Abstract
In order to improve the oxidation and wear resistance of blades tip of titanium alloys as well as the sealing performance of the gas turbine engine, a Ni/cBN abrasive coating was prepared on titanium alloys through composite electroplating. Oxidation, mechanical and tribological properties of the abrasive coating were investigated. Furthermore, the effect of the oxidation on the mechanical and tribological properties was also evaluated. Oxidation results revealed that the abrasive coating underwent slight oxidation within 700 °C. Meanwhile, some intermetallic compounds, Ni3Ti, NiTi and NiTi2, were formed at the coating/substrate interface during oxidation. Due to the pinning effect of cBN particles and the different thermal expansion coefficients of the coating and substrate, the coating/substrate interface was cracked after oxidation at 700 °C. Tensile results showed that the presence of coating reduced the strength of the alloy significantly at room temperature, while only marginal variations of the strength of the coated and uncoated specimens at elevated temperatures. Besides, the wear tests indicated that the coating had the excellent cutting ability and wear resistance, which can effectively protect the blades tip of titanium alloys. As the temperature increased, the wear resistance decreased due to the decrease in the mechanical properties of the Ni/cBN coating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to the low density and high specific strength, titanium alloys have been widely used in the manufacture of aero-engines, such as the blades and the casings of the compressors [1]. Furthermore, with the increase of the thrust-to-weight ratio of aero-engines, the proportion of titanium alloys in the compressors would further increase, which undoubtedly increases the potential risks.
During service, the blades tip of titanium alloys rubbed vigorously with the abradable coatings, and the linear velocity of blades tip is extremely high [2, 3]. Due to the poor wear resistance, it is easy to cause excessive wear of the blades, which further results in the decrease in the thrust and the fuel efficiency of the aero-engines [4, 5]. Additionally, a high affinity of titanium alloys for the oxygen limited their use at elevated temperatures. This could cause the formation of the hard and brittle oxygen diffusion zone (ODZ) in the underlying substrate, which would result in the decrease in fatigue resistance and tensile ductility [6, 7]. Thus, the formation of ODZ is critical to the service life of titanium alloys [6]. Furthermore, due to poor oxidation resistance and thermal conductivity, titanium alloys are highly prone to cause “titanium fire” accidents during the severe friction [8, 9]. Hence, the wear and oxidation seriously threaten the performance of blades of titanium alloys. Currently, the preparation of the abrasive coatings on the blades tip of titanium alloys is one of the most effective solutions for solving such problems [10, 11].
The service environment of the blades tip of titanium alloys is more awkward than that of blades body, where oxidation, wear and erosion might occur during the service [2]. Thus, the comprehensive performance requirements for the materials of the abrasive coatings are more stringent. Generally, the abrasive coatings manufactured at the tips need to satisfy two basic requirements: the resistance to in-service conditions under the service temperatures of aero-engines and the ability to rub the abradable coatings [12].
Some types of coatings could be applied to the titanium alloys to play an oxidation-resistant or wear-resistant role, such as MCrAlY, Ti–Al–X, thermal barrier coating (TBC) system and the composite electroplating coatings [11, 13]. However, due to their poor wear resistance, high adhesion to abradable coating and severe interdiffusion [13, 14], these coatings, such as MCrAlY, Ti–Al–X and thermal barrier coating (TBC) system, were not suitable for preparation on the blades tip. Therein, the structural design of the composite coatings that consisted of alloy matrix and ceramic particles not only could ensure sufficient cutting ability but also can reduce the adhesion of the transfer materials of abradable coatings [14]. Besides, some methods could be used to prepare these coatings, such as air plasma spraying, arc ion plating and electroplating [11, 13,14,15]. Among them, the electroplating technology is of particular significance as a technique for producing various composite coatings with ambient temperature, normal pressure, high deposition rate and low cost [16]. Therefore, it has been widely used in the coating preparation of substrates such as stainless steel, superalloys and titanium alloys [11, 17,18,19].
Researches have been carried out to investigate the effect of the abrasive coatings on titanium alloys, and various mechanisms were proposed. Watson and Liu et al. [11, 14] investigated the rubbing performance of blades tip coated with Ni/cBN coating. It was found that abrasive coatings could inhibit the materials transfer and reduce the densification of the abradable coating. The cBN and Ni matrix in the Ni/cBN electroplated coating were mechanical bonding, and thus one of the failure mechanisms of the coating is that the cBN particles were pulled out or fractured. Moreover, the bridging effect of the transfer layers between cBN particles also accelerated the failure of the coating. Fu et al. [20] prepared the cBN/Ti6Al4V composite coating and the NicBN/Ti6Al4V composite coating by the laser cladding technology. They found that the two composite coatings demonstrated much better wear resistance than the Ti6Al4V substrate. Besides, an interfacial reaction layer, namely TiN, formed between cBN and the Ti6Al4V matrix, which improved the interfacial bonding strength and effectively reduced the pulling-off of cBN particles. The high temperature generated by laser cladding inevitably led to the thermal cracking and decomposition of cBN particles, while the Ni plating on the surface of the cBN particles could decrease the thermal defects and increase the wear resistance of the composite coating. Nevertheless, due to the lack of research on this kind of coatings, the oxidation, mechanical and elevated temperature tribological performance of the abrasive coating used on titanium alloys is still unclear, especially the effect of the oxidation on the mechanical and the elevated temperature tribological properties.
Under the operating conditions, the failure process of the abrasive coatings is very complicated because it can be simultaneously affected by many factors. Therefore, it is necessary to make a comprehensive evaluation of the performance of the abrasive coatings. In this study, the Ni/cBN abrasive coating was prepared on titanium alloys by the composite electroplating, in which cBN particles were used as the strengthening phase to improve the wear resistance and cutting ability, and the nickel as the continuous phase to play a supporting role as skeleton materials. Nickel has a high melting point of 1480 °C and has superior high-temperature resistance up to 500 °C. Besides, cBN has a good chemical inertness and significant thermal stability, which makes it an ideal incorporated particle in the nickel matrix for high-temperature applications [21, 22]. Hence, it can be concluded that the Ni/cBN coating should have a superior oxidation resistance at elevated temperatures. This work aims to investigate the effect of the presence Ni/cBN abrasive coatings on the oxidation, mechanical performance and tribological properties of titanium alloys. The effect of the oxidation on the mechanical and the elevated temperature tribological properties was also evaluated. Moreover, the mechanisms were discussed in detail.
2 Experimental
2.1 Preparation of the Abrasive Coating
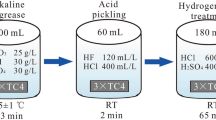
TC4 (Ti–6Al–4V) alloy was selected as the substrate material, which was cut into samples by a spark-discharging machine. The Ni/cBN coatings were fabricated on the substrate by composite electroplating as follows. First, the substrate was ground to 800-mesh SiC paper, blasted with 200-mesh glass balls and then ultrasonically cleaned in acetone and ethanol for 10 min, respectively. Afterward, the substrate was activated for 2 min, and the components of the activation solution were HF (4 mL), HCl (5 mL) and H2O (91 mL). Whereafter, a Ni active layer (2–3 μm) was deposited on the surface of the substrate from a Watt’s bath by electroplating. Subsequently, the cBN abrasive particles (90–100 μm) were anchored with a Ni layer. The thickness of the Ni matrix was controlled at about 70–80 μm by controlling the plating time. Therein, according to Refs. [11, 14, 23], the size of cBN particles was selected to be 90–110 μm and the distance between the particles was not more than twice the diameter of the particles. Moreover, the composition of Watt’s bath and the electrodeposition conditions can be found in Refs. [12, 14].
2.2 High-Temperature Oxidation Tests
Anisothermal oxidation test of the Ni/cBN coating was carried out by the ultra-high-temperature comprehensive thermal analyzer (STA449F3, German). Prior to the test, the Ni/cBN coating materials were cleaned in acetone and ethanol to remove surface contaminations and then dried. During the test, the coating materials were heated from the room temperature up to 750 °C at a rate of 10 °C/min and then immediately cooled to the room temperature at the same rate. Meanwhile, the airflow rate was set to 30 ml/min.
Isothermal oxidation tests were performed in static air at three different temperatures, i.e. 500 °C, 600 °C and 700 °C using a muffle furnace. The coated and uncoated specimens were placed in alumina crucibles inclinedly to ensure that each surface of the specimens was contacted fully with oxygen and oxidized at the test temperature. The oxidation kinetics curves were measured by discontinuous weighing method. Mass changes of the specimens were measured by an electronic balance with a precision of 0.01 mg. During the oxidation tests, at least three parallel specimens were adopted to obtain the average weight change. Prior to the experiments, the alumina crucibles were calcined at 1200 °C until their weight remained unchanged.
2.3 Tensile Tests
Tensile testing of the coated and uncoated specimens was carried out at three different temperatures, i.e. room temperature (RT), 500 and 600 °C, using a 50kN MTS E45.105 tensile testing machine. For the elevated temperature tests, to ensure temperature uniformity, the specimens were soaked at the desired test temperature for 10 min before starting the tensile loading. To ensure consistency in the results, at least three samples of each type were tested under the given conditions. The nominal crosshead speed was maintained at 1 mm min−1 for the tests. The schematic and morphologies of the tensile specimens are shown in Fig. 1.
2.4 Tribological Tests
Wear performance of TC4 and the abrasive coatings was determined by a high-temperature fretting tribometer (MFT-5000, Retc, USA). The tests were carried out at RT and 600 °C. The alumina balls were selected for the counter bodies with a diameter of 9.525 mm. The linear displacement, frequency and the normal load of all the tests were kept constant at 10 mm, 5 Hz and 20 N, respectively. The test duration was set at 30 min for each test. During the tests, the shear force (Fx) and normal load (Fz) were monitored and acquired by the data display and acquisition system continuously. The mass change of the specimens before and after tests was measured by an electronic balance with a precision of 0.01 mg. To ensure the accuracy of the test results, each test was repeated three times in the same experimental environment.
2.5 Characterization Techniques
Surface and cross-sectional morphologies of the specimens were investigated by a field-emission electron microscope (FE-SEM, Quanta, 200 F) equipped with an energy dispersive X-ray spectrometer (EDS). X-ray diffraction (XRD) was applied to determine the phases of coatings and oxides. The three-dimensional morphologies of the wear tracks of TC4 and Ni/cBN coatings were observed by a white-light interferometer (KLA-Tencor Corporation, America). The hardness of TC4 and the Ni/cBN coating was tested on a surface Rockwell hardness tester (WRHS-60D Beijing Science and Technology Co., Ltd.), and the selected scale was HR 15 N.
3 Results and Discussion
3.1 Microstructure of the As-deposited Coatings
Figure 2 presents the surface and cross-sectional morphologies of the as-deposited Ni/cBN coating. As shown in Fig. 2a, the electrodeposited cBN particles were uniformly anchored in the coating, and the surface of the Ni matrix was relatively smooth with no pores. As can be seen in Fig. 2b, cBN particles had a sharp and regular shape, which endowed them excellent cutting ability. It was found that the average 20–40% of each particle was exposed to the coating surface, while the remaining particle was embedded in the coating. This structure can ensure that the Ni matrix has enough holding force to cBN particles without reducing the cutting performance of the coating [14]. Besides, the hardness of TC4 and the Ni/cBN coating used in this study was about 54 and 91 h 15 N, respectively.
3.2 Oxidation Behaviors of TC4 and the Abrasive Coating
3.2.1 Oxidation Kinetic Curves and Oxidation Rate Constant
Figure 3 presents the anisothermal oxidation kinetic curve of the Ni/cBN coating in the air. The result revealed that the mass gain became significant from about 500 °C. From the preliminary result, isothermal oxidation of the Ni/cBN coating in the air at 500 °C, 600 °C and 700 °C was conducted.
Oxidation kinetic curves and corresponding oxidation rate constant (kp) of TC4 and the abrasive coating at different temperatures are shown in Fig. 4. In the initial stage (0–20 h), TC4 specimens experienced rapid oxidation due to their high affinity for oxygen (Fig. 4a) [24]. Then, the curve shifted to a steady stage maintaining a small mass gain (20–100 h), which was attributed to the formation of the oxide scale on the surface of TC4 specimens. However, the rate of mass gain of the Ni/cBN coating was larger than that of TC4 throughout the whole oxidation process, and the kp value of the coating was an order of magnitude higher than that of TC4 (Fig. 4d). Nevertheless, the difference between the two types of specimens decreased with increasing temperature. At 600 °C, the curves of the mass gain and the kp values of the coated and uncoated specimens were nearly coincident (Fig. 4b and e). At 700 °C, TC4 specimens showed a rapid oxidation rate during the whole oxidation process (Fig. 4c), and the kp values were an order of magnitude larger than that of Ni/cBN coating (Fig. 4f). The Ni/cBN coating experienced slower oxidation during the whole oxidation process, resulting in a lower mass gain than that of TC4 specimens eventually.
3.2.2 XRD Results
Figure 5 shows the XRD patterns of TC4 and Ni/cBN coating before and after oxidation. As shown in Fig. 5a, X-ray phase analysis revealed the presence of Al2O3 and TiO2 in the oxide scales of TC4 after oxidation. No V-rich phase could be detected, which could be due to the selective oxidation of elements [25]. Additionally, the diffraction peaks of Ti were detected, which was due to the penetration of X-ray beyond the oxide scale through the substrate [26]. Furthermore, the Ti peaks were shifted slightly left after oxidation, which might be caused by the increase in the lattice parameters due to the dissolution of oxygen in the oxygen diffusion zone. As can be seen in Fig. 5b, only an oxidation product, NiO, was identified on the surface of the Ni/cBN coating after oxidation. Furthermore, the relative intensity of NiO peaks gradually increased with increasing temperature. Additionally, no B2O3 phase was detected. This mainly because cBN could remain chemically inert to other elements due to its superior thermal stability, and thus it can strongly present in the XRD results after oxidation [27].
3.2.3 Effect of Oxide Scale on the Oxidation Process
For both the TC4 and the Ni/cBN coating, the oxidation rates were faster in the initial stage of oxidation, but with the extension of time, the oxidation curves were relatively flat, that is, the metal oxidation conformed to the parabolic law [24]. This finding revealed that the oxidation reactions were mainly controlled by the diffusion of metal ions and oxygen in the oxide scales [28].
Figure 6a–c shows the cross-sectional morphologies of TC4 after oxidation at different temperatures. The thickness of the oxide scale increased with increasing temperature. However, the thickness of the oxide scale at 700 °C was about 5 times that at the other two temperatures, indicating that TC4 was severely oxidized at 700 °C, which was consistent with the results in Fig. 4c and f. EDS demonstrated that the elemental composition of the oxide scale in Fig. 6c was 40.89 O–7.27 Al–51.84 Ti (wt%), suggesting the formation of Al2O3 and TiO2. Moreover, the oxide scale presented a layered structure, and several interlaminar cracks were observed (Fig. 6c) [29]. However, once the thickness of the oxide scale reached a critical value, the stress caused by the thermal stress and the growth stress would cause it to crack, which would accelerate the oxidation of the substrate. On the other hand, according to Fig. 4, the oxidation rate constant (kp) increased sharply with increasing temperature. Moreover, the rapid oxidation of TC4 could cause the formation of a thicker but defective oxide scale, which further led to the easy penetration of the oxygen into the underlying substrate [24]. Therefore, the protective effect of the oxide scale decreased with the increase in the temperature, which led to a sharp increase in the degree of oxidation of TC4.
The cross-sectional morphologies of Ni/cBN coating after oxidation at different temperatures are presented in Fig. 6d–f. Compared with TC4, the thickness of the oxide scale of the Ni/cBN coating at 700 °C changed little, which indicated that the oxidation degree of the coating did not increase sharply. Besides, the crack in Fig. 6f might be caused by polishing. During the oxidation, the formation of the NiO layer was due to the combination of the outward diffusion of nickel and the inward diffusion of oxygen. Moreover, according to the Ellingham diagram, the formation of the NiO was expected at the range of 500 to 700 °C at the oxidation environment [22]. Research on the oxidation of the pure nickel presented that the outward diffusion of cations by the short-circuit diffusion or the grain-boundary diffusion was the main mechanism that controlling the growth of the NiO scale during the elevated temperature oxidation [30, 31]. Hence, the upward migration of nickel from the coating to the surface of the oxide scale was the main mass transport process for the oxidation reaction, and the inward diffusion of oxygen was several orders of magnitude smaller than the outward diffusion of nickel to the surface [22]. The NiO layer formed on the surface of the Ni/cBN coating can effectively reduce the diffusion rate of oxygen and nickel. Therefore, with the growth of the NiO layer, the Ni/cBN coating shifted to a steady stage and maintained a small mass gain (Fig. 4).
3.2.4 Effect of Oxidation on the Interdiffusion Between the Coating and the Substrate
Figure 7 shows the cross-sectional morphologies of the coated specimens after oxidation at different temperatures. As shown in Fig. 7a, d and g, the coatings experienced a weaker degree of oxidation at 500 °C–700 °C, which indicated the coating could prevent the substrate from contacting with oxygen. As can be seen from the higher magnification image (Fig. 7b, e and h), obvious lamellar structures were observed at the coating/substrate interfaces, suggesting that the elemental interdiffusion occurred between the coating and substrate during oxidation, and the new intermetallic compounds were generated [32]. Furthermore, the chemical compositions of these intermetallics are listed in Table 1. EDS analysis indicated that Ni3Ti and NiTi were formed by the solid solution of Ti in Ni during oxidation at 500 °C and 600 °C (Fig. 7c and f). NiTi2 was also formed at 700 °C in addition to Ni3Ti and NiTi (Fig. 6i). It can be inferred that the NiTi layer grew by consuming the NiTi2 and Ni3Ti layer, namely 0.2 Ni3Ti + 0.4 NiTi2 = NiTi, so the consumption of NiTi2 is twice more than that of Ni3Ti for forming NiTi phase [32]. Thus, the thickness of NiTi2 and Ni3Ti layer was controlled by both their growth and their consumption of NiTi growing, which might be the reason why NiTi2 was not found after oxidation at 500 °C and 600 °C. As shown in Fig. 7g–h, a transverse crack was observed at the interface between the Ni layer and Ni3Ti layer after oxidation at 700 °C. This might be due to two reasons. On the one hand, the thermal expansion coefficient of these compounds, such as Ni3Ti and NiTi, was different from that of the Ni/cBN layer [16]. On the other hand, due to the pinning effect of cBN particles, it is difficult for the coating to release stress during oxidation, so the stress gradually increased with increasing temperature or oxidation time until the coating cracked [12].
During oxidation, due to the migration of metal atoms, a diffusion layer mainly composed of solid solution and intermetallic compounds, such as Ni3Ti, NiTi and NiTi2, was formed at the coating/substrate interface [33, 34]. In general, as the temperature increased, the metal migration rate increased, and then the diffusion layer became thicker. However, the thickness of the diffusion layer not only depended on the diffusion coefficient of each element but also depended on whether there are gaps at the coating/substrate interface. Since the coating and the substrate were mechanically combined, the interface bonding is not tight enough, which was not conducive to the formation of metal bonds of the metal atoms between the coating and the substrate. However, several different changes of the micro-gaps would occur during the oxidation process. When the temperature was low, the effect of thermal expansion was not obvious, the gap would not further expand, and the interdiffusion between the coating and substrate occurred which induced the increase in the bonding strength (Fig. 7b and e) [35, 36]. However, when the temperature was high, the gaps would increase due to the different thermal expansion coefficients and internal stress. At this point, the coating adhesion not only did not improve, and even deteriorated (Fig. 7h) [36].
3.3 Tensile Properties of the Coated and Uncoated Specimens
3.3.1 Tensile Properties
Figure 8 shows the tensile performance of the coated and uncoated specimens at different temperatures. As shown in Fig. 8a–d, whether at room temperature or elevated temperatures, the mechanical properties of the specimens changed to different degrees when the coating was prepared on them, but the variation amplitudes were all within 10%. The specified disproportionate elongation strength and the fracture elongation of the coated specimens were slightly reduced compared to the uncoated specimens (Fig. 8a and c). The average tensile strength of the coated and uncoated specimens at room temperature was about 1064 MPa and 958 MPa, respectively, with a variation range of about 10%. However, the average tensile strength of the two types of specimens at elevated temperatures was basically the same (Fig. 8b). It is worth noting that the coating was spalled from the substrate when tested at room temperature, which was the typical result from many of the results, and thus the corresponding reduction of the cross-section area was not given (Fig. 8d). At elevated temperatures, the reduction of the cross-section area of the coated specimens was slightly higher than that of the uncoated specimens, while the variation amplitude decreased with increasing temperature (Fig. 8d). According to Fig. 8e, the strength values (ultimate tensile strength and 0.2% yield strength) of the two types of specimens decreased as the temperature increased. The strength values of the coated specimens were lower than those of the uncoated specimens at room temperature, while they were basically the same at elevated temperatures.
3.3.2 Fracture Behaviors
Figure 9 presents the fracture morphologies of the specimens after tensile tests. It is quite evident that the microscopical fractures of all specimens showed the obvious necking, especially at elevated temperatures. Besides, obvious crack propagation zone and shear lip zone were observed in all fractures, which were typical characteristics of ductile fracture [37]. Therefore, it can be inferred that all of the fracture cracks nucleated inside the specimens [38], which indicated that the presence of the Ni/cBN coating on the TC4 alloy did not change the fracture behavior and fracture mechanism. It can be seen in Fig. 9d–f and Fig. 9j–l that a large number of dimples and a small amount of micro-voids are distributed on the surface of the fractures [37]. Moreover, the dimples tended to increase in size and number as the temperature increased, which indicated the fracture toughness increased with increasing temperature [37,38,39,40]. As shown in Fig. 9b, g, and i, when tested at elevate temperatures, the Ni/cBN coating did not spall from the substrate as it did at room temperature. It can be seen from the higher-magnification image inserted in Fig. 9g and i that the cracks appeared at the coating/substrate interface after the test at elevated temperatures, and the gap increased with increasing temperature [38].
3.3.3 Effect of Temperature on the Damage Mechanisms of the Coated Specimens
To summarize, it can be seen that the as-deposited coating and the substrate presented mechanical bonding with a clear boundary and no elemental interdiffusion (Fig. 2b). Furthermore, the pinning effect of cBN particles hindered the deformation of the coating [12], thus resulting in the deformation of the Ni/cBN coating being less than that of the TC4 alloy during tensile tests. Hence, the coating/substrate interface was the weakest part of the coating-substrate system. Under the continuous tensile load, the cracks nucleated and propagated here, further leading to the fracture of the coating. At elevated temperatures, the elemental interdiffusion occurred near the interface, and the interface reaction products were generated, thus increasing the bonding strength between the coating and the substrate. Moreover, the ductility of the Ni matrix was enhanced at elevated temperatures, and the pinning effect of the cBN particles was relatively weak at this time, so the coating can be deformed simultaneously with the TC4 alloy. Therefore, compared with the uncoated specimens, the elevated temperature tensile properties of the coated specimens did not change significantly.
3.4 Tribological Behavior of TC4 and the Abrasive Coating
3.4.1 Curves of Friction Coefficient
Typical curves of friction coefficient for TC4 and the abrasive coating at room temperature are presented in Fig. 10a. According to the curves, the friction coefficient of the Ni/cBN coating was lower than that of TC4. The friction coefficient of the TC4 maintained a steady and slow increase after the running-in stage (after 5 min). As for the Ni/cBN coating, the friction coefficient significantly fluctuated between 0.38 and 0.52 at the steady stage (after 4 min). Figure 10b shows the curves of the friction coefficient for TC4 and Ni/cBN coating at 600 °C. It can be seen that the friction coefficient of both was increased, especially that of Ni/cBN coating. Besides, contrary to the results at room temperature, the friction coefficient of Ni/cBN coating was higher than that of TC4 during the whole test process. With the increase in the temperature, the mechanical properties of alumina balls decreased [41]. This increased the incursion depth and the contact area of the Ni/cBN coating at elevated temperatures, further increasing the tangential force of the coating.
3.4.2 Mass Changes
Figure 11 shows the weight variation of the specimens and corresponding Al2O3 balls after wear tests at room temperature and 600 °C, respectively. All the values were obtained by using the original values minus the values measured after the tests. Thus, the positive values indicated weight loss, while negative values indicated weight gain. Whether at room temperature or 600 °C, the weight loss of TC4 was much larger than that of Ni/cBN coating, while that of the corresponding Al2O3 balls exhibited an opposite tendency, which indicated that Ni/cBN coating had superior wear resistance and cutting performance. In addition, compared with the results at the room temperature, the weight loss of TC4 and Ni/cBN coating increased by about 0.5 times and 20 times at 600 °C, which was associated with the oxidation and mechanical properties of the coating at elevated temperatures. Moreover, it is worth noting that the mass of Al2O3 balls increased when they wear with TC4 at 600 °C (Fig. 11b), which indicated that the materials of the TC4 transferred to the balls due to the increasing viscosity with increasing temperature.
3.4.3 Worn Surfaces
Figure 12 displays the surface morphologies and cross-sectional profiles for the wear tracks of TC4 and the abrasive coating at room temperature. For the TC4, a large number of abrasive grooves and a small amount of plastic deformation are found in Fig. 12a, which were the typical characteristics of adhesive wear and abrasive wear [17, 42]. Meanwhile, the wear scars were deep and presented wedge-shaped (Fig. 12c and e), which is because the presence of adhesive wear and abrasive wear triggered simultaneous damage mechanisms, further accelerating the wear process. As for the Ni/cBN coating, the protruding cBN particles acted as the main friction unit during the tests. Meanwhile, due to the extremely high hardness and excellent cutting ability of the abrasive coating, the corresponding counterpart was quickly removed, namely avoiding the direct contact between the Ni layer and the Al2O3 balls, which reduced the contact area of the tribopairs and thus protected the Ni matrix. Hence, the worn surface of the Ni/cBN coating was flat and only the protruding asperities of the Ni matrix were removed (Fig. 12b and d). Meanwhile, as can be seen from the higher magnification image inserted in Fig. 12b and d, no cBN was pulled out.
The surface morphologies and cross-sectional profiles for the wear tracks of TC4 and the abrasive coating at 600 °C are presented in Fig. 13. Compared with Fig. 12a, the worn morphology in Fig. 13a shows a large number of plastic deformation zones and a small number of abrasive grooves. This finding suggested that the wear mechanism was dominated by adhesive wear and supplemented by abrasive wear at elevated temperatures. The damage of adhesive wear is more than that of abrasive wear, and thus the weight loss of TC4 worn at elevated temperature was larger than that at room temperature [17, 43]. However, the metal material softened as the temperature increased, which caused the wear debris of TC4 to accumulate more easily in the contact zone. Then, the shear stress pushed these materials out of the wear track, causing these materials to accumulate at the edge of the track (Fig. 13c and e). Meanwhile, the soften debris was played a role in lubrication [44]. On the other hand, the oxides formed on the contact zone at elevated temperatures can also act as solid lubrication [45, 46]. Hence, these were the reasons why the variation amplitude of the weight loss of TC4 was smaller than that of Ni/cBN coating and the friction coefficient of TC4 was smaller than that of the abrasive coating at 600 °C.
Compared with Fig. 12f, the undulation of the track profile in Fig. 13f is smaller and exhibits an obvious groove, which might be associated with the decrease in the mechanical properties of Ni/cBN coating with increasing temperature [47]. Besides, the fractured cBN particles can be observed clearly in the higher magnification image inserted in Fig. 13b. On the other hand, the cracks can generate and propagate in the oxide scales on the surface of the Ni/cBN coating to result in the final spallation during the wear process. Therefore, these reasons caused the weight loss of the coating at 600 °C was much greater than that at room temperature. Moreover, the wear track width of the Ni/cBN coating was larger than that of the TC4 both at room temperature and 600 °C, which is because the wear track width should be governed mostly by the wear of the counterpart [41].
As mentioned above, the Ni/cBN abrasive coating has the excellent cutting ability and wear resistance, and thus it can reduce the wear of the blade tip, thereby prolonging the service life of the blade and avoiding the reduction of engine efficiency, which was consistent with the results of Ref. [20]. Moreover, due to the low thermal conductivity (about 10 W m−1 K−1) and specific heat capacity (503 J kg−1 K−1) of the titanium alloys [14, 48], “titanium fire” easily occurs, while the nickel in the abrasive coating has good thermal conductivity (about 70 W m−1 K−1) [49], which can effectively avoid the accumulation of friction heat, thus reducing the occurrence of “titanium fire” accidents.
4 Conclusions
A Ni/cBN abrasive coating was successfully prepared on titanium alloys through electroplating, and then subjected to oxidation, mechanical and wear tests. According to the experimental results, the conclusions can be drawn as follows:
-
1.
The abrasive coating underwent slight oxidation within 700 °C, and some intermetallic compounds, such as Ni3Ti, NiTi and NiTi2, were formed at the coating/substrate interface during oxidation, while the coating/substrate interface cracked after oxidation at 700 °C due to the pinning effect of cBN particles and the different thermal expansion coefficients of the coating and substrate.
-
2.
The tensile properties of the coated and uncoated specimens decreased dramatically with increasing temperature. Therein, the effect of coating on the mechanical properties of the TC4 substrate was the greatest at room temperature, while the mechanical properties of the two samples were almost the same at high temperatures.
-
3.
Whether at room temperature or elevated temperatures, the abrasive coating had excellent cutting performance, and its wear rate was much lower than that of TC4. However, as the temperature increased, the wear resistance of the Ni/cBN coating decreased due to the decrease in the mechanical properties.
References
R.R. Boyer, Mater. Sci. Eng. A 213, 103 (1996)
S. Rani, A.K. Agrawal, V. Rastogi, Case Stud. Eng. Fail. Anal. 8, 1 (2017)
N. Fois, J. Stringer, Wear 304, 202 (2013)
K. Laul, S. Rahemanji, Adv. Mater. Res. 170, 54 (2012)
R. Rajendran, Eng. Fail. Anal. 26, 355 (2012)
I. Gurrappa, J. Alloys Compd. 389, 190 (2005)
M. Berthaud, I. Popa, R. Chassagnon, O. Heintz, J. Lavková, S. Chevalier, Corros. Sci. 164, 108049 (2020)
D.G. Teer, F.B. Salem, Thin Solid Films 45, 583 (1977)
R. David, D. Mark, B. Justin, Mater. High Temp. 33, 1 (2016)
J.C. Williams, E.A. Starke, Acta Mater. 51, 5775 (2003)
M. Watson, N. Fois, Wear 338–339, 268 (2015)
Y.D. Liu, J. Sun, Z.L. Pei, W. Li, J.H. Liu, J. Gong, C. Sun, Corros. Sci. 167, 108486 (2020)
D. Kim, D. Seo, X. Huang, T. Sawatzky, H. Saari, J. Hong, Y.W. Kim, Int. Mater. Rev. 59, 297 (2014)
Y.D. Liu, J.P. Zhang, Z.L. Pei, J.H. Liu, W.H. Li, J. Gong, C. Sun, Wear 456–457, 203389 (2020)
B. Li, J.H. Jia, Y.M. Gao, H.J. Guo, M.M. Han, W.Z. Wang, Acta Metall. Sin. (Engl. Lett.) 30, 801 (2017)
C.F. Sun, Y.B. Wang, Q. Su, Z.G. Guo, L. Shi, Adv. Mater. Sci. Eng. 2016, 1 (2016)
J.H. Liu, Y.D. Liu, Z.L. Pei, W.H. Li, W.B. Shi, J. Gong, C. Sun, Wear 452–453, 203300 (2020)
Z.X. Ping, G.A. Cheng, Y.D. He, Acta Metall. Sin. (Engl. Lett.) 23, 137 (2010)
W. Wang, S.Q. Qian, X.Y. Zhou, J. Mater. Sci. 45, 1617 (2010)
S.R. Fu, L.J. Yang, P. Wang, S.P. Wang, Z.X. Li, Coatings 10, 702 (2020)
R. Tyagi, D.S. Xiong, J. Li, J. Dai, Wear 269, 884 (2010)
M.A. Farrokhzad, Surf. Coat. Technol. 309, 390 (2017)
J.R. Davenport, L. Mendez-Garcia, S. Purkayastha, M.E. Hancock, R.J. Stearn, W.J. Clegg, Mater. Sci. Technol. 30, 1877 (2014)
H. Guleryuz, H. Cimenoglu, J. Alloys. Compd. 472, 241 (2009)
R. Siab, G. Bonnet, J.M. Brossard, J. Balmain, J.F. Dinhut, Appl. Surf. Sci. 253, 3425 (2007)
H. Guleryuz, H. Cimenoglu, Surf. Coat. Technol. 192, 164 (2005)
K. Katuku, A. Koursaris, I. Sigalas, Corros. Sci. 64, 55 (2012)
D.B. Wei, P.Z. Zhang, Z.J. Yao, J.T. Zhou, X.F. Wei, P. Zhou, Appl. Surf. Sci. 261, 800 (2012)
H.L. Du, P.K. Datta, D.B. Lewis, J.S. Burnell Gray, Corros. Sci. 36, 631 (1994)
R. Peraldi, D. Monceau, B. Pieraggi, Oxid. Met. 58, 249 (2002)
R. Peraldi, D. Monceau, B. Pieraggi, Oxid. Met. 58, 275 (2002)
Y. Zhou, Q. Wang, D.L. Sun, X.L. Han, J. Alloys. Compd. 509, 1201 (2011)
G. Khosravi, M.H. Sohi, H.M. Ghasemi, A.K. Vafadar, Int. J. Surf. Sci. Eng. 9, 43 (2015)
P. Novak, P. Pokorný, V. Vojtech, A. Knaislova, A. Skolakov, J. Capek, M. Karlíkb, J. Kopecekc, Mater. Chem. Phys. 155, 113 (2015)
C. Velmurugan, V. Senthilkumar, S. Sarala, J. Arivarasan, J. Mater. Process. Technol. 234, 272 (2016)
Y.X. Xia, D. Li, Rare Met. Mater. Eng. 30, 388 (2001)
C. Parlikar, M.Z. Alam, D. Chatterjee, D.K. Das, Mater. Sci. Eng. A 682, 518 (2017)
S. Gao, J.S. Hou, F. Yang, Y.G. Guo, C.S. Wang, L.Z. Zhou, Mater. Sci. Eng. A 706, 153 (2017)
R. Braun, N. Laska, S. Knittel, U. Schulz, Mater. Sci. Eng. A 699, 118 (2017)
Z.P. Zhao, N.H. Tariq, J.R. Tang, C.L. Jia, X. Qiu, Y.P. Ren, H.H. Liu, Y.F. Shen, H. Du, X.Y. Cui, J.Q. Wang, T.Y. Xiong, Mater. Des. 185, 108249 (2020)
R. Aninat, N. Valle, J.-B. Chemin, D. Duday, C. Michotte, M. Penoy, L. Bourgeois, P. Choquet, Corros. Sci. 156, 171 (2019)
M. Franco, W. Sha, G. Aldic, S. Malinov, H. Çimenoğlu, Tribol. Int. 97, 265 (2016)
N. Sunwang, P. Wangyao, Y. Boonyongmaneerat, Surf. Coat. Technol. 206, 1096 (2011)
R.S. Magaziner, V.K. Jain, S. Mall, Wear 264, 1002 (2008)
M.C. Gui, S.B. Kang, J.M. Lee, Wear 240, 186 (2000)
J.H. Choi, G. Gyawali, D.R. Dhakal, B. Joshi, S.W. Lee, Acta Metall. Sin. (Engl. Lett.) 33, 573 (2020)
Z.H. Li, H.F. Zheng, Y.M. Zhu, Q.M. Yuan, Key Eng. Mater. 280–283, 1395 (2005)
W.H. Xue, S.Y. Gao, D.L. Duan, J.P. Zhang, Y. Liu, S. Li, Wear 410–411, 25 (2018)
R. Endo, M. Shima, M. Susa, Int. J. Thermophys. 31, 1991 (2010)
Acknowledgements
This work was supported by the National Science and Technology Major Project (No. 2017-VII-0012-0108) and the Natural Science Foundation of China (No. 51701157).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195.
Rights and permissions
About this article
Cite this article
Liu, Y.D., Liu, J.H., Gu, W.S. et al. Oxidation, Mechanical and Tribological Behaviors of the Ni/cBN Abrasive Coating-Coated Titanium Alloys. Acta Metall. Sin. (Engl. Lett.) 34, 1007–1020 (2021). https://doi.org/10.1007/s40195-020-01167-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-020-01167-0