Abstract
A new type of steel containing Sb based on the CASTRIP process had been designed to improve the corrosion resistance of high-strength steels in industrial atmospheres. The properties of the electrochemistry and rust layer (RL) were analyzed to evaluate the corrosion resistance of the ultra-thin cast strip (UCS) steel containing 0.10% Sb. Experimental results showed that the corrosion resistance of UCS steel containing Sb was doubled compared to that of traditional hot rolling strip (HRS) steel. It could be interpreted as an integral effect of CASTRIP process and alloying elements. The optimization for microstructure and inclusion of UCS steel can reduce the localized corrosion effects in initial corrosion processes. Cu, P and Sb reduced the anodic reaction and CuFeO2/Cu2O, CuO, FePO4 and Sb2O3 were formed, promoting the transition from γ-FeOOH to α-FeOOH. A (Cu, Sb, P)-enriched inner RL was generated and significantly improved protective ability of the RL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The economic losses produced by material corrosion in the atmosphere account for more than half of all environmental corrosion losses [1]. Steel structures such as tower, bridge, high-speed guardrail and other facilities are usually exposed to atmospheric corrosion during the long-term service process, resulting a decline in mechanical properties and a serious threat to the safe operation of steel structures. Despite of the fact that most steel structures have been applied with surface protection, chemical and electrochemical corrosion still occur due to the synthetical effects of multiple environmental factors [2]. So how to mitigate the corrosion of steel in the atmosphere has been an important issue in the metallurgical industry. Since Corten steels have been developed in the 1930s, they have been widely used in railways, bridges, buildings, and other industries without plating [3,4,5]. Other alloying elements, such as Mn, Ni, Mo, Al, Ti, Nb, Sn, and Sb, added into steels have been revealed to facilitate the formation of a protective layer, preventing the steel from rusting further [6,7,8,9]. Among all elements, Sb is an efficient element due to its improvement on the corrosion resistance to sulfuric, nitric, and hydrochloric acids at room temperature [10,11,12]. In the 1960s, Nippon Steel Corporation developed S-TEN 1 steel, which contained Cu and Sb elements and was resistant to sulfuric acid dewpoint corrosion. In 1992, a novel weathering steel named ND steel based on the alloy system of 09CrCuSb was successfully developed which was now widely utilized in electric power, metallurgy, petrochemical, and other industries due to its remarkable resistance to sulfuric acid dewpoint corrosion [11, 12].
Due to the development of industrial upgrading in recent years, higher strength, thinner and superior corrosion resistance steel has progressively demonstrated significant promise in new applications progressively [13, 14]. In order to meet the above requirements, a new type of ultra-thin cast strip (UCS) steel containing Sb produced by the CASTRIP process has been developed, which was featured by ultra-thinness, high-strength, superior corrosion-resistant and cost control [15,16,17,18,19]. In addition, the UCS steel is more environmentally friendly and energy efficient compared to the traditional hot rolling strip (HRS) [15, 20, 21].
Up to now, it has been reported that Sb could generate a protective substance (Sb2O3) that could enhance the compactness of rust layer (RL) [22, 23]. In an acidic environment where the pH value was less than 4, Sb2O3 was partially converted into more stable Sb2O5 to improve the corrosion resistance of RL [23]. Another effect was that Sb slowed the formation of RL by influencing the cathodic process [24]. It showed that Sb could consume the H+ in solution at the beginning of corrosion to prevent the effect of oxygen reduction reaction [22]. However, mechanisms of Sb for corrosion resistance enhancement have not been investigated in UCS steel. Meanwhile, researchers have mentioned that controlled rapid cooling of CASTRIP process has improved the corrosion resistance of steels [25], but the mechanism has not been clearly explained. Further research is needed to investigate the corrosion mechanism of UCS steel containing Sb.
In this study, a new type of UCS steel alloyed by Sb has been designed and produced. Evaluation corrosion properties were characterized by dry/wet cyclic tests and electrochemical tests. Difference of microstructure and inclusions was studied by ASPEX-scanning electron microscopy (ASPEX-SEM) and electron dispersive spectroscopy (EDS). The properties of the RL were analyzed by X-ray diffraction (XRD), electron probe microanalysis (EPMA), and micro-Raman spectroscopy. The purpose of this study was to describe the differences in corrosion performance and mechanisms, as a way to highlight the excellent corrosion resistance of UCS steels. Meanwhile, the mechanism of corrosion performance enhancement by Sb and its possible synergistic effect with other elements in UCS steel was elaborated through characterizing properties of the RL.
2 Experimental
2.1 Materials Preparation
This experiment designed a 500 MPa steel containing Sb based on CASTRIP process (0.10Sb UCS). Figure 1 shows the diagram of main process, which was developed by Shagang in 2018 as the first commercially production line in China. The experimental steel was treated by vacuum furnace vacuum degassing and secondary refining ladle furnace to get steel with a qualified composition. Then, the steel flowed gradually from the big ladle to the furnace hearth formed by the side sealing plate and two copper rolled through the long nozzle, tundish, and transition piece. Two copper rolls were fed with high-speed cooling water and the steel gradually solidified on the surface of copper rolls. A protective atmosphere was maintained through the “hot box” before the steel entered the hot rolling stand. The cast strip was transferred to the pinch rolls and was rolled to the target thickness (1.5 mm) by the hot rolling stand. The steel was subsequently cooled to the target temperature and then entered the coiler. To make the results more convincing, two steels (Sb-free HRS steel and Sb-free UCS steel) with the same strength level and similar alloying element were chosen as the comparison specimens. The chemical compositions of test steels are listed in Table 1.
2.2 Microstructure and Inclusion Distribution Analysis
Microstructures were observed by SEM (ZEISS SIGMA 300). Block samples were cut from the steel plates along the rolling directions and grounded with SiC papers, followed by polishing with diamond paste. The polished surfaces of samples were etched with 4% nital solution. Inclusion morphology was observed by SEM combined with EDS at an accelerating voltage of 20 kV. ASPEX-SEM was conducted to detect the size and number of inclusions by collecting the backscattered electron beam signal and analyzing the inclusion composition by simultaneous control of the X-ray energy spectrometer. The grinding and polishing process was the same as for SEM samples without etching. The test area was determined at 40 mm2.
2.3 Corrosion Tests
The electrochemical tests were conducted using a IVIUM CompactStat.h workstation in a three-electrode glass cell, with a platinum sheet as the counter electrode, a silver chloride as the reference electrode and the tested samples with an exposed area of 0.78 cm2 as the working electrodes. In the creation of the working electrodes, the backside of the samples was soldered with copper wire, and the samples were then sealed with epoxy resin. The exposed surfaces were subjected to abrasion with silicon carbide papers from 400 to 1000 grit. The open-circuit potential (OCP) was measured for 3600 s in a 0.01 M NaHSO3 solution. After the specimen reached stable OCP, electrochemical impedance spectroscopy (EIS) measurement was obtained with an amplitude of 10 mV in the frequency range from 100 kHz to 10 mHz. Potentiodynamic polarization curves were obtained at a scanning rate of 0.167 mV/s and a scanning range of ± 250 mv (Ag/AgCl) relative to open-circuit potential. Three replicate tests were carried out at room temperature (approximately 25 ℃) for each sample to guarantee that the test results were repeatable.
The dry/wet cyclic accelerated corrosion test was used to study the change of steels during atmospheric simulation test [26]. The tested samples with dimensions of 55 mm × 50 mm were grounded with SiC paper, washed by ethyl alcohol, and then dried by cool air to determine the original weight. The detailed test parameters are listed in Table 2. Based on the previous studies of other researchers [27, 28], an electrolyte solution consisting of 0.01 M NaHSO3 was used as the corrosion-accelerating solution to evaluate the corrosion resistance of industrial atmospheric corrosive environments. Each hour is one cycle and the corrosion time for the test samples was determined as 72, 168, 264 and 408 h. To guarantee the reliability of the results, three parallel specimens were used to perform each immersion experiment and another specimen to characterize the properties of RL.
Corrosion rate was determined by the weight loss method [29, 30]. The corroded samples were first cleaned ultrasonically in a solution consisted of 500 mL HCl + 500 mL H2O + 3.5 g hexamethylenetetramine for 10 min in accordance with the standard [27, 31]. Then, the samples were washed with alcohol and dried to obtain the final mass. The thickness loss (D, μm) and corrosion rate (CR, g/(m2·h)) were calculated with the following equation [32,33,34,35]:
where W was the weight loss measured after immersion test, g; A was the superficial area of the samples, cm2; ρ represented the density of the steel (7.8 g/cm−3); and T was the corrosion time, h.
2.4 Analysis Methods of RL
Corrosion products were scraped off from the specimens for the phase compositions of the RL, which were detected by the SmartLab with a Cu Kα target. The accelerated voltage and filament current were 40 kV and 150 mA, respectively. The scanning rate was 3°/min, and the scanning range was 10–80° (in 2θ). Moreover, semi-quantitative analysis was completed based on the diffraction intensity ratio of the test results and the standard card. EPMA was used to detect the distribution of elements in the RL of the test specimen. The accelerated voltage and filament current were 15 kV and 100 mA.
The phase distribution across the RL was analyzed by micro-Raman spectroscopy (Invia Qontor) equipped with the laser wavelength of 532 nm. The laser power was reduced below 0.1 mW so as to prevent the transformation of the detected compounds into α-Fe2O3 due to the laser heating [36]. The corresponding product for each point was differentiated by its Raman spectrum morphology and the strongest Raman band as referenced in previous research, for example, 248, 387 and 660 cm−1 for γ-FeOOH, α-FeOOH and Fe3O4 [37,38,39,40,41], respectively. To ensure the accuracy of the peak, the test range is within 1500 cm−1 and the acquisition time is 30 s. X-ray photoelectron spectroscopy (XPS) analysis was used to study the existence forms of Cu, P and Sb in RL. The binding energy value was corrected with reference to the standard C1s peak (284.8 eV) to avoid a possible charge effect.
3 Results
3.1 Microstructure
The microstructure of the prepared samples is shown in Fig. 2. The microstructure of Sb-free HRS steel included ferrite and pearlite. The microstructure of two UCS steels consisted of polygonal ferrite (αp), quasi-polygonal α (αq), bainitic ferrite (αB°) and acicular ferrite (AF) [42,43,44,45].
3.2 Inclusion Analysis
Figure 3 shows SEM images and EDS mapping of typical spherical inclusions. Since Sb had no obvious effect on the type of inclusion, Sb-free HRS steel and Sb-free UCS steel were counted by ASPEX, as shown in Fig. 4. A total of 1563 inclusions were detected in Sb-free HRS steel of which consisted of CaS, MnS and Al2O3. The typical composite CaS-MnS-Al2O3 inclusion with the size of more than 4 µm is shown in Fig. 3a. Micro-gaps existed between inclusion and matrix, and poor bonding leaded to matrix loss during grinding and polishing process. As a comparison, 1537 inclusions were detected in Sb-free UCS steel of which consisted of Al2O3, SiO2 and MgO. Typical ternary composite oxides were spherical, strongly bonding with the matrix with a size of about 2 μm, as shown in Fig. 3b.
The size distribution of the inclusions was statistically evaluated and sulfides or compound sulfides accounted for more than 95% in Sb-free HRS steel. As indicated in the ternary phase diagram in Fig. 4d, the majority of the inclusions in Sb-free UCS steel were consisted of SiO2, and the inclusions were reported to be distributed uniformly in UCS steel [46]. The size distribution of inclusions was also optimized in Sb-free UCS steel, as shown in Fig. 4a, c. The average size was 2.7 µm for Sb-free UCS steel, while the size was 3.1 µm for Sb-free HRS steel. The size of inclusions less than 2 µm accounted for 32%, while the value was only 19% in Sb-free HRS steel. To characterize the distribution of size range, a Gaussian function was used to fit the histograms of the size of inclusions in Sb-free HRS steel and Sb-free UCS steel. It can be inferred that the average size and size concentration was significantly better than that of Sb-free HRS steel. Overall, the inclusion was more significantly optimized in UCS steels.
3.3 Corrosion Properties
3.3.1 Electrochemical Properties
Figure 5a shows the Nyquist plots in a 0.01 M NaHSO3 solution. The equivalent circuit as shown in Fig. 5a was used to fit the EIS data to obtain the Rs (solution resistance), Rr (rust-layer resistance), Rct (charge transfer resistance), Cr (rust-layer capacitance) and Cdl (double-layer capacitance). The EIS results for the test steel showed two capacitive arcs, one for the high-frequency region and one for the low-frequency region. In the high-frequency region, the capacitive reactance arc reflected the double-electric-layer structure composed of the RL and solution. While in the low-frequency region, the capacitive reactance arc reflected the double-electric-layer structure of the interface between the substrate and the corrosive solution infiltrating the substrate surface. Since the NaHSO3 solution was weakly acidic, the Fe(OH)3 produced at the beginning of corrosion wrapped around the surface of the test steels. This indicated that the surface of the tested steel was covered by corrosion products and two sets of double-electric-layer structures existed between the sample and the solution. From the fitting results in Table 3, it could be seen that both Rr and Rct of Sb-free UCS steel were larger than those of Sb-free HRS steel. The Rr and Rct of UCS steel also increased after the addition of Sb. With the decrease in Rr and Rct, the transport resistance of the corrosive medium and charged particles decreased, which accelerated the electrochemical corrosion process [47]. This indicated that the 0.10Sb UCS steel had the densest rust layer at the beginning of corrosion, while the Sb-free HRS steel had the worst rust layer performance.
Figure 5b shows the polarization curves and the fitted parameters of the prepared steels. All steels had no obvious passivation characteristics in the anodic section. The electrochemical process exhibited an active dissolution reaction in the anodic region and combination of oxygen reduction and hydrogen precipitation reaction in the cathodic region. Moreover, the cathode curve shifted left with the direction of the arrow, which reflected the enhanced inhibiting effect on the cathodic process. It could be reasoned the CASTRIP process and the addition of 0.01% Sb both suppressed the cathodic reaction as well as anodic reaction. It was also reflected in a significant reduction in corrosion current density (icorr), which suggested that 0.1Sb UCS steel had the best corrosion resistance among three steels, as shown in the table of Fig. 5b.
3.3.2 Corrosion Kinetics and Corrosion Rate
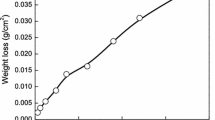
The thickness loss (Dt) and corrosion rate (CR) were determined from Eqs. (1) and (2) after various periods of dry/wet cyclic acceleration corrosion tests, as shown in Fig. 6. The thickness loss of three steels increased, while the corresponding corrosion rate decreased with corrosion time. The thickness loss was approximately the same in the initial stage of corrosion (approximately 70 h). The thickness loss of UCS tended to level off when an oxide layer was formed on the surface of the sample at the early stage of corrosion, while the thickness loss of Sb-free HRS steel increased linearly, as shown in Fig. 6a, which was similar to the study of some researchers on carbon and weathering steels [23, 48, 49]. When the corrosion time was up to 408 h, the corrosion rate difference between the three steels was obvious. The corrosion rate for Sb-free HRS steel and Sb-free UCS steel was 2.167 g/(m2 h) and 1.643 g/(m2 h) respectively, while that of 0.10Sb UCS steel was only 1.153 g/(m2 h), as shown in Fig. 6b.
In order to better evaluate the corrosion kinetic process of 0.10Sb UCS steel in simulated industrial atmosphere, the classical atmospheric corrosion power function model D = Atn [32, 50, 51] was used to fit the thickness loss, where D was the thickness reduction (μm) and t was the exposure time (h). The results are shown in Fig. 6a. A represented the initial corrosion performance of the steel grade, related to the environment and its surface properties. n represented the corrosion development trend of the material in this environment, mainly determined by the composition of RL. When n > 1, it meant that the RL had no protective effect, while the corrosion process was a deceleration process caused by the protective RL when n < 1 [51]. The A value of Sb-free HRS steel was much larger than that of two UCS steels, indicating that its initial corrosion rate rose more rapidly. n of all materials was less than 1, which demonstrated that all steels had a certain protective effect on RL. Surprisingly, the value of n for Sb-free HRS steel was close to twice the value for 0.10Sb UCS steel, confirming that the protective effect of its RL was much stronger than that of Sb-free HRS steel. Compared to Sb-free HRS steel, the corrosion rate of UCS was reduced with improving the stability of RL, and the addition of Sb had more effective effect.
3.4 Properties of RL
3.4.1 Cross-Sectional Elemental Analysis
To illustrate the influence of alloying elements on the RL, the cross-sectional morphology of the RL and the distribution of elements was scanned by EPMA, as shown in Fig. 7. Arounded by the yellow dashed line was the RL, which was clearly divided into the inner RL and outer RL by a microcrack. The RL of Sb-free HRS was consisted of a 200-μm-thick loose outer layer and a 75-μm-thick dense inner layer. Obvious broken areas and crack existed at the metal/rust interface and inner/outer layer. These cracks and pits provided a channel for corrosive media such as HSO3− to corrode the matrix, which was not conducive to the protection of the RL. Although enrichment of P and Cu elements was found in the inner RL, which may have a protective effect on the RL [6], the SO42− catalyzed acid regeneration in solution facilitated microcrack propagation due to the diffusion of S elements to the inner layer [52].
For Sb-free UCS steel and 0.10Sb UCS steel, the structure of RL was similar to that of Sb-free HRS, showing a bilayer structure with better adhesion and fewer defects existed at the metal/rust interface and inner/outer layer. In addition, the overall thickness of the RL decreased significantly and the proportion of internal RL increased significantly. Furthermore, the percentage of internal RL was slightly increased for UCS steel alloyed by 0.10% Sb, implying that the addition of Sb was effective in the generation of internal RL. As seen from the elemental surface mapping of Fig. 7c, a, Sb-rich layer appeared in the inner RL near the substrate. S was enriched in the outer RL and S barely entered the inner RL, thus blocking corrosion channels. It was generally believed that Sb was a favorable element interacting with Cu [53] to produce a RL with good corrosion resistance. Cu and Sb segregating toward the same area in 0.10Sb UCS steel was good evidence for this view. It could be reasonably speculated that the addition of 0.10% Sb participated in the corrosion process and prevented the intrusion of S by generating a Sb-rich layer, thus reducing the accelerated corrosion of the RL, which could also explain the relatively stable weight loss of 0.10Sb UCS steel in the late stage of corrosion in Fig. 6. Interestingly, P-rich layers were also found at the same location concentrated by Sb and Cu in the 0.10Sb UCS steel, suggesting that the formation of layers enriched by P, Cu, and Sb may contain some synergistic effects to improve the corrosion resistance, which will be discussed in the following.
3.4.2 Phase Composition and Distribution
The phase compositions of the RL of the tested steels after dry/wet cyclic tests for 408 h are shown in Fig. 8. The composition of the RL was mainly composed of spinel oxides (Fe3O4) and ferric oxyhydroxides (α-FeOOH and γ-FeOOH). It has been reported that the RL on low-alloy structural steel was composed of a large amount of amorphous substance and a small amount of α-FeOOH, γ-FeOOH, Fe3O4 and/or β-FeOOH in general [28]. The value of α/γ* was used as a protective ability index (PAI) to evaluate the influence of the complex RL composition on corrosion resistance, where α referred to the α-FeOOH content and γ* referred to the total amount of γ-FeOOH and Fe3O4 in the rust layer [54, 55]. The higher the value was, the better the protection performance of the RL had. The α/γ* value of three steels was obtained by semi-quantitative analysis of XRD. Figure 8b shows that the value of PAI increased sharply with 0.10% Sb addition in UCS, which was attributed to the highest proportion of α-FeOOH. Compared to γ-FeOOH and Fe3O4, α-FeOOH was an inactive substance with insulating and acted as the most stable hydroxy iron oxide preventing the invasion of foreign ions [6, 28]. These results also explained that the icorr, CR, n values for 0.10Sb UCS steel in simulated industrial environments significantly decreased with accelerated corrosion.
The cross-sectional phase distribution of the RL on the three steels after dry/wet cyclic acceleration corrosion for 408 h was studied by Raman micro-technique, as shown in Fig. 9. The strongest peak positions of α-FeOOH were at about 387 cm−1, those of γ-FeOOH were at around 251 cm−1 and those of Fe3O4 were at around 636–670 cm−1 [37,38,39,40,41]. For Sb-free HRS steel, the phases of internal RL mainly were γ-FeOOH, Fe3O4 and α-FeOOH (site 4 in Fig. 9a). In contrast, the internal RL of Sb-free UCS steel and 0.01Sb UCS steel consisted of Fe3O4 and α-FeOOH (site 4 in Fig. 9b and c). For the outer RL, Sb-free HRS steel had only γ-FeOOH, while Sb-free UCS steel and 0.01Sb UCS steel has γ-FeOOH and Fe3O4 (site 1 in Fig. 9a, b and c). The middle RL of Sb-free HRS steel showed γ-FeOOH and Fe3O4 (site 2 in Fig. 9a), while the inner RL showed α-FeOOH (site 3 in Fig. 9a). Compared to the Sb-free UCS steel where α-FeOOH appeared in the middle RL (site 2 in Fig. 9b), it indicated that the proportion of α-FeOOH was higher in the Sb-free UCS steel, which was consistent with the XRD results.
Meanwhile, for the 0.10Sb UCS steel, α-FeOOH and Fe3O4 were present in the middle and inner RL (sites 3 and 4 in Fig. 9c). On the contrary, untransformed γ-FeOOH was also present in the inner RL of the Sb-free UCS steel (site 3 in Fig. 9b). The results suggested that Sb can promote the formation of α-FeOOH in the RL, thus improving its corrosion resistance in industrial atmosphere, which was consistent with the XRD results.
In order to reveal the form of Sb elements in RL, detailed XPS spectra of Sb 4d were obtained and analyzed, as shown in Fig. 10. In addition, spectrum information on Cu 2p and P 2p was provided to elucidate the effect of alloying elements on the rust layer. Cu was present in RL as CuFeO2/Cu2O and CuO, which was consistent with the previous studies by Hao et al. [56]. The presence of P in the RL as FePO4 was consistent with the findings of previous researchers [57, 58]. In addition, the spectrum of Sb 4d was weak, indicating that Sb mainly existed in the form of Sb2O3 in an acidic environment. The reason why Sb2O5 was not detected in this experiment was that Sb2O5 was only formed in a highly acidic environment and the acidity of this experimental environment was not high enough [60]. The higher content of CuFeO2/Cu2O, CuO and FePO4 in Sb-free UCS steel than that in Sb-free HRS steel is observed in Fig. 10, indicating that the UCS steel promoted the enrichment of Cu and P in the RL. Meanwhile, Fig. 10c shows that the content of CuFeO2/Cu2O, CuO and FePO4 also increased after the addition of Sb, which was consistent with the results of EPMA. This result illustrated that the enrichment behavior of Cu, P and Sb in the inner rust layer had a synergistic effect.
4 Discussion
4.1 Influence of Process on Corrosion Induction
The above results show that Sb-free UCS steel has a lower corrosion rate compared to Sb-free HRS steel. The influence of the CASTRIP process on corrosion resistance is based on two main points, the specificity of the process parameters and the precise control of non-metallic elements such as C, Mn, S and Si. The reasons for the corrosion resistance enhancement will be explained in following two aspects:
From the result 3.2, the controlled rapid cooling and the casting of silicon deoxidized steel [25] facilitate the formation of fine and uniform sphere-like inclusions. The considerable Si content and lower Mn and S content make the inclusions type mostly oxide, which avoid the type MnS or CaS irregular inclusions. Non-metallic inclusion, especially MnS, acts as an important factor in inducing localized corrosion [61,62,63,64]. Non-metallic inclusions are insulators [62], the structural defects and thermodynamic stability around the non-metallic inclusions have been proved to be the real causes of local corrosion sprouting [62,63,64,65,66,67]. The structural defects may accumulate aggressive ions such HSO3−. CaS and MnS inclusions will preferentially form corrosion pits after chemical dissolution, resulting in an acidic, aggressive environment [63, 64]. From Fig. 3, it can be seen that inclusion of Sb-free HRS steel with micro-gaps around shows a poor integration with the matrix and UCS steel avoids MnS and CaS type inclusions. This results in more serious pitting pits in the RL (Fig. 6a) and faster dissolution of the matrix, which is benefit for a more uniformity and stability RL.
Another reason is the optimization of the microstructure, which has its roots in the integral effect of ultra-fast solidification process and ultra-low carbon. The CASTRIP process itself tends to produce ultra-low carbon steel, which avoids the introduction of cementite in the microstructure. At the same time, subrapid solidification creates conditions for AF and bainite in UCS steels. According to studies, residual cementite strongly contributes to local galvanic corrosion of ferrite-pearlite [68], which is explained by the following equation [69]:
where \(E_{\rm{e,\;c}}\) and \(E_{\rm{e,\;a1}}\) are equilibrium potentials of cathode and anode before electrical contact respectively, \(\beta_{\rm{a1}}\) and \(\beta_{\rm{c}}\) are Tafel slope of anode and cathode reactions respectively, \(I_{a1}\) is the anodic current density of ferrite after electrical–contact and \(I_{\rm{0,\;a1}}\) is the anodic exchange current density, \(A_{1}\) and \(A_{2}\) are the surface area of anode and cathode participating in the reaction respectively, \(I_{0,\;C1}\) and \(I_{0,\;C2}\) are the cathodic exchange current density of ferrite and cementite, respectively.
It is generally considered that the cathodic reduction reaction of depolarizer is very important. The cathodic Tafel slope (\(\beta_{\rm{c}}\)) is usually the same for ferrite and cementite, while the \(I_{0,\;C1}\) and \(I_{0,\;C2}\) are different. Since the electrochemical stability of cementite is higher than that of ferrite [68, 70], ferrite and cementite consist of point couples for the anode and cathode. Therefore, when the solution and the electric couple are determined, \(A_{2} /A_{1}\) is the key parameter to determining \(I_{\rm{a1}}\). As the acceleration corrosion time of Sb-free HRS steel increases, the anodic ferrite dissolved rapidly and the cathodic residual cementite collect rapidly on the steel surface [68]. This phenomenon leads to an increase in \(A_{2} /A_{1}\), which increases the anodic current density of ferrite. In contrast, the significant reduction of C content avoids ferrite-pearlite microstructure in UCS steel. To explain from Eq. (3), the substantial reduction of cementite induces a significant reduction in the number of ferrite-cementite type galvanic cell, resulting in a decrease in \(I_{\rm{a1}}\). On the other hand, the uniform distribution of αB° and AF in the matrix and their similar electrochemical activity makes the anodic and cathodic regions bind tightly to each other [71], reducing the electrochemical corrosion process. One important point to emphasize is that the effect of microstructure mainly happens at the beginning of corrosion process, where the microstructure affects the corrosion behavior of bare steel. However, the microregion galvanic corrosion effect decreases substantially after the steel surface is covered with corrosion products [72]. Similarly, the role of inclusions optimization on local corrosion is also reflected in the early stages of corrosion.
Therefore, at the beginning of corrosion process, the micro-zone corrosion is reduced and the proportion of uniform corrosion is increased by optimization of the microstructure and inclusions. This effect reduces the icorr and Rct of Sb-free UCS steel and the defects in the initial RL. As corrosion time increases, this effect promotes a dense and uniform RL, as shown in Fig. 7. Furthermore, this special RL structure also promotes the enrichment of Cu and P. The formation of layers enriched by Cu and P can effectively increase the resistance of RL to erosion. The role of weathering elements on the RL will be discussed in detail in the next section.
4.2 Influence of Alloying Elements on Stability of the RL
From the above results, it is obvious that Cu, P, and Sb elements all have significant effects on the simulated atmospheric corrosion behavior. It is necessary to discuss their respective roles as well as synergistic effects. The mechanism of the influence of alloying elements in 0.10Sb UCS steel on the initial corrosion electrochemical reaction and RL formation process is shown in Fig. 11. The anodic and cathodic reactions of the steel are as follows:
Anodic reactions:
Cathodic reactions:
As the reaction proceeded, Fe(OH)2 is oxidized in the presence of oxygen to form γ-FeOOH, which reacts as follows:
At the same time, HSO3− is easily oxidized in NaHSO3 solution. With the corrosion process, the steel surface will form a “nest” heaped up by sulfate such as ferrous sulfate, which makes the conductivity of the metal surface film enhanced, resulting in further reactions to enhance the solution acidity and intensify the corrosion process [73]. Reactions are as follows:
Electrochemical tests show that Sb has a significant effect on the cathodic process of steel, and the addition of Sb affects the cathodic process in the following ways [64]:
First, Sb consumes H+ in solution, which prevents the oxygen reduction reaction and inhibits the cathodic reaction. That is one of the reasons why the cathodic current density of 0.10Sb UCS steel is lower than that of Sb-free UCS steel. Then, SbH3 reacts with O2 to form Sb2O3, which is stable in acidic solution and makes RL more uniform, thus improving the corrosion resistance of the steel. At the same time, the reaction consumes oxygen, which effectively inhibits the transformation of Fe(OH)2 to γ-FeOOH (reaction 6) and the formation of ferrous sulfite, resulting in less γ-FeOOH production and thinner RL at the beginning of corrosion (Fig. 9). At the initial stage of corrosion process, Cu has a higher thermodynamic stability than Fe [73]. Its product is usually CuO/Cu2O or CuFeO2, forming a dense oxide film on the metal surface at the beginning of corrosion and has a protective effect on the anodic polarization process as well as in the intrusion of the corrosive medium. Phosphorus can be oxidized to PO43−. PO43− and H+ has a strong complexation. It reduces the cathodic hydrogen precipitation rate and anodic dissolution rate of steel, which can play the role of corrosion inhibitor [74].
Combined with the discussion in the previous three sections, it can be learned that the RL of 0.10Sb UCS steel has fewer defects in the early stage of corrosion due to the weakening of the local corrosion effect. And 0.10Sb UCS steel has a slower formation of γ-FeOOH oxide layer due to the limitation of weathering elements to numerous ionic reactions, and present a thinner initial RL. At the same time, more weathering elements in 0.10Sb UCS can form oxide films on γ-FeOOH to resist the invasion of corrosive ions in solution.
As the corrosion process proceeds, γ-FeOOH may transform to α-FeOOH or Fe3O4 because γ-FeOOH is a semi-conducting and electrochemically active [6, 75]. At this stage, the formation of rust is slow and the above changes from γ-FeOOH to α-FeOOH through amorphous iron hydroxide will be the main reaction [75]. The unstable corrosion product γ-FeOOH will be further transformed into the intermediate phase amorphous oxyhydroxide (FeOx(OH)3−2x). This intermediate phase will be transformed into the stable and protective α-FeOOH in the later stages of corrosion, which is amorphous as well as ultra-fine [66, 75]. The dense amorphous oxide layer located in the Cu-rich and P-rich inner layer is the main source of protection for weathering steels [71]. In this study, the so-called amorphous α-FeOOH is not considered because the peak of amorphous phase is not observed in the XRD pattern [59]. However, from the analysis of the RL above, this conclusion is quite reasonable.
The formation of this alloy-rich oxide layer of α-FeOOH can be explained in two ways. Firstly, Cu, P and Sb can effectively reduce the corrosion current density. Sb can consume H+ and reduce the local acidity, while the generation of CuFeO2/Cu2O, CuO, FePO4 and Sb2O3 will simultaneously act as a protective film to effectively hinder the invasion of corrosion ions. This also explains the greater Rr and Rct in the Nyquist fit parameters (Table 3). The result is that less γ-FeOOH is generated and the rust layer is thinner and denser. This provides a favorable environment for the transformation of γ-FeOOH to α-FeOOH. On the other hand, the transformation of γ-FeOOH to α-FeOOH by the catalytic action of Cu and P concentrated at the metallic surface [6, 76]. This conclusion also applies to Sb. The distribution position of Sb in the inner RL is the same as Cu and P (Fig. 7c). This indicates that CuFeO2/Cu2O, CuO, FePO4 and Sb2O3 promote the transformation of amorphous oxyhydroxide to ultra-fine α-FeOOH. Ultimately, a (Cu, Sb, P)-enriched amorphous α-FeOOH layer is generated in the inner RL, as shown in Fig. 12.
Previous studies have shown that recrystallized Sb or Cu oxides can be produced by the redeposition reaction of Cu2+ or Sb3+ [77]. With the increasing medium acidity, the redeposition process of Sb or Cu oxides was inhibited when only Sb3+ or Cu+ was present, while the redeposition process of Sb or Cu oxides was promoted when both Sb3+ and Cu+ were present [78]. In our study, the simulated industrial atmosphere is an acidic environment, and the microenvironment below the RL is more hostile due to the HSO3− induced acidification effect. Thus, the redeposition process of Sb and Cu oxides can occur, leading to a higher ratio of Cu oxides and the visible Sb oxides (Fig. 7c) in the RL of 0.10Sb UCS. This confirms the synergistic effect between Cu and Sb. The enrichment of P is also more obvious in the presence of Cu and Sb, indicating that Cu, P, and Sb have obvious synergistic effects in the same region of the inner RL. However, it is still unknown that whether the synergistic effect is achieved through the redeposition reaction of Cu2+ or Sb3+ to promote the deposition of the phosphide. Undoubtedly, there are still many studies to be carried out to prove in which way Cu, P and Sb contribute to each other.
All the above mechanisms significantly enhance the corrosion resistance of 0.01Sb UCS steel. There is no doubt that the addition of Sb is a good choice for improving the corrosion resistance of UCS steel. The dense corrosion product not only inhibits the anodic reaction, but also forms a dense oxide layer to enhance the protection of the rust layer.
5 Conclusions
The rust layer formed on the Sb-free HRS steel, Sb-free UCS steel and 0.10Sb UCS steel after exposed in simulated industrial atmosphere for 408 h was investigated by various characterization techniques. Experimental results showed that the corrosion resistance of UCS steel containing 0.1%Sb was doubled compared to that of hot rolling strip (HRS) steel. It can be interpreted as an integral effect of CASTRIP process and alloying elements.
CASTRIP process effectively reduces the localized corrosion effects and icorr of Sb-free UCS steel in initial corrosion processes by optimizing non-metallic element content and process parameters. Sb can consume H+ during corrosion process, which alleviates the local acidity and anodic reaction, thus preventing further dissolution of the steel and slowing down the corrosion rate. Cu, P and Sb can form CuFeO2/Cu2O, CuO, FePO4 and Sb2O3 to promote the transition from γ-FeOOH to α-FeOOH. A (Cu, Sb, P)-enriched inner RL is generated and significantly improves protective ability of the RL. All the above mechanisms significantly enhance the corrosion resistance of 0.01Sb UCS steel.
Change history
28 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40195-023-01607-7
References
M.V. Biezma, J.R. San Cristobal, Corros. Eng. Sci. Technol. 40, 344 (2005)
D. Xu, C. Lou, J. Huang, X. Lu, Z. Xin, C.L. Zhou, Prog. Org. Coat. 134, 126 (2019)
H. Kihira, M. Kimura, Corrosion 67, 095002–095011 (2011)
S. Vaynman, R.S. Guico, M.E. Fine, S.J. Manganello, Metall. Mater. Trans. A 28, 1274 (1997)
V. Krivy, V. Urban, K. Kreislova, Eng. Fail. Anal. 69, 147 (2016)
M. Morcillo, I. Díaz, B. Chico, H. Cano, D. de la Fuente, Corros. Sci. 83, 6 (2014)
Y. Zhou, J. Chen, Y. Xu, Z. Liu, Mater. Sci. Technol. 29, 168 (2013)
X. Cheng, Z. Jin, M. Liu, X. Li, Corros. Sci. 115, 135 (2017)
S. Jiang, F. Chai, H. Su, C.F. Yang, Corros. Sci. 123, 217 (2017)
A. Usami, M. Okushima, S. Sakamoto, S. Nishimura, T. Kusunoki, K. Kojima, Nippon. Steel. Tech. Rep. 90, 25 (2004)
X.X. Wang, Y.M. Gao, K. Li, J.B. Yan, Y.F. Li, J.B. Feng, Corros. Sci. 69, 369 (2013)
V.F.C. Lins, R.B. Soares, E.A. Alvarenga, Corros. Eng. Sci. Technol. 52, 1 (2017)
C. Liu, Y.L. Li, X.Q. Cheng, X.G. Li, Acta Metall. Sin. -Engl. Lett. 35, 1055 (2022)
J.C. Wu, Y. Fang, Y. Yu, Baosteel Technol. 04, 18 (2018)
D.J. Sosinsky, P. Campbell, R. Mahapatra, W. Blejde, Metallurgist 52, 691 (2008)
Y.X. Kelvin, Y. Lan, Z. Chen, M.C. Julie, R.K. Chris, J.B. Frank, G.W. James, Metall. Mater. Trans. A 42, 2199 (2011)
W. Blejde, R. Mahapatra, H. Fukase, Mater. Aust. 3, 32 (2000)
C.R. Killmore, D.G. Edelman, K.R. Carpenter, H.R. Kaul, J.G. Williams, P.C. Campbell, W.N. Blejde, Mater. Sci. Forum 654, 198 (2010)
K. Mukunthan, L. Strezov, R. Mahapatra, W. Blejde, Can. Metall. Quart. 40, 523 (2013)
R. Wechsler, J. Scand, Metals 32, 58 (2003)
N. Zapuskalov, ISLJ Int. 43, 1115 (2003)
S. Ahn, K.J. Park, K. Oh, S. Hwang, B. Park, H. Kwon, M. Shon, Met. Mater. Int. 21, 865 (2015)
W. Wu, Z.Y. Liu, Q.Y. Wang, X.G. Li, Corros. Sci. 170, 108693 (2020)
Y. Yang, C. Jiang, X.Q. Cheng, J.B. Zhao, X.G. Li, J. Mater. Eng. Perform. 29, 2648 (2020)
K.R. Carpenter, D.G. Edelman, P.C. Campbell, C.R. Killmore, H.R. Kaul, J.G. Williams, W.N. Blejde, AISTech-Iron and Steel Technology Conference Proceedings, 751 (2011)
F. Zhu, D. Persson, D. Thierry, C. Taxen. Corros 57, 582 (2001)
X.T. Lian, J.N. Zhu, R.Q. Wan, T.S. Liu, H. Dong, Metals 10, 1174 (2020)
M. Morcillo, I. Díaz, H. Cano, B. Chico, D. de la Fuente, Constr. Build. Mater. 213, 723 (2019)
W. Wu, X.Q. Cheng, J.B. Zhao, X.G. Li, Corros. Sci. 165, 108416 (2020)
W. Wu, W.K. Hao, Z.Y. Liu, X.G. Li, C.W. Du, W.J. Liao, J. Mater. Eng. Perform. 24, 4636 (2015)
ISO 8407: 2009, Corrosion of Metals and Alloys-Removal of Corrosion Products from Corrosion Test Specimens, 2009.
Y.T. Ma, Y. Li, F.H. Wang, Corros. Sci. 52, 1796 (2010)
ASTM G1-2011, Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens, 2011.
W. Wu, Z.Y. Dai, Z.Y. Liu, C. Lou, X.G. Li, Corros. Sci. 183, 109353 (2021)
ASTM G50-2015, Standard Practice for Conducting Atmospheric Corrosion Tests on Metals, 2015.
S. Li, L.H. Hihara, J. Electrochem. Soc. 159, C147 (2012)
J. Monnier, L. Bellot-Gurlet, D. Baron, D. Neff, I. Guillot, P. Dillmann, J. Raman Spectrosc. 42, 773 (2011)
D. Neff, L. Bellot-Gurlet, P. Dillmann, S. Reguer, L. Legrand, J. Raman Spectrosc. 37, 1228 (2006)
D. Faria, S.V. Silva, M.J. Oliveira, J. Raman Spectrosc. 28, 873 (1997)
D. Larroumet, D. Greenfield, R. Akid, J. Yarwood, J. Raman Spectrosc. 38, 1577 (2010)
E. Rocca, H. Faiz, P. Dillmann, D. Neff, F. Mirambet, Electrochim. Acta 316, 219 (2019)
P. Campbell, W. Blejde, R. Mahapatra, R. Wechsler, Metallurgist 48, 507 (2004)
K. Mukunthan, L. Strezov, R. Mahapatra, W. Blejde, Can. Metall. Quart. 40, 523 (2001)
C. Kristin, K. Chris, Metals 5, 1857 (2015)
T. Araki, I. Kozasu, H. Takechi, K. Shibata, M. Enomoto, H. Tamehiro, S. Yamamoto, M. Katumata, H. Okaguchi, K. Amao, Iron. Steel. Inst. Jpn. 1, 1 (1992)
D.G. Edelman, P.C. Campbell, C.R. Killmore, K.R. Carpenter, H.R. Kaul, J.G. Williams, W.N. Blejde, Iron. Steel. Technol. 6, 47 (2009)
T. Huang, X.P. Chen, X.D. Wang, B. Wang, F. Wang, X.Y. Li, J. Mech. Eng. 45, 53 (2017)
R.E. Melchers, Corros. Rev. 38, 515 (2020)
R. Lindsay, Shreir’s Corrosion, ed. by B.Cottis, M. Graham, S. Lyon, T. Richardson, D. Scantlebury, H. Stott (Elsevier BV, The Netherlands, 2010), p. 1693
X.Q. Cheng, Y.W. Tian, X.G. Li, C. Zhou, Mater. Corros. 65, 1033 (2014)
W. Han, C. Pan, Z.Y. Wang, G.C. Yu, Corros. Sci. 88, 89 (2014)
W. Wu, Z.Y. Liu, X.G. Li, C.W. Du, Z.Y. Cui, Mater. Sci. Eng. A 759, 124 (2019)
B.Y. Wu, W. Liang, A.H. Wang Brit, Corros. J. 48, 313 (2013)
T. Kamimura, S. Hara, H. Miyuki, M. Yamashita, H. Uchida, Corros. Sci. 48, 2799 (2006)
T. Kamimura, M. Yamashita, H. Uchida, H. Miyuki, J. Japan. Inst. Metals 65, 922 (2001)
L. Hao, S.X. Zhang, J.H. Dong, W. Ke, Corros. Sci. 54, 244 (2012)
W.M. Liu, J. Liu, H.B. Pan, F.B. Cao, Z.J. Wu, H.H. Lv, Z.Y. Xu, J. Alloy. Compd. 834, 155095 (2020)
F.Y. Mi, X.D. Wang, Z.P. Liu, B. Wang, Y. Peng, D.P. Tao, J. Iron. Steel. Res. Int. 18, 67 (2011)
T. Misawa, T. Kyuno, W. Suëtaka, S. Shimodaira, Corros. Sci. 11, 35 (1971)
D.P. Le, W.S. Ji, J.G. Kim, K.J. Jeong, S.H. Lee, Corros. Sci. 50, 1195 (2008)
C. Liu, R.I. Revilla, D.W. Zhang, Z.Y. Liu, A. Lutz, F. Zhang, T.L. Zhao, H.C. Ma, X.G. Li, H. Terryn, Corros. Sci. 138, 96 (2018)
C. Liu, X. Li, R.I. Revilla, T. Sun, J.B. Zhao, D.W. Zhang, S.F. Yang, Z.Y. Liu, X.Q. Cheng, H. Terryn, X.G. Li, Corros. Sci. 179, 109150 (2021)
C. Liu, R.I. Revilla, X. Li, Z.H. Jiang, S.F. Yang, Z.Y. Cui, D.W. Zhang, H. Terryn, X.G. Li, Corros. Sci. 124, 141 (2022)
T.Y. Zhang, Y.L. Li, X. Li, C. Liu, S.F. Yang, Z.G. Yang, X.G. Li, Corros. Sci. 208, 110708 (2022)
L.W. Wang, J.C. Xin, L.J. Cheng, K. Zhao, B.Z. Sun, J.R. Li, X. Wang, Z.Y. Cui, Corros. Sci. 147, 108 (2019)
I.I. Reformatskaya, I.G. Rodionova, Y.A. Beilin, L.A. Niselson, A.N. Podobaev, Prot. Met. 405, 447 (2004)
G.X. Li, L.W. Wang, H.L. Wu, C. Liu, X. Wang, Z.Y. Cui, Corros. Sci. 174, 108815 (2020)
X.H. Hao, J.H. Dong, I.N. Etim, J. Wei, W. Ke, Corros. Sci. 110, 296 (2016)
C.N. Cao, Principles of Electrochemistry of Corrosion, ed. by Z.B. Duan, F.Y. Sun (Chemical Industry Press, Beijing, 2008.), p. 81
D. Clover, B. Kinsella, B. Pejcic, R.D. Macro, J. Appl. Elelctrochem. 35, 139 (2005)
Y.B. Guo, C. Li, Y.C. Liu, L.M. Yu, Z.Q. Ma, C.X. Liu, H.J. Li, Int. J. Min. Met. Mater. 22, 604 (2015)
S.P. Qu, X.L. Pang, Y.B. Wang, K.W. Gao, Corros. Sci. 75, 67 (2013)
W. Ke, J.H. Dong, Acta Metall. Sin. 46, 1365 (2010)
T. Misawa, K. Asami, K. Hashimoto, S. Shimodaira, Corros. Sci. 14, 279 (1974)
M. Yamashita, H. Miyuki, Y. Matsuda, H. Nagano, Corros. Sci. 36, 283 (1994)
T. Misawa, Corros. Sci. 13, 659 (1973)
J.H. Hong, S.H. Lee, J.G. Kim, J.B. Yoon, Corros. Sci. 54, 174 (2012)
H. Lin, X. Wu, Metall. Mater. Trans. B 27, 157 (1996)
Acknowledgements
This work was financially supported by the Jiangsu Shagang Iron and Steel Research Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The original online version of this article was revised: In the original publication, the references citation number in the text appears incorrect.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Lian, X., Xi, Z. et al. A Study of Rust Layer of Ultra-Thin Cast Strip Steel Containing 0.10% Sb in Simulated Industrial Atmosphere. Acta Metall. Sin. (Engl. Lett.) 36, 1371–1384 (2023). https://doi.org/10.1007/s40195-023-01542-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-023-01542-7