Abstract
The permeability and diffusivity of hydrogen in directionally solidified polycrystalline and single crystal nickel foils were measured by gas permeation method. The results showed that both hydrogen diffusivity and permeability were higher in directionally solidified nickel specimen than those in single crystal one at the temperature ranging from 300 to 480 °C, and confirmed the existence of short-circuit diffusion along the grain boundaries (GBs) in the directionally solidified nickel. The results suggested that the rapid diffusion along GBs was more obviously characterized in terms of higher permeability rather than higher diffusivity. The contribution of grain boundary to hydrogen transportation was represented by the differences of diffusivity (and permeability) in single crystal nickel and directionally solidified nickel. By modifying the Fick’s first diffusion law and counting the grain boundary density, the hydrogen diffusivity and permeability of rapid diffusion along GBs were calculated. The results suggested both the diffusivity and permeability fit the Arrhenius relationship well at different temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solute hydrogen in polycrystalline nickel embrittles the material and leads to drastic losses of toughness and ductility by causing a sharp transition from ductile fracture to brittle intergranular fracture. The transportation and trapping of hydrogen plays an important role in embrittlement, where the involvement of diffusion along grain boundaries (GBs) is a key in understanding of hydrogen embrittlement phenomena [1, 2]. The grain boundary diffusion has been investigated by various techniques, and large inconsistence on diffusion coefficients has been reported. Among them, Tsuru and Latanision [3] charged cathodically the nickel specimen on one surface for a period of time, which was not long enough for hydrogen to permeate the specimen by lattice diffusion. Then, they observed the hydrogen segregation at GBs on the other surface using Ion microprobe (IMA) experiments, and explained it as an evidence of rapid diffusion along grain boundaries. Yao and Cahoon [4] augured that GBs played a role of retarding the hydrogen transport due to the trapping effect rather than the high diffusivity paths for hydrogen in nickel according to their results of electrochemical permeation experiments. However, this viewpoint was denied by the subsequent results of the same technique [5, 6], where accelerated diffusion was directly observed in terms of higher steady-state permeation flux and shorter transient time for samples with smaller grain size. The calculated diffusion coefficient along GBs was much higher (about 40 times [5] or 2–7 times [6]) than that of lattice diffusion at room temperature. The scanning electron microscopies (SEM) observation on embrittlement kinetics in the temperature range of 274–314 K also supported fast diffusion along grain boundaries, where the diffusivity about two times of the lattice diffusivity was observed [1]. The secondary ion mass spectrometry (SIMS) study [7] for nickel bicrystals indicated that the diffusion was enhanced by a factor of about 8–17.3 along the 39° (∑9) symmetric tilt boundaries but no enhancement was observed along the 129° (∑11), hence suggested that the dependence of grain boundary diffusivity on boundary types may account for the variability of the previous attempts to observe the enhanced grain boundary diffusivity. A recent study [8] on the grain size effect of hydrogen diffusion and trapping suggested that the hydrogen diffusion was accelerated in the vicinity of boundaries, while irreversibly trapping of hydrogen by the dislocations at boundaries, if there is a substantial dislocation density, may slow down the hydrogen diffusion.
However, the previous works have focused on hydrogen permeation tests at room temperature, and the measured diffusion coefficients along grain boundary show poor consistency. There is still a lack of information on the transportation and trapping of hydrogen along grain boundary at higher temperature for comprehensive understanding of the phenomenon. Since gas permeation method is valid for measurement of the transportation and trapping of hydrogen in nickel and nickel based alloys at higher temperature [9–13], we have, therefore, tried to quantify the contribution of grain boundary to hydrogen transportation and trapping in present work by comparing the diffusivity, permeability, and solubility of hydrogen in single crystal nickel with those in directionally solidified polycrystalline nickel measured by gas permeation method at a high temperature range of 300–480 °C. The values of the diffusivity and permeability of hydrogen along GBs have been derived from the measured data by modifying the Fick’s first law.
2 Experimental
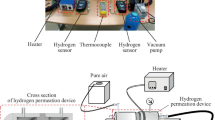
The directionally solidified polycrystalline and single crystal nickel rods of about 200 mm long and 19 mm in diameter were obtained by melting the electrolyzed polycrystalline Ni (with purity of 99.96%) and then directionally solidifying it by the high gradient liquid metal cooling (LMC) process [14]. The GBs were parallel to the rod axis in directionally solidified nickel rod, and the microstructure analysis showed the sizes of grains on the cross section of rod ranging from 200 μm to several mm. The disk shaped foil specimens were cut from the rods and polished for hydrogen permeation tests. Two types of specimens were cut from the directionally solidified polycrystalline rods to highlight the hydrogen transportation ability of grain boundary. Type 1 was cut with the surface basically perpendicular to the rod axis and denoted as radial specimen in the latter sections. There is only one grain along the axis of the specimen, i.e., all GBs could penetrate through the whole thickness of the foil. Type 2 was cut with surface parallel to the rod axis and denoted as axial specimen. Table 1 illustrates the dimensions of different specimens.
The solubility, diffusivity, and permeability of hydrogen were measured by gas permeation method. During the hydrogen permeation test, the entry side of specimen was exposed to pure hydrogen gas and the permeation flow through the specimen was detected by the quadrupole mass-spectrometer and associated software QUADERA, which significantly improved efficiency, sensitivity, and accuracy of the measurement. Before each test run, the input chamber was evacuated to base pressure (lower than several 10−6 Pa) and the specimen was degassed until the detected hydrogen flow was lower than the base value. The hydrogen gas permeation tests for the directionally solidified polycrystalline (both radial and axial) and single crystalline nickel foil specimens were performed at temperature range of 300–480 °C, and at various input hydrogen pressures from 10 to 35 kPa.
Based on the measured dynamic curve of hydrogen permeation flow, the diffusion coefficient D(T) was determined by the time-lag method [3, 15]:
where l was the thickness of the specimen disk and t was the characteristic time lag.
The permeation coefficients Φ(T) were defined in terms of steady-state hydrogen permeation flux by the following equation [9]:
where J ∞ was the steady-state permeation flux, A was the area of the planar foil exposed to hydrogen. P and P 0 were the hydrogen pressure on the entrance and exit sides respectively. In this study, the value of P 0 was neglected as P ≫ P 0.
The activation energy for hydrogen diffusion (H D) and permeation (H Φ ) and their prefactors (D 0 and Φ 0) were obtained by fitting the diffusion and permeation coefficients at different temperatures with the Arrhenius relationship: \( { \ln }D(\varPhi ) = { \ln }D_{0} (\varPhi_{0} ) - \frac{{H_{\text {D}} (H_{\varPhi } )}}{RT}, \) where R = 8.31 J/(mol K) was the molar gas constant.
3 Results and Discussion
3.1 Transportation of H in Single Crystal and Directly Solidified Nickel
The measured hydrogen flux (J) and time (t) in the permeation curves are normalized, respectively, by the steady-state flux (J ∞) and the characteristic time τ = l 2/D L (D L = 4.089 × 10−10 m2/s is the lattice diffusion coefficient at 400 °C [10]), for elimination of the effects of specimen thickness (l) and input hydrogen pressure for different specimens. Figure 1 shows the normalized permeation curves for different specimens measured at 400 °C. It can be seen that hydrogen diffusion is much more rapid in radial specimen than that in single crystal nickel during the whole transient state period, implying a short-circuit diffusion along grain boundary in the radial specimen. However, difference in diffusivity is hardly observed between the axial and the single crystal specimens. The results indicate that hydrogen diffusivity at transient state is significantly influenced by the orientation of GBs relevant to the specimen surface, which may account for the inconsistence in previous reported grain boundary diffusivity.
Figure 2 shows the hydrogen diffusion and permeation coefficients calculated by Eqs. (1) and (2) based on the measured dynamic curve of hydrogen permeation flow. The permeation coefficients of the radial specimens with different thickness are almost equal, indicating that the controlling process was indeed the diffusion of hydrogen through metal membranes. It can be seen that both the permeation and diffusion coefficients of hydrogen are higher in radial specimen of directionally solidified nickel than in single crystal nickel in the temperature range of 300–480 °C. For axial specimen, the diffusivity is almost equal to that in single crystal, whereas the permeability is higher than that in single crystal nickel but a little lower than that in radial specimen. These results confirm the following two facts: (1) the GBs are rapid paths for hydrogen diffusion and it is more obviously characterized in terms of higher permeability than higher diffusivity; (2) the contribution of grain boundary diffusion to steady hydrogen flux is considerable (higher than 40%) at temperature ranging from 300 to 480 °C, although there are only a few GBs in the radial specimen with grain size up to several millimeter.
Brass and Chanfreau [6] had measured no extra hydrogen flux in samples with grain size of 150 μm in comparison with that in single crystal at 298 K, and attributed the phenomenon to the small number of GBs in the samples with larger grain size. Yao and Cahoon [4] had predicted that the short-circuit effects cannot be observed when the grain size is above a critical value, which is about 10 μm when the binding energy of hydrogen to GBs is 11.6 kJ/mol. The significant increment in hydrogen diffusivity and steady-state flux in radial specimen may be attributed to the specific distribution of grain boundaries. The GBs penetrating through the whole thickness of the foil specimen act as an easy access for hydrogen diffusion. Actually, not all the GBs are helpful to deliver hydrogen through the foil. Those segments parallel to the foil surface not only hinder the hydrogen diffusion as tortuous paths but also trap the hydrogen atoms arrived from lattice diffusion with binding energy of 0.12 eV [4]. Therefore, for common polycrystalline samples with smaller grain size, the actual paths of grain boundary diffusion are much longer than the thickness of the specimen and the density of effective paths is much less than the density of GBs in the specimen. The side effect of the GBs parallel to the foil surface may exceed that of rapid diffusion along the penetrating grain boundaries, as observed in Ref. [6] that there is a delay in the “experimental” breakthrough in samples with smaller grain size of 25 μm. Furthermore, short-circuit diffusion is probably minimized by trapping effects of GBs and dislocations stored at the G Bs [6, 8].
The diffusion coefficient (D) and permeation coefficient (\( \varphi \)) at different temperatures fit well with the Arrhenius relationship, and the activation energy (with unit of kJ/mol) and the prefactors (with unit of m2/s) for different specimens are as follows.
For directionally solidified Ni (Radial specimen):
For single crystal Ni:
3.2 Trapping Effect of Grain Boundary
It is known that the prominent effect of trapping decreases the apparent diffusivity and increases the hydrogen solubility. To examine the trapping effect of grain boundary in pure nickel, the hydrogen solubility (S) is calculated according to the Sievert’s law, which is equal to the permeation coefficient divided by the diffusion coefficient as
where C represents the concentration of hydrogen in lattice, and P is the hydrogen pressure on the entrance side.
Figure 3 displays the calculated hydrogen solubility for three specimens at different temperatures. It can be seen that the hydrogen solubility in the two directionally solidified nickel specimens (radial and axial specimen) are comparable, but higher than that in single crystalline specimen. These results confirm the trapping effect of grain boundary in directionally solidified nickel and indicate that hydrogen solubility (or concentration) along GBs is higher than that in lattice, which is consistent with the ion microprobe IMA measurements by Tsuru and Latanision [3].
3.3 Contribution of Intergranular Transportation of Hydrogen
To quantitatively describe the intergranular transportation of hydrogen, it is necessary to quantify the contribution of GBs diffusion to diffusivity and steady-state flux. In present study, the contribution of grain boundary diffusion is regarded as the extra part of the measured diffusion (or permeation) coefficients in radial specimen with respect to those in single crystal specimen. The differences of diffusion (and permeation) coefficients are calculated for different temperature points at which the permeation test are performed for the two specimens. As shown in Fig. 4, each point represents the difference between the mean of measured values in one specimen and the extrapolated values from Eqs. (3)–(6) in the other specimen. It should be informed that the diffusion and permeation coefficients induced by grain boundary diffusion also fit well with the Arrhenius relationship as
The activation energy for diffusion 29.03 kJ/mol is consistent with the reported values 30–37.8 kJ/mol [1, 5]. The lower activation energy may imply the preferential diffusion path along grain boundaries.
3.4 Intergranular Diffusivity and Permeability
Considering the contribution of grain boundary diffusion to the hydrogen permeation flux, the Fick’s first law could be modified as
where D L and D GB are the diffusion coefficient in lattice and along grain boundary, respectively; C L and C GB are the concentration of hydrogen in lattice and at grain boundary, respectively; and f is the volume fraction of grain boundaries. The segregation coefficient, s, defined as the ratio of the concentration of hydrogen at the grain boundary to that inside the grains, varies exponentially with temperature:
where a is a pre-exponential factor, normally assumed as 1; E is the binding energy of hydrogen atom to grain boundary, experimentally determined as 11.6 kJ/mol [2]. Eq. (10) could be written as
The equation for the experimentally determined diffusion coefficient (D) and permeation coefficients (Φ) in this case become
where f = δ × ρ, here δ is grain boundary width effective for rapid hydrogen diffusion, assumed to be 1 nm [3, 4] and ρ represents the mean density of grain boundary, which is equal to l/A, l is the total length of all GBs on the surface of radial specimen, and A is the area of the surface. The calculated density ρ is 3.4 × 103 m/m2, and f is 3.4 × 10−6, much smaller than 1. Thus, Eqs. (13) and (14) could be approximated as
The contribution of grain boundary to hydrogen transportation in directionally solidified nickel could be described by Eqs. (15) and (16), with D L and Φ L being substituted by the measured values in single crystalline nickel, D GB and Φ GB could be calculated from Eqs. (8), (9), (15) and (16) as
4 Conclusions
Gas permeation method has been used to measure the diffusion and permeation coefficients in directionally solidified and single crystalline nickel foils at the temperature range of 300–480 °C. The results confirm the rapid diffusion of hydrogen along GBs in directionally solidified nickel foil. The phenomenon is more obviously characterized in terms of higher permeability than higher diffusivity. The hydrogen permeability is 40% higher in directionally solidified nickel specimen than in single crystal specimen at temperature high up to 450 °C, owing to the specific distribution of grain boundaries. The measured diffusion coefficients are significantly influenced by the orientation of the GBs to the surface of test foils. The contribution of grain boundary to hydrogen transportation is represented by the differences of diffusivity or permeability in single crystal nickel and directionally solidified polycrystalline nickel. The diffusivity and permeability of hydrogen along GBs are derived by modifying the Fick’s first diffusion law, and fit well with the Arrhenius relationship as \( D_{\text{GB}} = 6.09 \times 10^{ - 3} {\text{exp}}\left( {\frac{ - 40.63 \pm 0.89}{RT}} \right), \) and \( \varPhi_{\text{GB}} = 59.1 {\text{exp}}\left( {\frac{ - 52.97 \pm 0.1}{RT} } \right) \).
References
A. Kimura, H.K. Birnbaum, Acta Metall. 36, 757 (1988)
D.H. Lassila, H.K. Birnbaum, Acta Metall. 34, 1237 (1986)
T. Tsuru, R.M. Latanision, Scr. Metall. 16, 575 (1982)
J. Yao, J.R. Cahoon, Acta Metall. 39, 119 (1991)
T.M. Harris, R.M. Latanision, Metall. Trans. A 22, 351 (1991)
A.M. Brass, A. Chanfreau, Acta Mater. 44, 3823 (1996)
B. Ladna, H.K. Birnbaum, Acta Metall. 35, 2535 (1987)
A. Oudriss, J. Creus, J. Bouhattate, C. Savall, B. Peraudeau, X. Feaugas, Scr. Mater. 66, 37 (2012)
M.R. Louthan Jr, J.A. Donovan, G.R. Caskey Jr, Acta Metall. 23, 745 (1975)
Y. Ebisuzaki, W.J. Kass, M. O’keeffe, J. Chem. Phys. 46, 1378 (1967)
Q.Y. Wu, J. Zhang, X.F. Sun, Z.Q. Hu, Acta Metall. Sin. 32, 938 (1996) (in Chinese)
L.Y. Xiong, S. Liu, L.B. Wang, L.J. Rong, Acta Metall. Sin. 44, 781 (2008) (in Chinese)
H.H. Grimes, Acta Metall. 7, 782 (1959)
A.J. Elliott, S. Tin, W.T. King, S.C. Huang, M.F.X. Gigliotti, T.M. Pollock, Metall. Mater. Trans. A 35, 3221 (2004)
J.R. Phillips, B.F. Dodge, AlChE J. 14, 392 (1968)
Acknowledgments
Present work was financially supported by the National Natural Science Foundation of China (No. 51071154).
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://springerlink.bibliotecabuap.elogim.com/journal/40195
Rights and permissions
About this article
Cite this article
Wang, Y., Xiong, L. & Liu, S. Rapid Hydrogen Transportation Along Grain Boundary in Nickel. Acta Metall. Sin. (Engl. Lett.) 27, 615–620 (2014). https://doi.org/10.1007/s40195-014-0102-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-014-0102-y