead to the better glass-forming ability of the Co–Er–B alloys. The smaller magnetic anisotropy energy in the Co–Er–B metallic glasses results in a lower coercivity of less than 1.3 A/m. The Co–Er–B BMGs exhibit high-yield strength of 3560–3969 MPa along with distinct plasticity of around 0.50%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Since Duwez first prepared Au–Si amorphous alloys by melt quenching in 1960 [1], such materials with a distinctive structure have captivated people’s attention. The long-range disordered atomic arrangement inherent in the amorphous alloys (or called metallic glasses) gives them unique mechanical, physical, and chemical properties that cannot be achieved in crystalline counterparts [2,3,4]. The early developed metallic glasses commonly exist in the forms of ribbons, wires, or films due to their low glass-forming ability (GFA) [5]. Through rational compositional design strategies, such as Inoue’ three empirical rules [2], eutectic criteria [6], minor alloying [7], and so forth, the GFA of the alloys gets enhanced, and the glassy samples in bulk form have been successively discovered. Bulk metallic glasses (BMGs), typically defined for the alloys with a critical sample size of ≥ 1 mm, have been developed in Pd/Pt-based [8, 9], Zr/Ti/Cu-based [10,11,12], Mg/Ca/Ce/La-based [13,14,15,16], and Fe/Co/Ni-based [17,18,19] alloy systems. Although multi-component-alloyed alloys might possess higher GFA, it is important and valuable to develop binary or ternary BMGs. The long-range disorder atomic structure characteristic of the BMGs makes it challenging to analyze structural information, crucial for comprehending glass formation, while the BMGs with fewer types of components might offer convenience. For instance, the local atomic structure of the binary/ternary BMGs [20] can be analyzed through both experiments and simulations; nevertheless, the complexity dramatically increases when dealing with multi-component BMGs [21]. In addition, the binary or ternary BMGs can also serve as base alloys for developing BMGs with further enhanced GFA and other properties.
The Co-based BMGs can be roughly divided into two categories: One category possesses ultrahigh strength. For example, Co-based Co–Ta–B ternary BMGs possess fracture strength of over 6000 MPa, setting the highest record among all bulk alloys [22]. The other category exhibits excellent soft magnetic properties. Compared to Fe-based metallic glasses, the Co-based metallic glasses typically exhibit lower coercivity (Hc), higher permeability, and near zero saturation magnetostriction coefficient (λs), which make them more suitable for high-frequency applications. For example, Co-based Co–Y–B metallic glasses [23] possess lower Hc than Fe-based Fe–Y–B metallic glasses [24]. An amorphous Co-based Co–Fe–Si–B–Cr alloy has much lower λs and Hc than amorphous Fe–Ni–Si–B–C alloys [25]. In addition, the Co-based metallic glasses exhibit better corrosion resistance than the Fe-based alloys [26]. However, due to that the Co-based alloys suffer from weaker GFA than the Fe-based alloys, the Co-based BMGs usually feature low Co contents and/or composed of four or more elements with complex compositions. The low Co contents reduce ferromagnetic characteristic, and hence, most of the soft magnetic Co-based BMGs are multi-component with alloying Fe. The reported soft magnetic Co-based BMGs are typically found in Co–Fe–TM–B (TM: Transition Metal) [27, 28], Co–(Fe)–RE–(TM)–B (RE: Rare Earth) [23, 29], Co–Fe–TM–Si–B [30], Co–(Fe)–TM–P–B [31, 32], and Co–Fe–B–Si–P [33] systems. Although the Bs of the Co-based BMGs can be enhanced by alloying Fe, the λs is increased inevitably [29]. The binary or ternary Fe-free Co-based BMGs with soft magnetic properties have not been reported yet.
The GFA and thermal/mechanical/magnetic properties of the metallic glasses are closely correlated to their local atomic structure. Through ab initio molecular dynamics (AIMD) simulations, Jiang et al. studied the structure of Co-based Co–(P, C, B) amorphous alloys and found that the alloy with stronger atomic bonding and more icosahedral-like (ico-like) polyhedrons exhibits higher GFA [34]. Han et al. found that alloying an appropriate amount of Y into a Fe-based Fe–B alloy increases the fraction of stable atomic local packing and suppresses the formation of deformed body centered cubic (bcc) clusters, leading to enhanced GFA [35]. It is anticipated that alloying RE elements having strong chemical affinities with the constituents into Co-based Co–B alloys might generate more significant effect on enhancing GFA, benefiting from the potential improvements in structural stability and atomic packing density. We have confirmed that minor alloying Y into a Co75B25 alloy indeed improves the GFA and magnetic softness; however, obtaining the BMG samples remains a challenge [23]. In this work, a RE element Er was selected to add in the Co–B alloy due to its more negative mixing enthalpies with Co or B compared to other RE elements [36], and the GFA and thermal, magnetic, and mechanical properties of ternary Co–Er–B alloys were studied. Combining in situ high-energy synchrotron X-ray diffraction (HEXRD) with AIMD simulations, the local atomic structure of a Co71.5Er3.5B25 BMG was analyzed. For comparison, the GFA and thermal and magnetic properties of Co71.5RE3.5B25 (RE = Y, Sm, Gd) alloys were investigated, and the local atomic structure of a Co71.5Y3.5B25 counterpart was also studied to clarify the underlying reason of the different GFAs.
2 Materials and Methods
2.1 Experimental Procedure
Master alloy ingots with nominal compositions of Co62.5–78Er2–5B20–32.5 and Co71.5RE3.5B25 (RE = Y, Sm, Gd) were prepared by arc melting the mixtures of Co (99.98 wt%), RE (99.9 wt%), and B (99.5 wt%) under an argon atmosphere. The alloy ingots were re-melted four times to ensure chemical homogeneity. Alloy ribbons with a thickness of 20 μm and width of 1.5 mm were produced by melt spinning, and cylindrical rods with diameters of 1.0 and 1.5 mm, respectively, and a length of around 20 mm were prepared by copper-mold casting under the argon atmosphere. Structure of the samples was characterized by X-ray diffraction (XRD, Bruker D8 Focus) with Cu–Kα radiation and transmission electron microscopy (TEM, JEOL JEM-2010). A cross-section of the as-cast rods was etched and subsequently observed by optical microscopy (OM, Zeiss Primotech). The largest diameter of the XRD-detected glassy rod sample was determined as the critical diameter for glass formation (dc). When the dc was less than 1.0 mm, series of alloy ribbons with a thickness interval of 20 μm were produced, and the largest thickness of the glassy ribbons was set as the critical thickness for glass formation (tc). The in situ HEXRD experiments at room temperature (300 K) were conducted using the beam line 11-ID-C in the advanced photon source (APS), Argonne national laboratory (ANL), USA, to analyze the local atomic structure. The photon energy was 105.1 keV corresponding to an X-ray wavelength of 0.11798 Å, and the beam size was 0.5 × 0.5 mm2. The resultant 2D image files were integrated using the Fit2D program to obtain 1D intensity distributions I(q) as a function of the wavevector q [37], from which the S(q) and the G(r) were derived using the PDFgetX2 program [38]. Glass transition temperature (Tg) and the onset temperature of crystallization (Tx) of the metallic glasses were detected by differential scanning calorimetry (DSC, TA Ins. Q20) at a heating rate of 0.67 K/s. Liquidus temperature (Tl) was measured by a differential thermal analyzer (DTA, TA Ins. SDT650) under a heating rate of 0.33 K/s. Saturation magnetic flux density (Bs) and Hc were measured by a vibrating sample magnetometer (VSM, LakeShore-7400S) under a maximum applied field of 750 kA/m, and DC B-H loop tracer (Linkjoin MATS-2010SD), respectively. Before the magnetic properties measurements, the ribbons were vacuum-annealed for 300 s at temperatures of (Tx−60) K to release internal stress. Mechanical properties of the BMGs were examined by uniaxial compressive testing using an Instron 5582 mechanical testing machine. The gauge dimension of specimens was 1.0 mm in diameter and 2.0 mm in height, and strain rate was fixed as 5 × 10−4 s−1. The lateral and fracture morphology of the compressed specimens was observed by scanning electron microscopy (SEM, JEOL IT-5600LV). Mass densities of the metallic glasses were determined by an Archimedes method.
2.2 AIMD Simulations
AIMD simulations for Co71.5RE3.5B25 (RE = Er, Y) metallic glasses were performed based on density functional theory (DFT) by using the Vienna Ab initio Simulation package [39], combined with canonical NVT ensemble and Nosé thermostat [40]. The cubic simulation boxes with periodic boundary conditions contain 200 atoms, and the box size was determined by the experimental mass densities of the alloys at room temperature. Only the Γ point was adopted for the Brillouin zone sampling. All alloys were melted and equilibrated at 3000 K for 5000 AIMD steps to remove the memory from the initial configurations, after being relaxed for 10 ps, the alloys were sequentially quenched to 2000, 1500, 1100, 800, and 300 K, respectively, at a cooling rate of 1.67 × 1014 K/s to produce glassy structure. At each temperature, the equilibrium volume of the supercell was relaxed by approximately keeping the average internal pressure at ± 0.5 kBar, and the last 2000 configurations were used for statistical sampling. Pair distribution functions (PDFs) [41] and Voronoi tessellation analysis [42] were conducted by using VMD and OVITO software packages, respectively.
3 Results and Discussion
3.1 GFA and Thermal Stability
Figure 1a illustrates the compositional dependence of the GFA in Co-based ternary Co–Er–B alloys. The alloys within a Co68.5–71.5Er3.5–4B25–27.5 region (highlighted in orange) possess a dc of 1.0 mm, indicating the formation of the BMGs, while the remaining alloys exhibit a lower GFA with dc values below 1.0 mm. Figure 1b illustrates the XRD patterns of as-cast BMG rods. All exhibit a broad diffraction feature suggesting a single glassy phase. The inset-shown Co71.5Er3.5B25 rod sample displays a smooth and lustrous outer surface, which is typical appearance of the BMGs. The homogeneous optical morphology of the transverse cross-section (Fig. 1b(i)) and bright-field TEM image along with a diffuse selected-area electron diffraction (SAED) halo (Fig. 1b(ii)) further confirm the glassy structure in the entire Co71.5Er3.5B25 BMG.
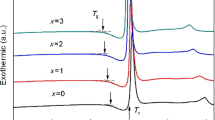
The DSC curves of the Co–Er–B BMGs all exhibit a distinct endothermic event corresponding to glass transition, followed by a supercooled liquid region and exothermic reactions caused by crystallization (Fig. 2a). The Tg and Tx roughly increase with lowering the Co content, ranging in 807–855 K and 832–882 K, respectively. The ΔTx (= Tx−Tg) fluctuates within 25–30 K. After detecting the Tl of the alloys in DTA curves (Fig. 2b), a GFA evaluating parameter Trg (= Tg/Tl) [6] was calculated, which ranges in 0.561–0.582. The XRD patterns and DSC curves of Co71.5RE3.5B25 (RE = Y, Sm, Gd) counterpart alloys can be seen in Fig. S1 (Supplementary Materials). The Tg, ΔTx, Tl, Trg, and dc (or tc) of the Co–Er–B BMGs and Co71.5RE3.5B25 (RE = Y, Sm, Gd) metallic glasses are listed in Table 1. It is seen that the GFA of the Co71.5RE3.5B25 (RE = Y, Sm, Gd) alloys is inferior (tc = 0.06–0.24 mm), and their Trg values are also lower than those of the Co–Er–B BMGs. As estimated from Ṫ = 10/R2 [43], where Ṫ (in K/s) is critical cooling rate for glass formation, and R (in cm) refers to dc or tc, the Ṫ of the Co–Er–B alloys is of the order of 103 K/s, much smaller than 104–105 K/s for the Co71.5RE3.5B25 (RE = Y, Sm, Gd) alloys.
3.2 Local Atomic Structures
The experimental results demonstrate that the Co–Er–B alloys have higher GFA than the Co–RE–B (RE = Y, Sm, Gd) counterparts. The local atomic structures of the Co71.5RE3.5B25 (RE = Er, Y) metallic glasses were studied to elaborate the mechanism behind it. The I(q) of the two alloys obtained from the HEXRD tests are plotted in Fig. S2 (Supplementary Materials), and the deduced structure factors S(q) are displayed in Fig. 3a, where the simulated results are also shown for comparison. The diffuse HEXRD synchrotron patterns (insets in Fig. 3a) confirm the amorphous feature of the two alloys. The shape and position of each peak in the simulated S(q) essentially align with the experimental results, revealing that the simulations can provide relatively accurate local atomic structures. The principle peaks in S(q) are located at 3.19 and 3.09 Å−1 for the Co71.5Y3.5B25 and Co71.5Er3.5B25 alloys, respectively. Reduced total pair distribution function G(r) transformed from the HEXRD results are shown in Fig. 3b. Upon examining the partially enlarged G(r) curves near the first main peak, it is evident that the peak position of the Co71.5Er3.5B25 metallic glass is 2.49 Å, smaller than 2.52 Å for the Co71.5Y3.5B25, indicating that the Er-bearing alloy possesses a higher atomic packing density as compared to the Y-containing alloy.
The simulated PDFs, coordination numbers (CNs), and chemical short-range order of the Co71.5RE3.5B25 (RE = Er, Y) metallic glasses were further analyzed to reflect the interactions and chemical affinities between different types of atoms. The PDFs g(r), which describe the probability of finding another atom at a certain distance (r) from the chosen atom, provide the atomic short-range ordering (SRO) of the metallic glasses [41]. Figure 4 shows the partial and total PDFs of different atomic pairs, and the positions of the first peak in g(r) curves representing the bond lengths of the atomic pairs are marked. The bond lengths of Co–B and Co–Co pairs are 2.04 and 2.45 Å, respectively, which correspond to the positions of the two sub-peaks in the first peak of the total PDFs (Inset in Fig. 4a), indicating that the SRO structures of the two alloys are mainly composed of metal-metalloid-type Co–B bond and metal–metal-type Co–Co bond. Although the bond lengths of Co–B, Co–RE, and Co–Co pairs are the same in the two alloys: the bond lengths of B–RE, B–B, and RE–RE in the Co71.5Er3.5B25 alloy are all shorter than those in the Co71.5Y3.5B25. The shorter bond lengths suggest a higher level of compact packing of the atoms. The total CNs were calculated by summing the partial CNs, which were determined by integrating over the first peak of the respective partial PDF g(r) [41]. The calculated total CNs of Co, RE, and B atoms in the two alloys are quite similar, which are around 13, 21, and 9, respectively (Fig. 5a). Warren–Cowley parameters reveal that the chemical affinities between each atom pair in the Co71.5Er3.5B25 alloy are stronger than Co71.5Y3.5B25 (Fig. 5b). Noting that the reductions of the Warren–Cowley parameters for the B–RE, RE–RE, and B–B atom pairs are more significant than those of the Co–B and Co–Co pairs, which is consistent with the shortened bond lengths. The findings demonstrate that the Er-bearing metallic glass possesses a more densely packed structure and strengthened atomic interactions as compared to the Y-bearing alloy, arising from the crucial role of the Er element [44].
Voronoi polyhedron (VP) analysis was conducted to study the local atomic packing of the Co71.5RE3.5B25 (RE = Er, Y) metallic glasses. The VP can be indexed as < n3, n4, n5, n6 > to describe the arrangements of the nearest-neighbor atoms around the center atom, where ni represents the number of i-edge faces on the VP [45]. Figure 6a and b shows the major VPs centered by Co and B atoms in the two alloys, respectively. The two alloys have similar configurations of the main VPs, i.e., the VPs centered by Co atom are composed of ico-like (< 0, 1, 10, 2 > , < 0, 0, 12, 0 > , < 0, 1, 10, 3 > , < 0, 1, 10, 4 >) and deformed bcc (< 0, 3, 6, 4 > , < 0, 3, 6, 5 >) polyhedrons, while those centered by B atom are mainly trigonal prisms (TP) (< 0, 3, 6, 0 > , < 0, 2, 8, 0 > , < 0, 3, 6, 1 >) and crystal-like structures (< 0, 4, 4, 0 > , < 0, 4, 4, 1 >). As determined from the fractions of the VPs, both the alloys are dominated by B-centered TP-type clusters (> 50%). It should be noted that the Co71.5Er3.5B25 alloy contains a significantly higher fraction of ico-like clusters but fewer deformed bcc and crystal-like VPs, as compared to the Co71.5Y3.5B25. The ico-like cluster, characterized by fivefold symmetry, is favorable for amorphous formation due to its incompatibility with periodic arrangement [46]. In contrast, the deformed-bcc and crystal-like VPs exhibit crystal symmetry, making them more prone to transforming into crystalline phases and hindering the formation of the amorphous phase [47, 48]. Consequently, the Co71.5Er3.5B25 alloy with more ico-like clusters and fewer deformed-bcc and crystal-like VPs exhibits superior GFA than the Co71.5Y3.5B25.
3.3 Dynamics Behaviors
Time-dependent mean square displacement (MSD) was calculated to study the dynamic behaviors of the Co71.5RE3.5B25 (RE = Y, Er) alloys at different temperatures. According to Einstein’s diffusion equation, a smaller slope of the MSD curve indicates a lower diffusivity of the atoms [46]. Figure 7a presents the MSD curves versus time of the two alloys at different temperatures. As the temperature decreases, the slopes of the curves monotonically decrease, indicating a gradual increase in viscosity and a simultaneous reduction in atomic diffusion coefficient of the alloys. Below 1100 K, it is hardly to observe long-range diffusion behavior. Noting that the slopes for the Co71.5Er3.5B25 alloy are consistently smaller than those for the Co71.5Y3.5B25 alloy throughout the entire cooling process (Fig. 7a and b). The MSD results suggest that the Co71.5Er3.5B25 alloy has higher alloy melt viscosity and lower atomic diffusion capacity, which are consistent with its stronger bond strengths and denser local atomic packing structure. These can hinder the long-range diffusion of atoms during solidification and lead to a deceleration of crystallization, corresponding to its better GFA.
3.4 Magnetic and Mechanical Properties
The static magnetic properties tests reveal that the Co–Er–B metallic glasses exhibit a typical soft magnetic characteristic with quite low Hc values of around 1.3 A/m, and the Bs gradually decreases from 0.68 to 0.53 T with the enrichment of non-magnetic B and Er. The detailed values of the Co–Er–B alloys along with the data for Co71.5RE3.5B25 (RE = Y, Sm, Gd) alloys are listed in Table 1. Figure 8a compares the hysteresis loops inset with partially enlarged B-H curves near zero applied field of the Co71.5RE3.5B25 (RE = Er, Y) metallic glasses. The hysteresis loops of the Co71.5RE3.5B25 (RE = Y, Sm, Gd) metallic glasses can be seen in Fig. S3 (Supplementary Materials). Noting that the Co–Er–B metallic glasses have lower Hc than the Co71.5RE3.5B25 (RE = Y, Sm, Gd) alloys. Since the metallic glasses are structurally isotropic and free of crystalline defects like grain boundaries, the Hc is closely related to the magnetic anisotropy induced by internal stress, magnetostriction, and other factors [49]. Although the internal stress produced during melt-spinning can be alleviated by annealing, it is unable to eliminate the magnetic anisotropy completely even through a long-time thermal treatment [50]. The magnetic anisotropy energy (MAE), representing the absolute value of the maximum difference in total energy in different directions, has been employed to characterize the magnetic anisotropy [51]. Following the calculation method in [51], the MAE of the Co71.5RE3.5B25 (RE = Er, Y) metallic glasses were calculated to explain the better magnetic softness of the Co–Er–B alloys. As shown in Fig. 8b, the calculated MAE for the Co71.5Er3.5B25 metallic glass is 0.75 meV, which is smaller than that of the Co71.5Y3.5B25 (0.80 meV), aligning with the lower Hc. The Co71.5Er3.5B25 metallic glass with higher GFA possesses a higher atomic packing degree (refer to the subsequent discussion on local atomic structure), which might reduce the number density of quasi-dislocation dipole defects in the alloy, considered the main source of elastic stress [52], resulting in decreased MAE and consequently lower Hc. On the other hand, the Bs of the Co71.5Er3.5B25 metallic glass is smaller than that of the Co71.5Y3.5B25 (Table 1). In order to elucidate the underlying reasons, the average total magnetic moment (μtotal) and local magnetic moments of Co (μCo), Er (μEr)/Y (μY), and B (μB) in the two alloys were calculated, and the results can be seen in Table 2. The μEr is more negative than μY, and the μCo in the Co71.5Er3.5B25 alloy is also smaller than that in the Co71.5Er3.5B25. Hence, the μtotal is lower for the Co71.5Er3.5B25 alloy, and the Bs is inferior.
a Hysteresis loops of Co71.5RE3.5B25 (RE = Er, Y) metallic glasses inset with partially enlarged B-H curves near zero applied field and b differences (E-\(\overline{E}\) ) between total energy (E) in different magnetization orientations of cubic supercells and average energy of all orientations (\(\overline{E}\)) for Co71.5RE3.5B25 (RE = Y, Er) metallic glasses. MAE was determined from the difference between the maximum and minimum E
The compression tests demonstrate that the Co–Er–B BMGs possess high yielding strength (σy) of 3560–3969 MPa with distinct plastic strain (εp) of up to 0.50% (Table 1). Taking Co71.5Er3.5B25 and Co71Er4B25 BMGs as examples, both the alloys undergo an initial elastic strain of about 2% followed by yielding and distinct plastic deformation until failure (Fig. 9). Observing Fig. 9i–iii, the presence of numerous shear bands on the lateral surface is indicative of serrated flow during compression, which contribute to the plasticity [53]; vein-like patterns on the fracture surface characterize the excellent toughness of the BMGs [53, 54].
4 Conclusion
The Co–Er–B alloys demonstrate better GFA than the Co–RE–B (RE = Y, Sm, Gd) counterparts, and the BMG samples can be obtained in a composition region of Co68.5–71.5Er3.5–4B25–27.5. In comparison with the Co–Y–B alloys, the Co–Er–B metallic glasses possess stronger atomic bond strengths and denser local atomic packing structure characterized by a higher proportion of ico-like clusters but fewer deformed-bcc and crystal-like VPs, leading to enhanced structural stability and impeded ordered diffusion of atoms during solidification, which are the key factors contributing to the better GFA. The Co–Er–B BMGs exhibit excellent soft magnetic properties featuring low Hc of around 1.2 A/m and good mechanical properties with high σy of 3560–3969 MPa and distinct εp up to 0.50%. The exceptionally low Hc of the Co–Er–B metallic glasses can be attributed to their minimized MAE. The obtained ternary Co–Er–B BMGs can serve as foundational alloys for advancing the development of Co-based BMGs with further enhanced GFA and excellent soft magnetic properties.
References
W. Klement, R.H. Willens, P. Duwez, Nature 187, 869 (1960)
A. Inoue, Acta Mater. 48, 279 (2000)
W.L. Johnson, JOM 54, 40 (2002)
B. Wang, X.Q. Gao, J.C. Qiao, Rare Metal Mat. Eng. 53, 70 (2024)
A. Inoue, H. Koshiba, T. Itoi, A. Makino, Appl. Phys. Lett. 73, 744 (1998)
D. Turnbull, Contemp. Phys. 10, 473 (1969)
Z.P. Lu, C.T. Liu, J. Mater. Sci. 39, 3965 (2004)
A.J. Drehman, A.L. Greer, D. Turnbull, Appl. Phys. Lett. 41, 716 (1982)
H. Kato, T. Wada, M. Hasegawa, J. Saida, A. Inoue, H.S. Chen, Scr. Mater. 54, 2023 (2006)
W.Q. Li, Z.W. Zhu, G.J. Li, L. Zhang, Z.K. Li, H.M. Fu, H.W. Zhang, H. Li, A.M. Wang, H.F. Zhang, Acta Metall. Sin. -Engl. Lett. 33, 1407 (2020)
Y.C. Kim, W.T. Kim, D.H. Kim, Mater. Sci. Eng. A 375, 127 (2004)
K. Yang, X.H. Fan, B. Li, Y.H. Li, X. Wang, X.X. Xu, Acta Metall. Sin. -Engl. Lett. 31, 290 (2018)
B. Gun, K.J. Laws, M. Ferry, Mater. Sci. Eng. A 471, 130 (2007)
K. Amiya, A. Inoue, Mater. Trans. 43, 81 (2002)
B. Zhang, M.X. Pan, D.Q. Zhao, W.H. Wang, Appl. Phys. Lett. 85, 61 (2004)
N. Nagendra, U. Ramamurty, T.T. Goh, Y. Li, Acta Mater. 48, 2603 (2000)
M.A. Rahmani, Y.Z. Lu, Q. Luo, S.J. Qu, F.X. Ye, Y.X. Wu, J. Shen, Acta Metall. Sin. -Engl. Lett. 29, 834 (2016)
Y.M. Zhao, X. Li, X.B. Liu, J.Z. Bi, Y. Wu, R.J. Xiao, R. Li, T. Zhang, J. Mater. Sci. Technol. 86, 110 (2021)
S. Yi, T.G. Park, D.H. Kim, J. Mater. Res. 15, 2425 (2000)
F.Y. Chen, C.C. Cao, Q. Zhong, J.N. Liu, L.P. Yang, Z.Z. Chen, Mater. Today Commun. 27, 102207 (2021)
J. Zhou, Q.Q. Wang, X.D. Hui, Q.S. Zeng, Y.W. Xiong, K.B. Yin, B.A. Sun, L.T. Sun, M. Stoica, W.H. Wang, B.L. Shen, Mater. Des. 191, 108597 (2020)
J.F. Wang, R. Li, N.B. Hua, T. Zhang, J. Mater. Res. 26, 2072 (2011)
X.Y. Liang, Y.H. Li, F. Bao, Z.W. Zhu, H.F. Zhang, W. Zhang, Intermetallics 132, 107135 (2021)
C.Y. Lin, H.Y. Tien, T.S. Chin, Appl. Phys. Lett. 86, 162501 (2005)
V. Zhukova, M. Churyukanova, S. Kaloshkin, P. Corte-Leon, M. Ipatov, A. Zhukov, J. Alloy. Compd. 954, 170122 (2023)
A. Pardo, M.C. Merino, E. Otero, M.D. López, A. M’Hich, J. Non-Cryst. Solids 352, 3179 (2006)
X.L. Zhang, Y. Wu, R.R. Liu, J.H. Liu, H.T. Zhou, J. Non-Cryst. Solids 619, 122562 (2023)
A.H. Taghvaei, R. Farajollahi, J. Bednarcík, J. Eckert, M. Pahlevani, J. Alloy. Compd. 963, 171271 (2023)
J.Y. Zhang, S. Ma, H. Wang, T. Kubota, Y.H. Li, H. Kato, R.Y. Umetsu, M. Yao, W. Zhang, J. Alloy. Compd. 968, 171875 (2023)
A. Inoue, B.L. Shen, Mater. Trans. 43, 1230 (2002)
L.Y. Bie, Q. Li, D. Cao, H.X. Li, J.J. Zhang, C.T. Chang, Y.F. Sun, Intermetallics 71, 7 (2016)
N. Aihemaiti, Q. Li, M.C. Li, T. Wang, C.T. Chang, X. Ma, Intermetallics 123, 106834 (2020)
D.W. Huang, Y.H. Li, Y.P. Yang, Z.W. Zhu, W. Zhang, J. Alloy. Compd. 843, 154862 (2020)
J.W. Jiang, Q. Li, H.M. Duan, H.X. Li, Comput. Mater. Sci. 130, 76 (2017)
J.J. Han, W.Y. Wang, X.J. Liu, C.P. Wang, X.D. Hui, Z.K. Liu, Acta Mater. 77, 96 (2014)
A. Takeuchi, A. Inoue, Mater. Trans. 46, 2817 (2005)
A.P. Hammersley, S.O. Svensson, M. Hanfland, A.N. Fitch, D. Hausermann, High Pressure Res. 14, 235 (1996)
I.K. Jeong, J. Thompson, T. Proffen, A. Perez, S.J.L. Billinge, J. Appl. Crystallogr. 34, 536 (2001)
W. Kohn, L.J. Sham, Phys. Rev. 140, 1133 (1965)
S. Nosé, J. Chem. Phys. 81, 511 (1984)
Y.Q. Cheng, E. Ma, Prog. Mater. Sci. 56, 379 (2011)
J.L. Finney, Proc. R. Soc. A 319, 479 (1970)
X.H. Lin, W.L. Johnson, J. Appl. Phys. 78, 6514 (1995)
S.P. Pan, J.Y. Qin, T.K. Gu, J. Appl. Phys. 107, 033503 (2010)
N.N. Medvedev, J. Comput. Phys. 67, 223 (1986)
Y.C. Hu, F.X. Li, M.Z. Li, H.Y. Bai, W.H. Wang, Nat. Commun. 6, 8310 (2015)
A. Hirata, Y. Hirotsu, T. Ohkubo, E. Matsubara, A. Makino, J. Microsc. 223, 191 (2006)
S. Trady, A. Hasnaoui, M. Mazroui, J. Non-Cryst. Solids 468, 27 (2017)
M. Tejedor, J.A. García, J. Carrizo, L. Elbaile, J.D. Santos, J. Mira, J. Rivas, J. Appl. Phys. 96, 5658 (2004)
T. Bitoh, A. Makino, A. Inoue, Mater. Trans. 44, 2020 (2003)
M. Tejedor, J.A. García, J. Carrizo, L. Elbaile, J.D. Santos, J. Magn. Magn. Mater. 202, 485 (1999)
S. Ma, Y.Z. Ran, X.Y. Liang, L. Jiang, Y.H. Li, X.D. Wang, M. Yao, W. Zhang, J. Alloy. Compd. 902, 163637 (2022)
G.Y. Zhang, H. Zhang, S.Q. Yue, R.J. Cheng, A.D. Wang, A.N. He, Y.Q. Dong, H.W. Ni, C.T. Liu, Intermetallics 107, 47 (2019)
M.M. Trexler, N.N. Thadhani, Prog. Mater. Sci. 55, 759 (2010)
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos. 52171153 and 51871039). The HEXRD tests used the beamline 11-ID-C at APS, ANL, USA. APS is supported by the Department of Energy (DOE) Office of Science (DE-AC02-06CH11357). Q. Zeng acknowledges financial support from the Shanghai Science and Technology Committee, China (Grant No. 22JC1410300) and the Shanghai Key Laboratory of Material Frontiers Research in Extreme Environments (MFree), China (Grant No. 22dz2260800). The authors also acknowledge the National Supercomputing Center in Shenzhen.
Author information
Authors and Affiliations
Contributions
JL: Investigation, simulation, and writing–original draft. YL: Formal analysis and writing–review and editing. SM: Simulation. WL and FB: Investigation and Validation. ZZ: Formal analysis. QZ: Investigation and discussion. HZ and MY: Discussion. WZ: Supervision, Writing–review and editing, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., Li, Y., Ma, S. et al. Novel Soft Magnetic Co-Based Ternary Co–Er–B Bulk Metallic Glasses. Acta Metall. Sin. (Engl. Lett.) 37, 1633–1642 (2024). https://doi.org/10.1007/s40195-024-01722-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-024-01722-z