Abstract
Stress relaxation resistance is one of the most significant properties that are critical to the service life of the fasteners. In this study, the stress relaxation behavior of a Nb-stabilized austenitic stainless steel was investigated. It was revealed that the homogenization treatment at 1250 ℃ could make more primary NbC particles dissolved back into the matrix and consequently get a great number of nano-sized secondary NbC carbides. Therefore, the stress relaxation resistance will be enhanced due to the effective pinning of such higher density of nano-sized secondary NbC carbides on the dislocation movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fasteners are the key to ensure reliable contact between connectors, while some of fasteners are consistently subjected to harsh service conditions, such as high temperature or vibration; hence, the metals designed for manufacturing fasteners are required to have excellent comprehensive properties, such as superior stress relaxation resistance [1,2,3], which significantly affects the service life of the fasteners.
Under certain stress and temperature, the phenomenon of stress relaxation shows that the total deformation remains constant, and the elastic stress gradually decreases with time. It is generally believed that the high-temperature stress relaxation behavior of metals can be regarded as a creep process of continuous stress reduction, which is essentially the same as the constant load creep behavior [4, 5]. Dupeux et al. [6] studied the stress relaxation behavior of nickel-base alloys at 700 and 1000 ℃ and pointed out that the dislocation movement played an important role on the stress relaxation. Some scholars believed [7] that dislocation movement is the direct cause of stress relaxation, and the dislocation movement mechanism dominates the whole relaxation process, during which the substructures do not change. While others argued that [8] the stress relaxation process is essentially a redistribution process of dislocations. During the stress relaxation process, the total number of dislocations remains unchanged, but the number of movable dislocations will decrease, and the microstructure tends to be stable. In conclusion, there was still a divergence in understanding the nature of stress relaxation characteristics, but all the theories acknowledge that stress relaxation was closely related to the behavior of dislocations at elevated temperature. Therefore, the key to improve the stress relaxation resistance of materials is to effectively limit the movement of dislocations.
Nb-stabilized austenitic stainless steels such as AISI 347H are widely used as structural materials in nuclear power plants at elevated temperatures. Due to the effective pinning of dislocations by nano-sized NbC carbides, the Nb-stabilized austenitic stainless steels have excellent creep-rupture strength [9,10,11,12]. In addition, NbC can also increase the intergranular corrosion resistance by suppressing the M23C6 precipitation at grain boundaries [13].
The precipitation strengthening effect is directly correlated to the size and density of NbC particles [14, 15], a decrease in the average size and an increase in density can lead to an increase in precipitation strengthening. While the coarse NbC particles not only provide insufficient precipitation strengthening, but also are detrimental to the service performance of fasteners. Xie et al. [16] studied a kind of Nb-stabilized austenitic stainless steel and proved that the nucleation of micro-cracks located at the NbC/γ interface during the initial stage of deformation was caused by the plastic strain incompatibility. Ghalambaz et al. [17] pointed out that the coarse NbC particles are the origin of thermal fatigue cracks in an AISI 347 stainless steel pipe after 60,000 of working cycles. Hence, it is crucial to manipulate the precipitation of NbC in order to obtain a better service performance.
It was found that enough supersaturation of Nb in austenite matrix is the prerequisite for higher density of secondary NbC carbides [18], while the coarse primary NbC particles would precipitate in the interdendritic spacing during the solidification process [19]. The primary NbC particles consume a large amount of Nb, and consequently, the precipitation of nano-sized secondary NbC carbides is suppressed in the forging and subsequent solution treatment. Therefore, adjusting both size and density of primary NbC particles is crucial to manipulate the precipitation behavior of NbC carbides.
High-temperature homogenization refers to heating the ingot to a certain temperature below the liquidus temperature for long-term insulation. This heat treatment is an effective method to adjust the size and density of primary NbC particles by accelerating the elements diffusion between NbC and the steel matrix, as well as changing the equilibrium value of [Nb] and [C] for the partial dissolution of NbC [20]. In our previous study [21], we carried out homogenization treatment of a Nb-stabilized austenitic steel at different temperatures, and the results indicated that after homogenization treatment at 1250 ℃, most of the primary carbides could dissolve in the matrix, and it is beneficial for the precipitation of nano-sized NbC carbides in the subsequent treatment.
In this study, the stress relaxation behavior at 550 ℃ of a Nb-stabilized austenitic stainless steel with different densities of nano-sized NbC carbides was investigated. The effect of nano-sized NbC carbides on stress relaxation was analyzed, and the correlative mechanisms between precipitation and dislocation were discussed.
2 Experimental
2.1 Material and Heat Treatment
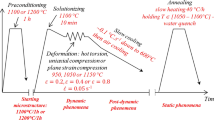
An austenitic stainless steel ingot with weight of 50 kg and diameter of 220 mm was produced by vacuum induction melting, and the nominal composition is 0.1C-15Cr-11Ni-3Si-Nb-Mo, in which the mass ratio of Nb/C is 9. The ingot was divided into two parts with the same weight. One ingot was homogenized at 1250 ℃ for 4 h followed by air cooling, while the other was not treated as the above. The two ingots were forged to rods with diameter of 25 mm after soaking at 1150 ℃ for 2 h, followed by air cooling, and then were solid solution treated at 1050 ℃ for 1 h followed by water cooling. Figure 1 shows the schematic of the heat treatment process. For convenience, they were referred to HFS (homogenization-forging-solid solution) and FS (forging-solid solution) steels, respectively.
2.2 Stress Relaxation Test
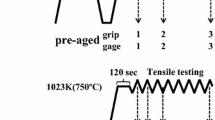
The geometric dimensions of stress relaxation test specimens following the ASTM standard E328-13 are shown in Fig. 2, and the specimens were machined from HFS and FS rods in the direction parallel to that of forging. The equipment for the stress relaxation test is a RMT-D5SC electronic high-temperature stress relaxation test machine.
The stress relaxation test was carried out according to GB/T 10120-2013 standard. The yield strength of HFS and FS steels at 550 ℃ is 208 and 200 MPa, respectively, as shown in Fig. 3. To keep the strain of experimental steels in the elastic range, the stress 120 MPa (about 60% of the yield strength) with a rate of 0.002 mm/s was loaded before the stress relaxation test. After that, the strain under the corresponding state was kept constant at 550 ℃ for 1000 h.
2.3 Microstructural Characterizations
Samples for microstructural observation were ground and polished to create a flat- and mirror-like surface and then etched in 30% aqua regia. The microstructure was examined by optical microscopy (OM, LEICA DMIRM), field emission scanning electron microscopy (FE-SEM, TESCAN MIRA3), and electron backscatter diffraction (EBSD, NordlysMax3, Oxford Instruments, UK). Chemical micro-analysis of the precipitates was conducted by an energy-dispersive X-ray system (EDS) coupled to the FE-SEM. Moreover, samples for EBSD measurements were prepared using electrolytic polishing in an electrolyte solution containing 10% perchloric acid and 90% alcohol under a voltage of 20 V for 300 s at − 30 ℃. EBSD measurements were then performed using a step size of 0.2 μm. Subsequently, all the raw data of EBSD were processed and analyzed using the HKL Channel 5 software. To further examine the precipitates in the steel matrix, EDS measurements and selected area electron diffraction (SAED) analyses were performed on a Talos 2100 transmission electron microscope (TEM) operated at 200 kV. Samples for TEM observation were prepared on thin wafers of 3 mm in diameter that was twin-jet electro-polished at 28 V in an electrolyte solution of 8% perchloric acid and 92% alcohol at – 25 ℃.
3 Results and Discussion
3.1 NbC Morphologies Before and After Homogenization
Figure 4 shows SEM micrographs of the as-cast steel. Equiaxed dendritic structures were observed, and eutectic-like structures were found in the interdendritic regions, which is consistent with the Nb-stabilized alloys [22, 23]. Higher magnification morphologies in Fig. 4b show that the primary precipitate was a eutectic-like microstructure. Furthermore, EDS analysis revealed that the eutectic-like microstructure was obviously enriched in Nb (Fig. 4c); thus, the primary precipitates were identified as NbC, which has been observed in the other as-cast Nb-stabilized austenitic stainless steel [16, 24].
Figure 5 shows the microstructures of the steel after homogenization treatments at 1250 ℃. A significant reduction in the size of primary NbC particles is observed, which is evidenced by the changes in morphology from initial eutectic-like morphology (Fig. 4) to granular at grain boundaries. It is concluded that homogenization treatment at 1250 °C could result in a noteworthy dissolution of primary NbC particles.
Figure 6 shows the distribution of primary and secondary NbC particles and grain size in HFS and FS steels after forging and solid solution treatment characterized by SEM and OM. Forging caused the primary NbC particles to be distributed in a chain along the longitudinal direction (Fig. 6a and d). Since most of the primary NbC particles were dissolved in the matrix after homogenization treatments (Fig. 5a), the primary NbC particles in HFS steel were in a short-chain distribution while they were long in FS steel, as shown in Fig. 6a and d. At the same time, by using Image Pro Plus software, 10 SEM images were selected for counting the density and average size of the secondary NbC carbides in two steels (Fig. 6b and e), as listed in Table 1. Based on the statistical data, we can conclude that there was a large difference between HFS and FS steels in the density of the secondary NbC carbides, which were 3.26 and 1.45 per square micron, respectively, but the size was almost the same. Despite the homogenization treatment made the grain size of the cast ingot larger, as shown in Fig. 5a, owing to the forging deformation and recrystallization, the grain size of two steels is almost the same (Fig. 6c and f).
3.2 NbC Evolution After Homogenization and Forging
During the homogenization, the thermodynamic driving force for dissolving NbC is from the difference in the chemical potential between Nb and C in the austenite matrix. Therefore, the equilibrium solubility product equation of NbC in the austenite is given as follows [21]:
where [Nb] and [C] are the solid solubilities of Nb and C in the austenite matrix, and T is the Kelvin temperature. According to the solubility product equation, homogenization treatment temperature could affect the equilibrium value of [Nb] [C] product, as shown in Fig. 7.
The equilibrium solid solubility product of NbC varies exponentially with the inverse of temperature, and the higher the temperature, the larger the equilibrium solid solution product. Assuming that Nb-to-C ratio in NbC is stoichiometric, the mass fraction of Nb and C used to form NbC could be calculated according to Eq. (2):
where \({X}_{\mathrm{t}}\left(\mathrm{Nb}\right)\) is 0.9, and \({X}_{\mathrm{t}}\left(\mathrm{C}\right)\) is 0.1, which represent the mass fractions of Nb and C in the experimental steel, and \({M}_{\mathrm{Nb}}\) and \({M}_{\mathrm{C}}\) are the atomic masses of Nb and C, respectively. For HFS steel, the temperature of homogenization treatments was 1250 ℃, and FS steel was 1150 ℃ before forging. According to Eqs. (1) and (2), the calculated amount of NbC is 0.72 wt% at 1150 ℃, while at 1250 ℃, the amount significantly decreases to 0.6 wt%. Hence, it is believed that the homogenization treatment at 1250 ℃ is beneficial for the dissolution of NbC, and a certain amount of Nb remains in the matrix. Since the supersaturation of Nb was enhanced in the matrix, the density of the nano-sized secondary NbC carbides in HFS steel was higher than that in FS steel.
3.3 Stress Relaxation Behaviors
The stress–time curves of the two experimental steels for 1000 h of stress relaxation at an initial stress of 120 MPa at 550 ℃ are shown in Fig. 8. In order to accurately determine the mechanical behavior of the experimental steel after long-term relaxation and eliminate the influence of data fluctuation during the experiment, the Origin software (v8.0) was used to fit the relaxation curve of the experimental steel.
Figure 8 intuitively shows that the stress relaxation curves of the two experimental steels are typical logarithmic decay curves, which are similar to the other studies [25, 26]. The curves are mainly divided into the rapid stress drop stage (Stage I) and the stress stabilization stage (Stage II). The Stage I is in the early stage of stress relaxation. By comparing the curves of two steels, the relaxation rates in the Stage I were approximately the same, but the Stage I of HFS steel ended earlier, entering the Stage II after about 300 h, while 500 h for FS steel. In the Stage II, the stress of the experimental steels dropped slowly and finally approached stable values, that is, the stress relaxation limits. The stress relaxation limit is the key factor to determine whether a fastener can maintain the fastening force during the high-temperature service. Due to the difference in the Stage I, the stress relaxation limit of HFS steel was 12 MPa higher than that of FS steel after 1000 h, improved by 21.8%. This indicates that HFS steel has greater resistance to the stress relaxation.
3.4 Dislocation Activation Volume
It is generally believed that the stress relaxation behavior can be regarded as a creep process of continuous stress reduction, and the mechanism of the creep process includes dislocation creep and grain boundary migration creep. In fact, the grain boundary could not migrate at such experimental conditions, this is mainly determined by low stress, low temperature, and low diffusion coefficient of austenitic steel. As shown in Fig. 9, the EBSD band contrast images indicate that the grain size in HFS steel was basically unchanged after 1000 h. So, it is reasonable to believe that there was no obvious grain boundary migration under the experimental condition, and the mechanism of creep was mainly the dislocation creep. The stress relaxation behavior of the experimental steel reflected a thermal activation movement between the precipitates and the dislocations during relaxation.
As mentioned above, the initial strain is elastic, and the elastic strain can gradually turn into plastic strain during the process of stress relaxation. The total strain, which is composed of elastic strain and plastic strain, remains constant during the stress relaxation, so the instantaneous total strain rate is always zero. According to the Hooke's law, the elastic strain rate is the stress relaxation rate divided by the elastic modulus. Therefore, the plastic strain rate and stress relaxation rate meet the following equations:
where \(\dot{\varepsilon }_{{\text{e}}}\) is the elastic strain rate, \(\dot{\varepsilon }_{{\text{p}}}\) is the plastic strain rate, and E is the elastic modulus.
The stress relaxation curve is of logarithmic decay type, and the reduction of the stress \((\Delta \sigma )\) and the relaxation time (t) meet the following Eq. [27]:
where \({\sigma }_{0}\) is the initial stress, \({\sigma }_{t}\) is the instantaneous stress at the time t, and s and c are the material constants, reflecting the rate of stress reduction. According to the stress relaxation curves in Fig. 8, the s values of HFS and FS steels can be calculated as 9.549 and 12.876, respectively.
The dislocation movement often encounters various obstacles during the deformation. The resistance to impede the dislocation motion can be classified into two categories [28]. The first category is the long-range resistance, which is generated by the elastic stress field of all dislocations within grains. If the external stress is less than the long-range resistance, the dislocation cannot slip. Only through the recovery to reduce the dislocation density, can the dislocation slip. The other is the short-range local resistance mainly from solid solution atoms or precipitate particles. The dislocations overcome short-range local resistance mainly through the thermal activation of atoms, thereby releasing stress. The short-range resistance of dislocation can be quantitatively described by dislocation thermal activation volume (V) [29,30,31,32,33]:
where M is the Taylor factor, k is the Boltzmann constant, and T is the Kelvin temperature.
It follows that during the stress relaxation at 550 ℃, the dislocation thermal activation volume of HFS steel is 1.35 times that of FS steel. The higher density of the secondary NbC carbides increases the resistance to dislocation movement and prolongs the time of dislocations to overcome barriers, so the activation volume of the dislocation motion is larger.
In-depth analysis of the relaxation curves, the threshold stress could be measured in the stress relaxation process, which proves that the precipitates hindered the thermally activated motion of dislocations. The threshold stress is the minimum stress required to overcome the second-phase particles for dislocation slip [34] and is equal to the Orowan stress. It can be solved by the curve of log plastic strain rate and log stress relationship, as shown in Fig. 10. The stress corresponds to the inflection point where the strain rate shows a nearly 90° vertical decrease in the curve. It can be seen from the curve that the threshold stress of HFS steel is 20.9% higher than that of FS. This proves that the density of NbC carbides in the experimental steels had a significant effect on the threshold stress, and further indicates that NbC carbides could impede the thermally activated motion of dislocations.
3.5 Interaction of Nano-sized NbC and Dislocations
Figure 11 shows the TEM observation of precipitates and dislocations in the matrix of HFS steel after the stress relaxation test. It can be seen that the nano-sized NbC carbides play a significant role in pinning the dislocations during the stress relaxation process. Since the nano-sized NbC carbides are non-shearable, they are difficult to be overcome by dislocations even at high temperatures [35]. It can be also observed that the dislocations attached at the departure side of the particles [36], as shown in Fig. 11a. It means that a short length of dislocation line was trapped at the interface and unable to glide away. While the other mode, as shown in Fig. 11b, usually occurred on the large size nano-sized NbC carbides. Some of the dislocations could pass through the NbC carbides by the Orowan mechanism and form dislocation tangles around the NbC carbides. The entangled dislocation reduced the spacing between the NbC carbides, and it was more difficult for subsequent dislocations to bypass the particles [37]. Accordingly, in the stress relaxation process, since the dislocations were pinned by high-density NbC carbides, the elastic deformation in HFS experimental steel was difficult to transform into the plastic deformation, which reduced the rate of stress relaxation, and the stress could maintain at a high level.
Furthermore, the austenitic stainless steel has low stacking fault energy. Therefore, the perfect dislocations are prone to decompose to extended dislocations, including two incomplete dislocations as well as the stacking faults (SF) [38]. Figure 12 shows the slip process of the extended dislocations on the main slip plane in the austenite matrix of the two experimental steels. As shown in Fig. 12a and d, the density of nano-sized NbC carbides in FS steel was low compared with that of HFS steel. Besides, there were a large number and large size of extended dislocations in FS steel. Figure 12b shows the initial state of the extended dislocations. During the deformation process, the leading dislocations slip forward, making the intermediate stacking faults longer [39]. Therefore, there were a large number of long-range extended dislocations with size of submicron or micron level in the matrix after slipping (Fig. 12c). The slip of the extended dislocations reduced the energy in the matrix and released the stress generated by deformation. Figure 12d–f shows the micromorphology of HFS steel. In addition to the perfect dislocation lines, there are also a certain number of extended dislocations. However, compared with FS steel, the size of extended dislocation lines in HFS steel is smaller, and the intermediate stacking fault is shorter. The main reason for this phenomenon is the hindering and limiting effect of nano-sized NbC carbides. The leading extended dislocations were stopped (Fig. 12e) or twisted (Fig. 12f) by the repulsive force on the front NbC carbides during the slipping process. So, the leading extended dislocations would stop or slow down in the slip process, while the following dislocations were not hindered and continued to slide, resulting in the shortening of the intermediate stacking faults. The hinder effect of the nano-sized NbC carbides generated greater stress concentration; thus, the extended dislocations would recombine as the perfect dislocations, and the stress in the deformation process could not be released completely.
In the process of the stress relaxation, as for the nano-sized NbC carbides, both the pinning effect on the perfect dislocations and the limiting effect on the extended dislocations would prevent the effective release of stress at high temperature and thus reduce the stress relaxation rate of the experimental steel. Increasing in the density of the secondary NbC carbides led to the significantly pinning and limiting effects. Therefore, compared with FS steel, the dislocation activation volume and threshold stress of HFS steel were relatively larger, and the relaxation limit should be higher.
4 Conclusions
In this work, stress relaxation experiment at 550 ℃ was carried out on a Nb-stabilized austenitic stainless steel under different heat treatments. The main conclusions can be summarized as follows:
-
1.
About 1250 ℃ homogenization treatment could effectively reduce the volume fraction of primary NbC particles in the cast Nb-stabilized austenitic steel and promote the precipitation of nano-sized NbC carbides in the steel matrix.
-
2.
Under constant strain, the stress in Nb-stabilized austenitic steel presented a typical logarithmic decay. Compared with FS experimental steel, HFS experimental steel had a higher relaxation limit after 1000 h with initial stress of 120 MPa.
-
3.
The nano-sized NbC carbides in the steel hindered the movement of dislocations mainly by pinning perfect dislocations and limiting the slip of extended dislocations. The higher density of nano-sized NbC carbides led to the more significant hindering effect for dislocations. The calculation results show that increase in the density of particles causes the increase in dislocation activation volume, which increases the stress relaxation limit, and reduces the stress relaxation rate of the experimental steel.
References
N.G. Pai, D.P. Hess, J. Sound Vib. 253, 585 (2002)
M. Zheng, Z. Liu, X. Yan, N. Niu, T. Zhang, Y. Li, Eng. Fail. Anal. 134, 106021 (2022)
G. Wang, H. Liu, K. Song, Y. Zhou, C. Cheng, H. Guo, Y. Guo, J. Tian, J. Mater. Res. Technol. 19, 3579 (2022)
D.A. Woodford, JSME Int. J. 45, 98 (2002)
F.V. Ellis, D.R. Sielski, R. Viswanathan, J. Press. Vessel. Technol. 123, 70 (2000)
M. Dupeux, J. Henriet, M. Ignat, Acta Metall. 35, 2203 (1987)
K. Hirai, I. Takeuchi, Y. Takigawa, T. Uesugi, K. Higashi, Mater. Sci. Eng. A 551, 19 (2012)
J. Zhao, J. Gong, A. Saboo, D.C. Dunand, G.B. Olson, Acta Mater. 149, 19 (2018)
L. Sun, X. Liu, X. Xu, S. Lei, H. Li, Q. Zhai, J. Iron. Steel Res. Int. 29, 1513 (2022)
Y. Chen, D. Wu, D. Li, Y. Li, S. Lu, Acta Metall. Sin. -Engl. Lett. 36, 637 (2022)
X.F. Guo, Y.Y. Ni, J.M. Gong, L.Y. Geng, J.Q. Tang, Y. Jiang, X.K. Jia, X.Y. Yang, Acta Metall. Sin. -Engl. Lett. 30, 829 (2017)
C.W. Hong, Y.U. Heo, S.J. Kim, Mater. Charact. 178, 111212 (2021)
S. Zhang, X. Shi, Y. Liang, H. Xu, C. Yan, W. Yan, L. Rong, K. Yang, J. Mater. Res. Technol. 23, 4280 (2023)
J.H. Shin, B.S. Kong, H.J. Eom, C. Jang, H. Do, D. Jang, Mater. Sci. Eng. A 806, 140816 (2021)
C.W. Hong, Y.U. Heo, N.H. Heo, S.J. Kim, Mater. Charact. 124, 192 (2017)
A. Xie, S. Chen, Y. Wu, H. Jiang, L. Rong, Mater. Charact. 194, 112384 (2022)
M. Ghalambaz, M. Abdollahi, A. Eslami, A. Bahrami, Case Stud. Eng. Fail. Anal. 9, 52 (2017)
M.H. Jang, J.Y. Kang, J.H. Jang, T.H. Lee, C. Lee, Mater. Charact. 137, 1 (2018)
J. Erneman, M. Schwind, H. Andren, J. Nilsson, A. Wilson, J. Agren, Acta Mater. 54, 67 (2005)
Y. Zhang, H. Zou, J. Iron. Steel Res. Int. 30, 82 (2022)
H.T. Xu, C.G. Yan, S.Z. Zhang, X.B. Shi, W. Yan, H.C. Jiang, Press. Vessel Technol. 39, 9 (2022)
H. Xu, C. Shi, X. Zhu, S. Wang, J. Li, S. Wang, J. Iron. Steel Res. Int. 29, 1823 (2022)
K.G. Buchanan, M.V. Kral, Metall. Mater. Trans. A 43, 1760 (2012)
S. Yu. Kondrat’ev, E.V. Sviatysheva, G.P. Anastasiadi, S.N. Petrov, Acta Mater. 127, 267 (2017)
L. Xiao, J.L. Bai, Mater. Sci. Eng. A 244, 250 (1998)
S. Khayatzadeh, D.W.J. Tanner, C.E. Truman, P.E.J. Flewitt, D.J. Smith, Eng. Fract. Mech. 175, 57 (2017)
T. Xiao, X.F. Sheng, Q. Lei, J.L. Zhu, S.Y. Li, Z.R. Liu, Z. Li, Acta Metall. Sin. -Engl. Lett. 33, 375 (2019)
M.M. Mostafa, G.S. Al-Ganainy, A.M.A. El-Khalek, R.H. Nada, Phys. B Condens. Matter 336, 402 (2003)
E. Majewski, Int. Geophys. 323 (2001)
G. Regazzoni, U.F. Kocks, P.S. Follansbee, Acta Metall. 35, 2865 (1987)
J. Buha, R.N. Lumley, A.G. Crosky, Mater. Sci. Eng. A 492, 1 (2008)
Y. Xu, L. Zhan, L. Xu, M. Huang, Mater. Sci. Eng. A 682, 54 (2017)
J. Ren, Y. Zhang, D. Zhao, Y. Chen, S. Guan, Y. Liu, L. Liu, S. Peng, F. Kong, J.D. Poplawsky, G. Gao, T. Voisin, K. An, Y.M. Wang, K.Y. Xie, T. Zhu, W. Chen, Nature 608, 62 (2022)
R. Kaibyshev, O. Sitdikov, I. Mazurina, D.R. Lesuer, Mater. Sci. Eng. A 334, 104 (2002)
M. Heczko, B.D. Esser, T.M. Smith, P. Beran, V. Mazánová, D.W. McComb, T. Kruml, J. Polák, M.J. Mills, Mater. Sci. Eng. A 719, 49 (2018)
M. Heczko, B.D. Esser, T.M. Smith, P. Beran, V. Mazánová, T. Kruml, J. Polák, M.J. Mills, J. Mater. Res. 32, 4342 (2017)
A.S. Kirtikar, A.H. King, Mater. Sci. Eng. A 148, 155 (1991)
Z. Zhang, M.M. Mao, J. Wang, B. Gludovatz, Z. Zhang, S.X. Mao, E.P. George, Q. Yu, R.O. Ritchie, Nat. Commun. 6, 10143 (2015)
X. Song, C. Huang, J. Jia, J. Liu, J. Iron. Steel Res. Int. 29, 1004 (2022)
Acknowledgements
The present research was financially supported by the LingChuang Research Project of China National Nuclear Corporation, the Natural Science Foundation of Liaoning Province (2023-MS-019), and the project of "Novel corrosion-resistant low-active 1000 MPa Grade high strength steel for water resources and electric engineering" from Guangdong Polytechnic of Water Resources and Electric Engineering.
Funding
All authors disclosed no relevant relationships.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Shi, X., Su, Y. et al. Stress Relaxation Behavior of a Nb-Stabilized Austenitic Stainless Steel at 550 ℃. Acta Metall. Sin. (Engl. Lett.) 36, 2079–2088 (2023). https://doi.org/10.1007/s40195-023-01611-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-023-01611-x