Abstract
Duplex stainless steels generally solidify with a ferritic microstructure, followed by a partial transformation to austenite during cooling. Rapid cooling may render the transformation incomplete. The too-low or too-high cooling rate can also result in the formation of intermetallic phases and precipitates which can have a detrimental effect on the pitting corrosion resistance. The possibilities of achieving a microstructure with 30–70% ferrite in duplex stainless steel (1.4462, DX 2205) autogenously welded with a novel plasma welding equipment were investigated. Welding was performed as a square butt joint, with a 0-mm nominal gap size, on a 6-mm plate thickness, without the use of filler metal. The plasma torch uses a focus gas that allows it to constrict the arc in addition to the conventional constriction by the plasma nozzle. The three separated gas flows (plasma, focus and shielding gas) allow the gas composition to be tailored in the different gas flows. The effect of argon, nitrogen and helium content on the phase balance related to the gas flow channels was studied. The presence of nitrides as well as the sigma phase was investigated, and the joint quality was evaluated. The microstructure of the welded samples, investigated by image analysis, was correlated to pitting corrosion resistance. It was shown that high joint quality, quality level B, with favourable ferrite/austenite phase balance can be achieved through autogenous single-pass welding of duplex stainless steel 1.4462, DX 2205 (6.0 mm) using the novel plasma torch. With N2 additions in, most notably, the plasma gas and the shielding gas, it was possible to get a ferrite phase fraction in the weld metal close to 50%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The weld process and parameter selection highly influence the mechanical properties and corrosion resistance of welded stainless steel. One of the main challenges associated with the welding of stainless steel is to provide a weld that matches the properties of the base material while meeting requirements for manufacturing productivity [1].
A metallurgical phenomenon that impacts the mechanical and corrosion properties of welded duplex stainless steel includes the balance between ferrite and austenite, as well as the precipitation of undesirable phases such as intermetallic compounds and nitrides [2,3,4]. Subsequently, application standards for welding duplex stainless steel attempt to partly base their requirements on the post-weld microstructure. A clear trend for allowed ferrite content in the weld metal is a range between 30 and 70% [5,6,7,8]. In similarity to the requirement of acceptable ferrite content, standards also have limits on intermetallic phases and precipitates, which can significantly impact toughness and corrosion resistance. ISO 1516–3 (oil and gas) and DNV-FS-101 (submarine piping) state an upper limit of a maximum of 1% for intermetallic phases, nitrides and carbides combined and a maximum limit of 0.5% for sigma phase when examined at 400 × magnification in a light optical microscope (LOM) [6, 9]. Norsok M-601 (piping welds) accepts a quantitative limit of < 0.5% intermetallic phases; furthermore, it states that “any presence of intermetallic phases and/or precipitates shall be reported” in the 2016 version [7]. ISO 17781 (petrochemical) has another approach to the required criteria and states, “discrete/standalone intermetallic particles are acceptable given that the maximum dimension of these is ≤ 10 µm” [5].
Plasma arc welding (PAW) utilises a non-consumable tungsten electrode which is placed in a nozzle with a separate plasma gas flow. The weld method is usually automated and is characterised by its high-energy density arc, created through forcing the plasma gas and arc through a constricting nozzle which transmits a plasma jet onto a workpiece. The high density of energy in a small area makes keyhole formation possible. Welding in keyhole mode provides welds with narrow, deep penetration with a low and even cap and root. The result is higher possible welding speeds and therefore increased productivity if compared to, for example, GTAW (gas tungsten arc welding) [2]. A single-pass keyhole mode can be used with success for material thickness up to 8 mm [10]. Laser beam welding (LBW) can also be performed in keyhole mode, with extremely narrow deep penetration weld profiles as a result. The cooling rate for LBW is however generally much faster if compared to PAW which can pose a problem during autogenous welding of duplex stainless steel due to impairment of austenite transformation and a weld microstructure containing more ferrite than recommended [11].
In the PAW process, a separate shielding gas flows through an outer nozzle to protect the weld pool and weld metal from oxidation [10]. Pure argon (Ar) or Ar with additions of hydrogen (H2), for non-ferritic materials due to the risk of cold cracking, are often used as plasma and shielding gas. An addition of 20–30% helium (He) facilitates fluidity and typically permits a higher travel speed due to an increase in arc voltage and therefore arc energy [12]. To promote austenite formation or compensate for element segregation, the use of a suitable filler wire is one option [13]. However, adding filler wire to a process adds complexity as well as an increase in cost. Subsequently, there is often a desire to mediate the problem with an unfavourable microstructure by other means than incorporating filler wire. An example is the addition of nitrogen (N2). It promotes austenite formation, and by utilising N2, it is possible to compensate for the loss occurring in the molten material during autogenous welding.
Linde Gas has developed a new design for the welding torch used in the PAW equipment ARCLINE®. One of the new features of the torch is that it utilises three different gas flows (plasma, focus and shielding) instead of the conventional two (plasma and shielding). The purpose of the focus gas is to further support arc constriction, in addition to the constricting effect of a conventional plasma gas nozzle. The separated gas flow channels are shielding gas, plasma gas and focus gas, displayed in Fig. 1. The composition of the gases used in the individual gas flow channels may be customised.
The aim of this investigation was to produce single-pass welds with the novel plasma torch and examine the influence of nitrogen additions in the separated gas flow channels to achieve a favourable microstructure and sufficient corrosion resistance. The investigation focused on 6-mm autogenously welded duplex stainless steel (1.4462, DX 2205), which was examined microstructurally and with pitting corrosion testing.
2 Experimental
Arc energy, AE, is defined [14] according to the equation:
where Um is the arithmetic mean value of voltage (in volts), Im is the arithmetic mean value of current (in amperes), and v is the welding speed (in mm/s). The arc energy (supplied energy to the arc per weld length unit) is related to the heat input, HI, through the equation:
where η is the process efficiency factor. In PAW with keyhole mode, this factor is rather high; η = 0.9 is used in [2]. In this, paper arc energy has been calculated.
2.1 Design of experiment
Design of experiment (DoE) using the software JMP (JMP Statistical Discovery from SAS, version 14.0) was applied to create a definitive screening design with all interactions. The null hypothesis is that N2 and He do not influence the ferrite content. A p-value (statistical measurement to validate the hypothesis against observed data) less than 0.05 is defined as significant, i.e. showing that N2 and He do influence the ferrite content. The N2 and He contents of the gas composition for the plasma, focus and shielding gas flow channels were designed to have low, medium and high percental values. The percentage values were selected based on commercially available gases (Ar + 1.8% N2) for autogenous PAW welding of duplex stainless steels and earlier experience with helium additions [15]. All gases were mixed by Linde for this investigation. The DoE resulted in a test matrix consisting of 20 test runs in total, whereof the last 3, as shown in the matrix, were repetitions for verification of the previous test; see Table 1.
As ferrite/austenite balance was the sole result parameter for the DoE and the investigation focused on the influence of the gas compositions and gas channel configuration, it was important to limit other factors which affect the phase balance. Ferrite content is highly dependent on the cooling rate and therefore also the arc energy. It was subsequently determined to aim for constant arc energy without altering the current, without taking the gas content and gas channel configuration into account. The reason for maintaining a constant current was that an alteration would affect the arc pressure. Experimental test set-up trials with high (50%) He content in the plasma gas flow channel showed that the arc pressure was able to suffice keyhole formation at the given arc energy, although the travel speed had to be increased to compensate for the increased arc voltage when aiming for constant arc energy.
2.2 Material and welding
Weld trials were performed at Linde, Unterschleißheim, Germany. Duplex stainless steel (1.4462, DX 2205) with a 1D (hot rolled, heat treated, pickled) surface at a thickness of 6 mm was a water cut to 500 × 70 mm. The chemical composition of the base metal can be seen in Table 2.
The joint preparation was a square butt joint. Although a zero mm gap was targeted, the butt joint gap sizes varied between 0.05 and 0.2 mm during the trials. The stand-off distance (distance between plasma nozzle and workpiece) was 5.0, mm and the nozzle diameter was 3.0 mm. All samples were cleaned with isopropanol prior to welding and clamped to a water-cooled copper fixture. Information regarding the gas flows used during weld trials is presented in Table 3.
The weld parameters with the calculated arc energy are displayed in Table 4.
2.3 Microstructural evaluation
2.3.1 Phase balance
All specimens had cross-sections, placed 80 mm from weld start, used for phase balance analysis. Specimen number 1 had a cross-section at the start before the welding process was stable (1A), a cross-section at 80 mm from the weld start (1B), and a cross-section at the end of the weld (1C).
The cross-sections were wet ground and polished with the diamond suspension to 1 μm. Etching of the surface was performed with modified Beraha II etchant (60 ml H2O + 30 ml HCl + 1 g K2S2O5) for approximately 3 s, after which the samples were rinsed in ethanol and dried with a blow dryer. The length of time between the steps of polishing, etching, and viewing in a light optical microscope (LOM) was kept to a minimum, and the etchant was used directly after being mixed. The etchant causes the microstructure to be exposed as a dark-coloured ferrite matrix with lighter-coloured austenite grains when viewed in LOM. Images for phase balance analyses were placed in accordance with Fig. 2.
The majority of the locations of images, heat affected zone (HAZ) and location of images 1–5, were placed along a horizontal line 2 mm from the surface of the samples, and locations of images 6 and 7 were placed vertically towards the root. All images of the different locations used in phase balance analysis were captured in 500 × magnification, with the scale indication removed.
The cross-section images were analysed using ImageJ (version 1.52a) to determine the phase fraction of ferrite/austenite. Using the ImageJ “threshold” feature, the light phase, in this case, austenite, can be extracted from the picture. The border between what is considered austenite in the picture has been determined using the default IsoData algorithm. In Fig. 3, the austenite is extracted and marked in red. The red fraction of pixels is representative of the austenite volume fraction.
The method assumes that the investigated area only contains ferrite and austenite. The ferrite fraction is calculated as 100% minus the area fraction of austenite. It should be noted that possible nitrides are included in the area fraction for ferrite.
2.3.2 Presence of sigma phase and nitride precipitations
The samples were polished with diamond paste to 0.25 µm and subsequently polished (Colloidal Silica polishing suspension) during 15 min using an oxide suspension and water. Am FEG-SEM (field emission gun – scanning electron microscope) Zeiss Gemini 450 with Symmetry EBSD camera was used. The EDS (energy-dispersive X-ray spectroscopy) detector was a 150 mm2 Ultim Extreme SDD (silicon drift detector) from Oxford Instruments. The settings were EHT: 10 kV, working distance 9 mm, back-scattered detector.
2.4 Corrosion testing
Prior to corrosion testing, the samples were chemically pickled in HNO3/HF (16/4) at 40 °C for 20–30 min. Samples were water cut to 25 mm (along the weld length) × 50 mm (across the weld length). Non-investigated surfaces (e.g., cut edges and root side or cut edges and top side) were masked off using aquarium silicon. The cut edges were masked off to prevent crevice corrosion. If crevice corrosion occurs prior to pitting corrosion, it will provide cathodic protection to the area close to the crevice corrosion, which prevents the formation of pits and therefore inhibits accurate determination of critical pitting temperature (CPT) [16]. Corrosion testing was solely performed on the top/root surfaces of the samples because they are the surfaces most likely to be exposed to corrosive media in a finished application.
Corrosion testing was performed with a modified test method based on ASTM G150, which measures the CPT for stainless steels. The use of an ordinary Avesta cell is inappropriate due to the uneven and large area of interest of the welded samples; instead, a larger, open stainless steel vessel filled with an electrolyte was used as the electrochemical cell, making it possible to expose all surfaces of interest to the electrolyte. A large net of platinum was used as the counter electrode. This counter electrode was electrically connected to the stainless steel vessel enlarging the counter electrode surface even further. The corrosion test starts with pouring the electrolyte, a 1 M NaCl solution, with a temperature close to 0 °C into the vessel. When the temperature of the electrolyte reached the lowest stable temperature in the cell (< 4 °C), each sample was put under potentiostatic control at 700 mV S.C.E and the controlled heating of the electrolyte was started with a temperature increase rate of 1 °C/min.
2.5 Weld quality assessment
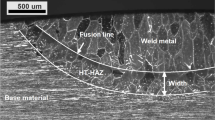
Information about the welds was obtained through scanning data using a weld geometry laser scanning system Micro-Epsilon laser scanCONTROL 2950–50 with a z-axis distance of 70 to 125 mm and a resolution of 4 µm (software developed by Winteria) and from measurements from cross-section images. Scanning was performed between 10 and 134 mm from the start of each welded specimen. Although the scanning distance does not cover the entire weld length, it is more than one-third of the length, which is a criterion for continuous indications in EN ISO 5817:2014 [17]. The scanning provided information about weld toe radius, continuous and intermittent undercut and weld sagging. Cross-section at 80 mm from the weld start of each specimen provided information about excess weld metal, weld toe width, excess penetration and weld root width, as indicated in Fig. 4.
3 Results
3.1 Microstructural evaluation
3.1.1 Phase balance
The average phase fraction between 7 images per cross-section and the HAZ image can be seen in Table 5.
Ferrite in the weld varied 43–69% and the HAZ 65–79%. The width of the HAZ varied some between cross-sections and locations around the bead but was typically between 1.0 and 1.5 mm wide.
3.1.2 Presence of nitride precipitations and sigma phase
Duplex stainless steels exposed for a sufficient time at 700–900 °C can precipitate intermetallic phases such as the sigma phase [18]. The unwanted intermetallic phases may impair toughness as well as corrosion resistance due to the depletion of Cr. To be sure to be on the safe side, it was therefore decided to examine specimen number 15 for the possible presence of sigma phase and/or nitrides with SEM. Sigma phase may occur during prolonged cooling times from high temperatures; therefore, specimen number 15 which was welded with the highest calculated arc energy (1.49 kJ/mm) was thought to have a higher risk. It was also believed that the welds could contain nitrides, especially in HAZ which exhibited a coarser microstructure rich in ferrite when compared to the weld metal and some of the other specimens. Specimen number 15 contained one of the highest ferrite contents in the HAZ and was welded with a high level (3.6%) of N2 in the plasma and shielding gas and was selected for the evaluation of nitride precipitation in the weld metal.
SEM analysis did not indicate any presence of the sigma phase in the cross-section of sample 15. In the HAZ and at higher magnification images in Fig. 5, the nitrides can be seen as dark needle-shaped structures.
Although no quantification of the total area fraction of nitrides was performed, they were shown to be evenly distributed inside the ferrite grains of the HAZ and not detectable to a significant extent inside the weld metal. The largest single nitrides present in HAZ were up to 1 µm in length.
3.2 Influence of gas flow composition and gas channel configuration on ferrite/austenite fraction
The correlation between gas content and gas channel configuration was evaluated with phase balance as the response parameter. The response was average ferrite fraction in the weld metal, excluding HAZ. The factors were N2 content in the plasma gas channel, N2 content in the focus gas channel, N2 content in the shielding gas channel, He content in the plasma gas flow channel, He content in the focus gas flow channel, and He content in the shielding gas flow channel. Arc energy should be treated as an uncontrolled factor, as it was not evenly distributed over the factors. In the model, all interactions were included except for interactions with arc energy. Factors with p-value ≤ 0.05 were kept in the model, as p-values greater than 0.05 are to be interpreted as insignificant. The significant factors and interactions were kept, and a model was created by linear regression in SAS JMP software. Significant factors on the average ferrite fraction in the weld metal were in order as follows.
-
1.
N2 content in the plasma gas flow channel.
-
2.
N2 content in the shielding gas flow channel.
-
3.
N2 content in the focus gas flow channel.
-
4.
A slight interaction between N2 content in the plasma gas flow channel and He content in the shielding gas flow channel.
The weld metal showed variations in ferrite content mainly depending on the N2 content of the plasma gas flow channel. Other significant factors were the N2 content of the shielding and, to a lesser extent, the focus gas flow channel. The lowest ferrite content in the weld metal is to be expected for specimens welded with high (3.6%) N2 in the gas flow channels.
The plot of the response on average phase fraction excluding HAZ can be seen in Fig. 6.
The actual predicted plot and effect summary of influence on phase balance in the weld metal. Note that the numbers for N2 (0, 3, 6) represent the DoE values of low, medium and high. During the trials, the gas flows contained either 0, 1.8 or 3.6% N2, as seen in Table 1
The model has an R2 of 0.97 which means that the model is able to accurately predict the ferrite content of the weld metal depending on gas composition and configuration based on the tests made for the samples within the trials.
As can be seen in Fig. 7, most of the expected ferrite content inside the weld metal can be explained by the amount of N2 content in the plasma gas flow channel, although N2 content in the shielding gas flow channel and N2 content in the focus gas flow are also significant.
The slight interaction between N2 content in the plasma gas flow channel and He content in the shielding gas flow channel should be considered with particular care. Although efforts were made to avoid it, the heat input varied slightly. The interaction may therefore likely be due to a variation in heat input rather than an interaction between N2 content in the plasma gas flow and He content in the shielding gas flow.
3.3 Weld quality assessment
The result parameter for the DoE matrix was the ferrite/austenite balance, and the aim was therefore to keep arc energy constant. The quality of the welds was consequently not the main focus, as adjustment of weld parameters for the different gases and combinations would have improved the quality of the individual specimens. It was however decided to compare the quality of the welds to some of the visual examination criteria in EN ISO 5817:2014 to determine the quality level. Investigated imperfections which were determined to pose a possible problem included undercut, excess penetration and weld sagging with incompletely filled grooves. Requirements for quality level B according to EN ISO 5817:2014 [17] are shown in Table 6.
Although an end crater pipe was present in all specimens, it was not considered in the quality investigation, as no effort was put into avoiding it during the weld trials. The results of the quality assessment are found in Table 7.
3.4 Corrosion testing
As evident from the DoE model, the N2 content of the plasma gas flow had the greatest impact on the ferrite content of the weld metal. A comparably minor effect of N2 content in the shielding gas flow was noted. Although, no direct impact of the focus gas content was apparent. It was therefore decided to examine the CPT of specimens welded with 0%, 1.8% and 3.6% N2 in the plasma gas flow, with no addition of N2 or He in the shielding gas flow. The specimens used in corrosion testing can be seen in Table 8.
Three samples were tested per ID and examined surface; non-examined surfaces were masked off (cut edges and top or root side). The root side for ID 12 was tested, as it was welded with the commercially available standard gas content of 1.8% N2. The average CPT of three samples and the location of the pitting attack can be seen in Table 9.
The location of the pitting attack occurred either in the base metal of the samples at approximately 8 mm from the fusion line and in the weld metal. The width of the HAZ was around 1–1.5 mm, and an attack at approximately 8 mm from the fusion line was therefore determined to be outside the low-temperature HAZ. The root side (of ID12) showed almost the same CPT value as the parent material (Ref.). The CPT values for the top side of the welded samples were slightly lower, and the pitting corrosion attack occurred mainly in the weld metal. The lowest CPT values, even if very close to ID12 and ID13, were found for the top side of weld ID 4 that had the highest ferrite content (welded without any nitrogen addition to the plasma gas).
The standard deviation for the test method ASTM G150 is ± 3 °C for ground surface specimens [16] and was found to be similar in this investigation.
4 Discussion
Only a few welding processes are capable of welding duplex stainless steels for plate thickness of around 8 mm autogenously in a single pass without joint preparation. Examples of processes that are capable of this are laser, plasma arc welding (PAW) and high-current keyhole TIG. The cooling rate in laser welding is very rapid, and low austenite formation and nitride formation therefore become issues [19,20,21]. In multi-pass welding, parts of the weld are reheated and precipitation of intermetallic phases may therefore become a concern, particularly for duplex stainless steels with high Cr content [3, 22].
4.1 Phase balance
We summarised the average ferrite fraction varied between a minimum of 43% and a maximum of 69% in the weld metal for the investigated cross-sections, i.e., excluding the HAZ. The higher ferrite fraction levels were achieved with argon as plasma gas. This is in line with reported results using single-pass high-current TIG welding with similar arc energy levels, as used in this study (using deep penetration in keyhole mode) [21, 23, 24].
In laser welding duplex stainless steels, the cooling rate is typically higher, and consequently, the resulting austenite fraction is lower [19, 25, 26]. In this study, the ferrite fraction was generally found within the targeted fraction (30–70% ferrite) in the weld metal of the samples. The ratio can be modified through additions of N2, most notably in the plasma gas and, to some extent, the shielding gas, where a higher austenite fraction was obtained with increasing levels of N2. Uptake of nitrogen from the shielding gas and the effect on promoting austenite formation has been observed in, for instance, TIG and laser welding of duplex stainless steels [27,28,29]. In this study, specimens welded with high (3.6%) N2 in the plasma and shielding gas flow were specimens 11, 13 and 18, which had a phase balance microstructure of close to or 50/50 ferrite/austenite in the weld metal. Specimen 15 and the repetition specimen 20 contained a high level (3.6%) of N2 in all three different gas flows and contained a slight majority of austenite inside the weld metal. Test ID 1 which was examined at different locations indicates that the ferrite fraction in the weld metal does not tend to vary significantly over the weld length.
The HAZ was narrow, less than 1–1.5 mm wide, which also has been found in other investigations with PAW using arc energy of the same order of magnitude as in this study [20], and only one image of the HAZ for each sample was analysed. The ferrite fraction percentage should be treated with care due to the location of the image in the HAZ and instead be viewed as a general trend. The majority of the investigated cross-section had a ferrite fraction below 60%; however, the high-temperature HAZ generally exhibited a significantly coarser and more ferrite-rich structure compared to the images captured in the weld metal. The higher ferrite content can be explained by the rapid cooling of the HAZ (between 1500 and 1200 °C), which impairs austenite growth. Grain growth at higher temperatures results in a coarser ferrite structure consisting of larger ferrite grains compared to the weld metal microstructure, which suppresses austenite formation [2]. Moreover, no evident correlation between the decrease in ferrite fraction in the HAZ and N2 additions to the different gas flows could be seen. The uptake of nitrogen to the HAZ directly from the plasma gas and focus gas is probably very limited since there is a molten layer between the HAZ and the keyhole as well as the small part of the HAZ that is in contact area with the shielding gas at the top side. It has been reported that the diffusion and loss of nitrogen from the HAZ to the weld metal could be reduced by increased nitrogen content in the weld metal [30, 31], but no such conclusion could be made from this study. The HAZ ferrite fraction was therefore often well above the average of the rest of the weld and occasionally above 70%. The higher ferrite content of the HAZ, even when outside the recommended 30–70% range, does not necessarily have to be detrimental. The standard EN 13,445–4:2014 “unfired pressure vessels – part 4: fabrication” relaxed the requirements of ferrite content in HAZ to a maximum of 85% from the fusion line to a location at a distance of two grain diameters [8]. A suggested way of ensuring that a sufficient amount of austenite has been achieved in the HAZ is to perform pitting corrosion tests in order to prove the weldment is fit for purpose [10].
4.2 Presence of nitride precipitations and sigma phase
The lack of a significant amount of nitrides in the weld metal and the clear presence of nitrides in HAZ may be explained by the rapid cooling and high ferrite content of the HAZ. Prevention of austenitic nucleation and growth causes oversaturation of nitrogen in the ferritic phase, which has a very low nitrogen solubility, resulting in nitride precipitates [2, 32,33,34]. The arc energy used during the weld trials, ranging from 1.31 to 1.49 kJ/mm, is however well inside the recommendations (0.5–2.5 kJ/mm [35] and therefore also inside the recommended cooling rate range for duplex stainless steels. The presence of nitrides is dependent on the chemical composition of the material, which makes some materials more prone to nitride precipitation, especially in areas with a coarse ferrite-rich microstructure [28, 32, 36]. Quantification of nitrides is rather impractical, which has caused some standards to change the methodology for evaluation. EN ISO 17781:2017 (petroleum, petrochemical and natural gas industries—test methods for quality control of microstructure of ferritic/austenitic (duplex) stainless steels) specifies that “nitride precipitates which are observed shall be reported and documented, and the acceptance shall be based upon meeting the impact and corrosion requirements,” in addition to the maximum dimension requirement of ≤ 10 µm for discrete/standalone intermetallic particles [2]. Common standards state that examination should be performed in LOM “at a magnification sufficient to observe” [5], “suitably etched and examined at X 400” [6] or “the micro-structure shall be etched in a 20/40% NaOH/KOH solution and examined at 400 or 500 X magnification” [7]. In this case, the investigation was performed with SEM at significantly higher magnifications.
No sigma phase was found. The time at elevated temperature for duplex stainless steel (DX 2205, 1.4462) is long compared to the cooling time at critical precipitation temperature in single-pass welding [3, 20, 32].
4.3 Corrosion testing
The reference exhibited the highest CPT when compared to the welded specimens. A CPT of approximately 49 °C for un-welded 1.4462, DX 2205 is in line with previous CPT (approximately 50 °C) obtained through traditional ASTM G150 testing with an Avesta Cell on ground surface specimens [16]. Ground surface specimens usually exhibit slightly higher CPT than pickled surface specimens [16]. The root of ID 12 had an average CPT of 47 °C which can be explained using N2 backing gas during the weld trials. The pitting attack of ID 12, root, was located approximately 8 mm from the fusion line of the root, where some residue weld oxides were visible. The top side of ID 12 and ID 13 obtained rather similar CPT values, independent of the higher ferrite content in ID 12. ID 4 with the highest ferrite content in the weld metal also obtained a CPT close to that of the other tested samples. All specimens exhibited pitting corrosion attack mainly in the weld metal, except for two of the ID 12 samples which exhibited minor pitting attack in the base metal at approximately 8 mm from the fusion line. In the corrosion testing, only the surface microstructure is tested, which can explain the slight difference in CPT between the specimens, independently of the difference in average ferrite content of the weld metal. No prominent austenite layer was visible in LOM on the top surface of the weld metal or HAZ for any of the corrosion-tested samples, which could also explain the similar CPT values. From the corrosion-tested samples in this study, no correlation between N2 content in the gas flows, nor the resulting ferrite fraction, can be drawn based on the CPT values. A possible reason for obtaining a rather similar CPT for the welded samples could be that all tested samples have an average ferrite fraction between 51 and 69%, which is within the recommendations for the material.
5 Conclusions
-
A ferrite fraction within the range of 30–70% is achievable through autogenous single-pass welding of duplex stainless steel 1.4462, DX 2205 (6.0 mm) using a novel plasma torch with separated gas flow channels for plasma, focus and shielding gas. All welded specimens obtained an average ferrite fraction in this range without any nitrogen additions.
-
The ferrite/austinite ratio of the weld metal can however be beneficially tailored further towards a 50/50 balance by N2 additions in, most notably, the plasma gas flow and the shielding gas flow.
-
The highest tested N2 content (3.6%) in the gas flow channels (ID 15 and ID 20) provided the lowest ferrite (44% and 43%) content in the weld metal.
-
The ferrite content of the HAZ was not correlated to the gas flow channel content nor gas channel configuration.
-
The constructed DoE model can accurately predict the ferrite content of the weld metal depending on gas composition and gas channel configuration based on the tests made.
-
Although not the main focus, the quality of the welds was satisfactory, with the majority of the welds fulfilling requirements for weld class B (EN ISO 5817:2014) for the examined imperfections.
Data availability
Not available.
References
Stainless Steel Advisory Service (2001) Stainless steel advisory service British stainless steel association welding of stainless steel
Ureña A, Otero E, Utrilla MV, Múnez CJ (2007) Weldability of a 2205 duplex stainless steel using plasma arc welding. J Mater Process Technol 182:624–631. https://doi.org/10.1016/j.jmatprotec.2006.08.030
Mohammed GR, Ishak M, Aqida SN, Abdulhadi HA (2017) Effects of heat input on microstructure, corrosion and mechanical characteristics of welded austenitic and duplex stainless steels: a review. Metals (Basel) 7
International Molybdenum Association (IMOA) (2014) Practical guidelines for the fabrication of duplex stainless steels 2, 3rd ed. Int Molybdenum A (IMOA)
International Organization for Standardization (2017) ISO 17781:2017 Petroleum, petrochemical and natural gas industries — test methods for quality control of microstructure of ferritic/austenitic (duplex) stainless steels. 7–13
International Organization for Standardization (2019) International Standard ISO 15156–3:2015 Technical circular 3-petroleum and natural gas industries-materials for use in h2s-containing environments in oil and gas production, part 3: cracking-resistant CRAs (corrosion-resistant alloys) and other alloys. 6
NORSOK (2016) Welding and inspection of piping M-601:2016. 14–21
European committee for standardization (2021) EN 13445–4:2021 Unfired pressure vessels – part 4: fabrication. 33
Det Norske Veritas (2021) DNV-ST-F101 Submarine pipeline systems. 166
Outokumpu Stainless AB (2010) Plasma arc welding (PAW). In: Welding Handbook, 1st ed. Outokumpu Oyj. Avesta 29
Pekkarinen J, Kujanpää V (2010) The effects of laser welding parameters on the microstructure of ferritic and duplex stainless steels welds. In: Physics Procedia. Elsevier B.V 517–523
Outokumpu Stainless AB (2010) Plasma arc welding (PAW). In: Welding Handbook, 1st ed. Outokumpu Oyj. Avetsta 29–30
Westin EM, Serrander D (2012) Experience in welding stainless steels for water heater applications. Weld World 56:14–28. https://doi.org/10.1007/BF03321346
Ramesh Singh (2016) Physics of welding. In: Applied Welding Engineering, 2nd ed. Elsevier 131–162
Outokumpu Stainless AB (2010) Shielding and backing gases. In: Welding Handbook , 1st ed. Outokumpu Oyj. Avesta 46–47
Outokumpu Stainless AB (2009) Delivery testing and acceptance testing. In: Corrosion Handbook 75th Anniversary Edition, 75th ed. Outokumpu Oyj I:40
European Committee for Standardization (2015) SS-EN ISO 5817:2014 Welding – fusion-welded joints in steel, nickel, titanium and their alloys (beam welding excluded) – quality levels for imperfections
Chan KW, Tjong SC (2014) Effect of secondary phase precipitation on the corrosion behavior of duplex stainless steels. Materials 7:5268–5304
Baghdadchi A, Hosseini VA, Hurtig K, Karlsson L (2021) Promoting austenite formation in laser welding of duplex stainless steel-impact of shielding gas and laser reheating. Weld World 65:499–511. https://doi.org/10.1007/s40194-020-01026-7/Published
Taban E, Kaluc E (2011) Welding behaviour of duplex and superduplex stainless steels using laser and plasma arc welding processes. Weld World 55:48–57. https://doi.org/10.1007/BF03321307
Cui S, Pang S, Pang D et al (2021) Numerical simulation and experimental investigation on 2205 duplex stainless steel K-TIG welded joint. Metals (Basel) 11. https://doi.org/10.3390/met11081323
Hosseini V (2016) Influence of multiple welding cycles on microstructure and corrosion resistance of a super duplex stainless steel. Licentiate Thesis, University West
Cui S, Yu Y, Tian F, Pang S (2022) Morphology, microstructure, and mechanical properties of S32101 duplex stainless-steel joints in K-TIG welding. Materials 15. https://doi.org/10.3390/ma15155432
Cui S, Shi Y, Cui Y, Zhu T (2018) The impact toughness of novel keyhole TIG welded duplex stainless steel joints. Eng Fail Anal 94:226–231. https://doi.org/10.1016/j.engfailanal.2018.08.009
Karlsson L, Arcini H (2012) Low energy input welding of duplex stainless steels. Weld World 56
Omura T, Kushida T, Komizo Y (2000) Microstructural features and corrosion properties in laser welded duplex stainless steels. Weld Int 14:257–260. https://doi.org/10.1080/09507110009549176
Asuncion M, Bermejo V, Pandian KT et al (2021) Microstructure of laser metal deposited duplex stainless steel: influence of shielding gas and heat treatment. Weld World. https://doi.org/10.1007/s40194-020-01036-5/Published
Kotecki DJ (2010) Some pitfalls in welding of duplex stainless steels (Algumas Armadilhas na Soldagem de Aços Inoxidáveis Duplex). Soldag insp 15:336–343
Sales AM, Westin EM, Jarvis BL (2017) Effect of nitrogen in shielding gas of keyhole GTAW on properties of duplex and superduplex welds. Weld World 61:1133–1140. https://doi.org/10.1007/s40194-017-0486-1
Hosseini V (2018) Super duplex stainless steels-microstructure and properties of physically simulated base and weld metal
Varbai B, Adonyi UY, Baumer R et al (2019) Weldability of duplex stainless steels-thermal cycle and nitrogen effects. Weld J 98:78–87. https://doi.org/10.29391/2019.98.006
Karlsson L, Rigdal S, Bergquist EL, Arcini H (2007) Int. Conf. Duplex 2007. In: Effects of alloying elements on properties of duplex Weld Metal
Holländer Pettersson N, Lindell D, Lindberg F, Borgenstam A (2019) Formation of chromium nitride and intragranular austenite in a super duplex stainless steel. Metall Mater Trans A Phys Metall Mater Sci 50:5594–5601. https://doi.org/10.1007/s11661-019-05489-2
Varbai B, Pickle T, Májlinger K (2019) Effect of heat input and role of nitrogen on the phase evolution of 2205 duplex stainless steel weldment. Int J Press Vessel Pip 176. https://doi.org/10.1016/j.ijpvp.2019.103952
Outokumpu Stainless AB (2010) Duplex (austenitic-ferritic) stainless steels. In: Welding Handbook , 1st ed. Outokumpu Oyj. Avesta 20
Hosseini AV, Karlsson L (2019) Physical and kinetic simulation of nitrogen loss in high temperature heat affected zone of duplex stainless steels. Materialia 6. https://doi.org/10.1016/j.mtla.2019.100325
Acknowledgements
Outokumpu provided the material. Rickard Aldén (currently AFRY, formerly Swerim AB) design and interpretation of DoE. Oskar Karlsson (Swerim AB) SEM analysis. Jesper Flyg (Swerim AB) design and analysis of corrosion testing.
Funding
The investigation was financed by the Centre for Joining and Structures (CJS) at Swerim AB.
This research was partially sponsored by XPRES (Centre of Excellence in Production Research) – a strategic research area in Sweden.
Author information
Authors and Affiliations
Contributions
Conceptualisation: all authors; methodology: all authors; welding: Erwan Siewert, Nakhleh Hussary, Michael Pfreuntner; analysis and investigation: Klara Trydell, Kjell-Arne Persson; writing—original draft preparation: Klara Trydell; writing—review and editing: Klara Trydell, Kjell-Arne Persson, Nuria Fuertes.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trydell, K., Persson, KA., Fuertes, N. et al. Ferrite fraction in duplex stainless steel welded with a novel plasma arc torch. Weld World 67, 805–817 (2023). https://doi.org/10.1007/s40194-022-01447-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40194-022-01447-6